Abstract
We have examined the detailed kinetics and thermodynamics of the association of Ergosta-5,7,9(11),22-tetraen-3β-ol (dehydroergosterol, DHE) with lipid bilayers prepared from 1-palmitoyl-2-oleoylphosphatidylcholine (POPC), a 1:1 binary mixture of POPC and cholesterol (Chol), and a 6:4 binary mixture of egg sphingomyelin (SpM) and Chol. Association of DHE with all three membranes was shown to be entropically driven, most so in the case of SpM-Chol bilayers. Equilibrium partition coefficients for partitioning of DHE between the lipid phase and the aqueous phase were shown to be similar for POPC and POPC-Chol bilayers between 15 and 35°C. Partitioning into the SpM-Chol bilayer is favored at higher temperatures and there is a crossover in solubility preference at ∼25°C. Insertion (k+) and desorption (k−) rate constants were shown to be very similar for POPC and POPC-Chol bilayer membranes, but were lower for SpM-Chol bilayers. Similar results were previously reported by us for the association of other amphiphiles with these membranes. We propose a model for the microscopic structure of a POPC-Chol (1:1) bilayer membrane that is consistent with these observations.
INTRODUCTION
The distribution of sterols, and in particular, of cholesterol, between domains of coexisting phospholipid phases in a membrane, between different types of cell membranes, and between membranes and other organized lipid assemblies, is a matter of vital importance in animal physiology (1–4). The basal noncatalyzed distribution of sterols between different lipid environments in a cell or in multicellular organisms (domains in a membrane, membranes in a cell, or other organized lipid assemblies) can be accurately predicted if the equilibrium thermodynamic and kinetic parameters that describe the association of the sterol with the lipid environment are known. The information is also fundamental in evaluating the efficiency of putative catalyzed processes in sterol homeostasis. This concept has prompted several laboratories over the last 30 years to examine the kinetics and thermodynamics of the interaction of sterols with membranes and lipoproteins ((5–27) and references cited therein; for a review of the literature before 1987, see (5)). Curiously, despite the large volume of literature on the subject, the only kinetic rate constants available for the interaction of sterols with membranes are the rate constants for their exchange between a donor and an acceptor species (both usually lipid assemblies). These exchange rate constants have been equated with the rate constants for desorption of the sterol from the donor lipid assembly on the assumption that the rate constant for its insertion into the acceptor lipid assembly is diffusion-limited. As we have shown elsewhere (28–33), this assumption is not valid for phospholipid-derived fluorescent lipid amphiphiles. The exchange rate constants are, in fact, a function of the rate constants for insertion, desorption, and translocation (flip-flop) of the sterol with regard to both the donor and the acceptor species (see, for example, Eqs. 5 and 6 and the relevant comments in Results). Detailed quantitative modeling of sterol homeostasis in a cell or in a whole animal requires knowledge of the values of equilibrium partition coefficients for partitioning of the sterols between the lipid and aqueous phases (or between coexisting lipid phases) and all of the kinetic rate constants for the partitioning process and is not possible when only the exchange rate constants are known.
In recent years we have attempted to obtain detailed kinetic and thermodynamic information concerning the partitioning of fluorescent phospholipid derivatives between the aqueous phase and lipid bilayer membranes in liquid-ordered (lo) and liquid-disordered (ld) phases (28,29) and between the aqueous phase and lipoproteins (30). To do these studies it was necessary to develop a strategy (32,33) that would permit us to work with fluorescent phospholipid derivatives at concentrations in the range of ∼10−6 M in the aqueous phase without having to worry about the complex self-aggregation behavior that is often characteristic for these amphiphiles. The strategy involves use of a binding equilibrium with a binding agent, usually serum albumin, whose concentration can be adjusted to values at which the amphiphile is mostly in the bound state in equilibrium with a very small amount (far below the critical aggregation concentration) of the amphiphile in aqueous solution. Addition of the lipid assemblies (liposomes or lipoproteins) then results in two competing processes for binding of the amphiphile in the aqueous phase, namely, association with the lipid assembly and binding to the albumin. Prior knowledge of the equilibrium association and kinetic rate constants for binding to albumin allows extraction of the kinetic and equilibrium parameters that characterize the association with the lipid assemblies. The temperature-dependence of these results permits us to obtain a complete thermodynamic description of the process as well. We have also made an attempt to explain the mechanism of the association of amphiphiles with lipid bilayers, in which the association rate constant is clearly not diffusion-limited as had been generally assumed in the literature, based on these detailed results (33).
In this article, we report our results on the association of dehydroergosterol (DHE), a naturally occurring fluorescent sterol, with 100 nm unilamellar liposomes prepared from 1-palmitoyl-2-oleoylphosphatidylcholine (POPC), in the ld phase; and from mixtures of POPC and cholesterol (1:1 molar ratio), and egg sphingomyelin (SpM) and cholesterol (Chol, 6:4 molar ratio). Of the latter two membrane systems, those prepared from the SpM-Chol binary mixture are clearly in an lo phase (34), and it is generally assumed that the POPC-Chol membranes are in the lo phase as well (35,36). Our results indicate that POPC-Chol membranes are probably better characterized as ld phase membranes with a microheterogeneous structure that results from a cosmophilic/cosmophobic (order-loving/order-hating) amphiphilicity of the POPC molecule in the presence of flat rigid surfaces such as those presented by cholesterol.
MATERIALS AND METHODS
Bovine serum albumin (BSA), essentially free of fatty acids (∼0.005%), ergosta-5,7,9(11),22-tetraen-3β-ol (dehydroergosterol, DHE), and egg yolk sphingomyelin (SpM) were obtained from Sigma-Aldrich Química (Sintra, Portugal). Cholesterol (Chol) was from Serva/Boehringer Ingelheim (Heidelberg, Germany), and 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) was obtained from Avanti Polar Lipids (Alabaster, AL). All reagents used were of the highest commercially available purity.
Phospholipid concentrations were determined using a modified version of Bartlett's phosphate assay (37) and cholesterol concentrations were determined by the Lieberman-Burchard method as described by Taylor et al. (38). BSA concentrations were determined using the method of Lowry et al. (39) or by their absorbance at 279 nm using an extinction coefficient of 0.667 mg−1 mL cm−1 (40), and DHE concentration was determined by spectrophotometry using a molar extinction coefficient at 340 nm of 8100 M−1 cm−1 in acetone. Absorption spectra were recorded on a Unicam UV530 UV/Vis spectrophotometer and fluorescence measurements were performed on a Cary Eclipse fluorescence spectrophotometer equipped with a thermostated multi-cell holder accessory (Varian, Cary, NC). The samples were stirred continuously during measurements.
Aqueous suspensions of lipids were prepared by evaporating a solution of the desired lipid or premixed lipid mixture in chloroform/methanol (87/13, v/v) solution by blowing dry nitrogen over the heated (blowing hot air over the external surface of the tube) solution and then leaving the residue in a vacuum desiccator for at least 12 h at 23°C. The dry residue and the hydration solution (0.11 M NaCl, 1 mM EDTA, 0.02% NaN3, pH 7.4) were preheated, in a water bath, at 65°C. The hydration volume used was calculated to obtain a final lipid concentration of ∼5 × 10−3 M. The samples were submitted to several cycles of vortex/incubation at the specified temperature for at least 1 h. The resulting multilamellar vesicle (MLV) suspensions were extruded (41) through two stacked polycarbonate filters (Nucleopore) with a pore diameter of 0.1 μm using a minimum of 10 passes. During the extrusion the water-jacketed extruder (Lipex Biomembranes, Vancouver, British Columbia, Canada) was maintained at 65°C (for membranes prepared from mixtures of SpM and Chol) and at 23°C (for other membranes). The large unilamellar vesicle (LUV) suspensions obtained after extrusion were diluted in buffer to obtain the desired lipid concentration for fluorimetric experiments.
Preassociation of DHE with BSA was done by squirting an acetone solution of DHE into an aqueous buffered (0.02 M sodium phosphate, 0.11 M NaCl, 1mM EDTA, 0.02% NaN3, pH 7.4) solution of BSA at the desired concentration while vortexing the latter. Vortexing was carried on for a further 5 min. The solution was then allowed to attain equilibrium over a period of 0.5–1 h at the desired temperature (between 15 and 45°C). The final concentration of acetone was always 1% (v/v). The kinetics of association of DHE with BSA was studied essentially as described by Abreu et al. (32), using 2,4-dinitrobenzene (42) as the energy transfer partner for DHE.
The kinetics of association of DHE with LUV were studied by addition of the desired amount of LUV (between 1 × 10−10 to 2 × 10−9 M) to a solution of DHE (∼2 × 10−7 M), which had been previously equilibrated with BSA (∼2.7 × 10−4 M) as described above. Fluorescence emission changes at 374 nm (excitation at 324 nm) were followed in time. Data was analyzed using Excel and Solver (MicroSoft, Seattle, WA).
RESULTS
Binding of DHE to bovine serum albumin
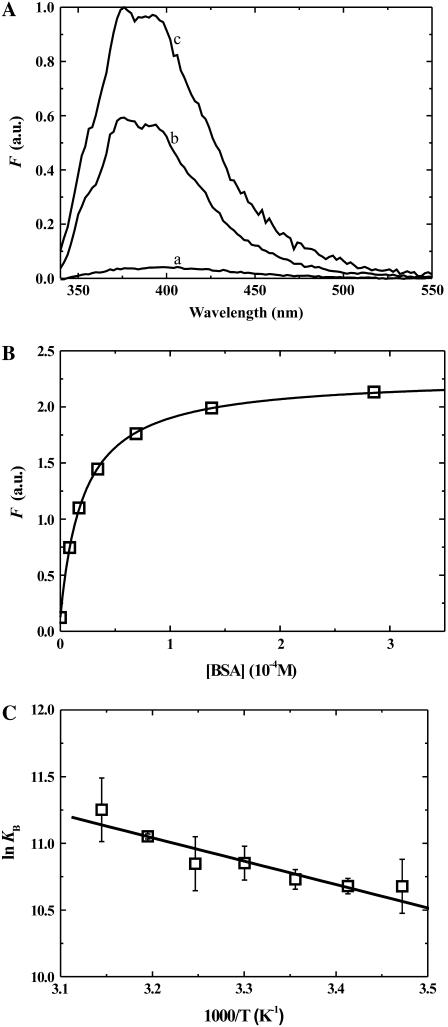
DHE, like many amphiphiles in aqueous solution, has a very low solubility and forms aggregates at concentrations as low as 2 × 10−8 M (43,44). DHE also binds to bovine serum albumin (BSA) with an increase in its fluorescence quantum yield compared to the aqueous solution as seen in Fig. 1 A. This change in fluorescence quantum yield was exploited to determine the equilibrium binding constants (KB) by equilibrium titration as shown in Fig. 1 B. KB was determined at various temperatures between 15 and 35°C, the corresponding van 't Hoff plot is shown in Fig. 1 C.
FIGURE 1.
(A) Comparison of the fluorescence emission spectra of DHE (2 × 10−7 M) in aqueous solution at pH 7.4 (spectrum a); associated with POPC LUV (2 × 10−9 M, spectrum b) and bound to BSA (2.6 × 10−4 M, spectrum c) at 35°C. (B) Equilibrium titration of a solution of 2 × 10−7 M DHE with BSA, in buffer at pH 7.4 at 35°C (KB ∼5 × 104 M−1). (C) Van 't Hoff plot for the association of DHE with BSA. The results shown represent the mean ± SD of three independent experiments.
In the equilibrium titrations of DHE with BSA, we added an acetone solution of DHE to an aqueous solution of BSA at the desired concentration and followed the change in fluorescence intensity with time. Equilibrium was attained in several seconds. If these solutions were allowed to equilibrate over long periods of time (hours), a slow reduction in fluorescence intensity was observed. A similar, although much faster, reduction in fluorescence intensity was observed when no BSA was present and the rate of reduction in fluorescence intensity was also seen to be dependent upon the BSA concentration, slower at higher BSA concentrations. We attributed the reduction in fluorescence to a slow aggregation of DHE in aqueous solution since the total DHE concentration in these titrations was approximately an order-of-magnitude higher than its reported critical aggregation concentration of ∼2 × 10−8 M. Under conditions where DHE was transferred between BSA and LUV (see below), the reduction of fluorescence intensity was ∼5% after 24 h.
We studied the kinetics of association of DHE with BSA (data not shown) using a methodology previously described by us (32). The characteristic time for the association process is ≤4 s under conditions where the binding step is pseudo-first order with respect to BSA.
Transfer of DHE between albumin and LUV
When LUV are added to a mixture of DHE and BSA at equilibrium with each other, the DHE in the aqueous phase is free to associate with the LUV as well as with the BSA. The two competing equilibria can be written as
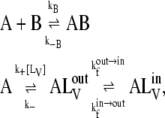 |
(1) |
where A is DHE, B is BSA, LV is the LUV, and AB and ALV are DHE associated with BSA and LUV, respectively. The subscripts B, −B, +, −, and f refer to the association/dissociation reactions with BSA, with LUV, and to the transmembrane translocation processes (flip-flop), respectively. The superscripts in and out refer to the inner and outer monolayers of the LUV. In the second equilibrium displayed in Eq. 1, the association of a molecule of DHE with an LUV does not reduce the capacity of that LUV to accommodate more molecules of DHE under the conditions of our experiment. The pseudo-first order rate constant for the forward process in this equilibrium is, therefore, k+[LV]. The relevant mass balances in the experiments reported here are given by
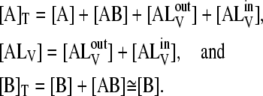 |
The LUVs have a diameter of 100 nm so that the ratio of inner monolayer surface to the outer monolayer surface is 0.92. For purposes of simplicity we considered 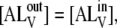 so that
so that 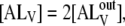 and
and 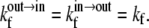 With these approximations, it can be shown that
With these approximations, it can be shown that
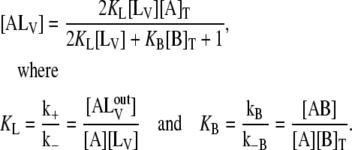 |
(2) |
The kinetic scheme shown in Eq. 1 is described by the following set of differential equations:
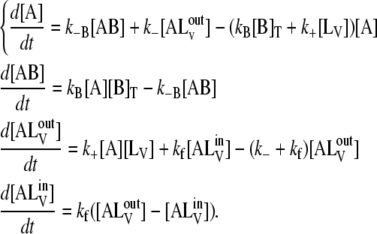 |
Assuming rapid equilibrium for the association between A and B, and between 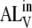 and
and 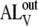 (sterol translocation rates have been reported to have characteristic times from ≤1 s (17) to ≤50 s (45)), it can be shown that
(sterol translocation rates have been reported to have characteristic times from ≤1 s (17) to ≤50 s (45)), it can be shown that
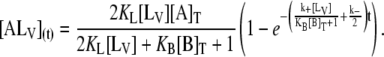 |
(3) |
The fluorescence intensity of DHE associated with lipid vesicles is shown in Fig. 1 A. After addition of LUV to an equilibrated mixture of DHE and BSA, a decrease in fluorescence intensity in time is observed that relates to the extent of transfer of DHE from BSA to LUV through the aqueous phase. At any given time, the fluorescence intensity of the total reaction mixture is given by
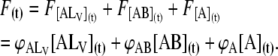 |
(4) |
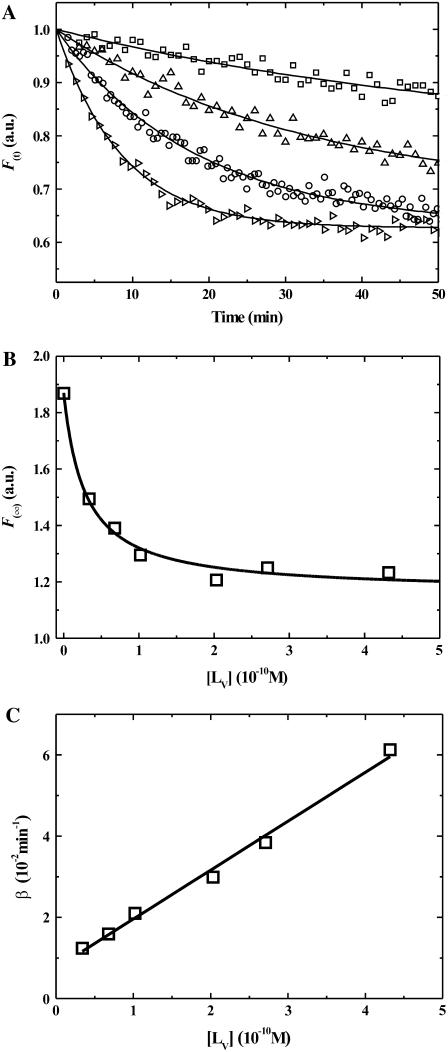
Here, ϕi is the proportionality constant that relates the fluorescence intensity to the concentration of the species i. Since ϕA is negligible in comparison with ϕALV and ϕAB, and [A] is also negligible as compared to [AB] and [ALV], the ϕA [A](t) term in Eq. 4 may be neglected. Fig. 2 A shows typical traces of the temporal evolution of F(t) for POPC-Chol LUV at 25°C. These traces are mono-exponential decays that may be fitted by an expression of the form
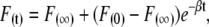 |
(5) |
where β is a transfer rate constant.
FIGURE 2.
(A) Time course of the association of DHE (2.2 × 10−7 M) preequilibrated with BSA (2.7 × 10−4 M) in buffer at pH 7.4, with POPC-Chol LUVs (from top to bottom: 3.4 × 10−11, 1.0 × 10−10, 2.7 × 10−10, 4.3 × 10−10) at 25°C. (B) Equilibrium titration of POPC-Chol LUV with DHE previously equilibrated with 2.7 × 10−4 M BSA. The points are experimental, the line is a theoretical best fit. (C) Dependence of the transfer rate constant, β, upon the LUV concentration, [LV], at 25°C and the best fit of Eq. 6 to the data.
The concentrations of the relevant species at t = 0 and t = ∞ are given by
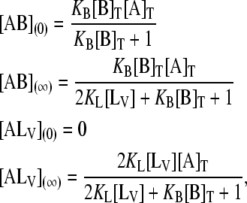 |
from which F(∞), F(0), and β can be defined as
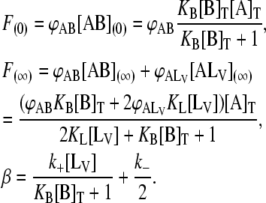 |
(6) |
From the values of F(∞) obtained by fitting curves of the type shown in Fig. 2 A using different concentrations of lipid vesicles ([LV]) we obtained titration curves for the association of DHE with the lipid vesicles (Fig. 2 B). From these titration curves, values of KL and their temperature dependence were independently extracted for each of the three lipid vesicle systems examined in this work. Further, at each temperature, β was determined for several values of [LV] between 3 × 10−11 and 2 × 10−9 M (between three and six ab initio repetitions). The dependence of β on [LV] at 25°C is shown in Fig. 2 C. The values of k+ and their standard deviations were obtained (Eq. 6) from the slopes of plots of β versus [LV] similar to that shown in Fig. 2 C. The values of k− could, in principle, be obtained from the intercept of the same plots but the error involved in these determinations was too large. We, therefore, determined the values of k− from the independently determined values of KL and k+ (k− = k+/KL). Experiments were performed between 15 and 35°C. Table 1 lists the kinetic and equilibrium constants at 15 and 35°C for association of DHE with three types of lipid bilayer LUV. The equilibrium partition coefficient, KP(L/W), for the partitioning of DHE between the lipid phases examined and the aqueous phase was determined from the respective values of KL as described previously (28). The values of KP(L/W) are also listed in Table 1.
TABLE 1.
Kinetic constants for the association of DHE with lipid bilayer membranes at 288 and 308 K
| POPC
|
POPC-Chol (1:1)
|
SpM-Chol (6:4)
|
||||
|---|---|---|---|---|---|---|
| 288 K | 308 K | 288 K | 308 K | 288 K | 308 K | |
| k+ (M−1 s−1) | 4.8 ± 0.4 × 106 | 5.1 ± 0.5 × 107 | 6.3 ± 0.6 × 106 | 6.4 ± 0.6 × 107 | 6.3 ± 0.5 × 105 | 1.3 ± 0.1 × 107 |
| k− (s−1) | 4.7 ± 1.1 × 10−5 | 1.0 ± 0.2 × 10−3 | 4.5 ± 1.4 × 10−5 | 6.4 ± 2.1 × 10−4 | 1.4 ± 0.4 × 10−5 | 6.9 ± 2.1 × 10−5 |
| KL (M−1) | 1.0 ± 0.2 × 1011 | 5.1 ± 1.1 × 1010 | 1.4 ± 0.4 × 1011 | 1.0 ± 0.3 × 1011 | 4.6 ± 1.3 × 1010 | 1.9 ± 0.6 × 1011 |
| KP(L/W) | 2.6 × 106 | 1.3 × 106 | 3.6 × 106 | 2.5 × 106 | 1.2 × 106 | 4.8 × 106 |
Temperature-dependence of the equilibrium and kinetic rate constants
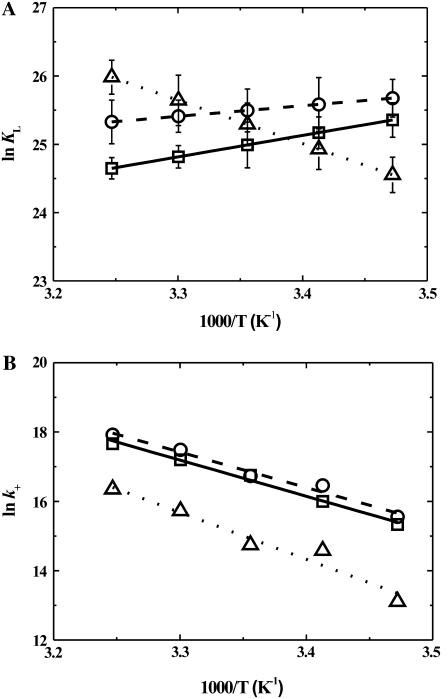
All experiments were performed between 15 and 35°C at 5°C intervals. The equilibrium constants, KL, determined from titration curves at different temperatures, were used to generate van 't Hoff plots (Fig. 3 A) from which the corresponding values of ΔHo were obtained. The values of the derived thermodynamic parameters (ΔGo, ΔHo, and TΔSo) at 35°C are listed in Table 2. The association of DHE with all three lipid bilayers has a large entropic component as might be expected due to the hydrophobic effect. This result is qualitatively similar, although not quantitatively identical, to our previous results with the association of two lipid-derived amphiphiles, NBD-DMPE and NBD-lysoMPE, with the same set of membranes (28,29). In particular, the large endothermic component that characterizes the association of DHE with SpM-Chol bilayers suggests that this lipid bilayer phase is not capable of adequately “wetting” the DHE solute.
FIGURE 3.
(A) van 't Hoff plots for the association of DHE with LUV made from: (□) POPC; (○) POPC-Chol (1:1); and (Δ) SpM-Chol (6:4), showing the respective standard deviations. (B) Arrhenius plots for the insertion of DHE into: (□) POPC; (○) POPC-Chol (1:1); and (Δ) SpM-Chol (6:4) LUVs. The values shown are average values of between three and six independent experiments. The standard deviations are within the size of the symbols.
TABLE 2.
Thermodynamic constants for the association of DHE with lipid bilayer membranes at 308 K
| POPC | POPC-Chol (1:1) | SpM-Chol (6:4) | |
|---|---|---|---|
| ΔG° (kJ mol−1) | −63 | −65 | −67 |
| ΔH° (kJ mol−1) | −26 ± 5 | −13 ± 10 | 53 ± 7 |
| TΔS° (kJ mol−1) | 37 ± 5 | 52 ± 10 | 119 ± 7 |
| Eact (desorb) (kJ mol−1) | 113 | 98 | 60 |
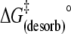 (kJ mol−1) (kJ mol−1) |
93 | 94 | 100 |
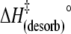 (kJ mol−1) (kJ mol−1) |
110 ± 7 | 95 ± 12 | 57 ± 16 |
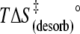 (kJ mol−1) (kJ mol−1) |
17 ± 7 | 1 ± 12 | −43 ± 16 |
| Eact (insert) (kJ mol−1) | 87 | 86 | 113 |
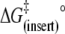 (kJ mol−1) (kJ mol−1) |
30 | 30 | 34 |
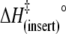 (kJ mol−1) (kJ mol−1) |
84 ± 5 | 83 ± 7 | 110 ± 14 |
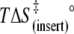 (kJ mol−1) (kJ mol−1) |
54 ± 5 | 53 ± 7 | 77 ± 15 |
Arrhenius plots of the rate constants for insertion of DHE into the bilayer membranes examined are shown in Fig. 3 B and the results at 35°C are listed in Table 2. The thermodynamics of the activation process (the transition state is one in which most of the probe is out of the bilayer; see details in (28)) was analyzed using transition state theory (46).
Hypothetical partitioning between coexisting lipid phases
As discussed elsewhere (28,29), the measured lipid phase/aqueous phase equilibrium partition coefficients, KP(L/W), can be used to determine hypothetical partition coefficients between the lipid phases assuming that they existed in the same lipid bilayer. According to the published phase diagram of the POPC/SpM-Chol ternary system in excess water (36), the POPC-Chol and SpM-Chol phases we have studied here are mutually miscible at all proportions but we have reasons (W. L. C. Vaz, M. J. Moreno, and E. Melo, unpublished results) to doubt this. (Note: Specifically, we have examined the long-range translational diffusion of two fluorescent lipid-derived probes in bilayers prepared from ternary mixtures of egg SpM, POPC, and Chol at a constant 0.5 molar fraction of Chol with a varying POPC/SpM ratio between 1:0 and 0:1 at intervals of 0.2. One of the probes had a preference for partitioning into liquid-disordered and the other into liquid-ordered membrane phases. Both probes show percolation behavior with a percolation threshold at a POPC/SpM molar ratio of between 0.30 and 0.35. This percolation behavior is characteristic of a system in which there are two coexisting liquid phases where the theoretical threshold is expected to be 0.33 ± 0.02 (57,58). In our case, the two coexisting liquid phases would be a SpM:Chol-rich liquid-ordered phase and a POPC:Chol-rich liquid-disordered phase. Thus, contrary to the published phase diagram for the ternary mixture of POPC/SpM/Chol (36), our result suggests that these three-component membranes are heterogeneous at 50 mol % Chol.) We, therefore, report the hypothetical partition coefficients for DHE between the lipid phases we have examined in Table 3. The endothermic association of DHE with SpM-Chol bilayers compared with the exothermicity of its association with POPC and POPC-Chol bilayers makes the hypothetical partitioning of DHE between POPC and SpM-Chol, or POPC-Chol and SpM-Chol membranes, different at 15 and 35°C. As seen in Table 3, DHE would partition preferentially into POPC or POPC-Chol bilayers at 15°C but would prefer the SpM-Chol bilayers at 35°C. At 25 °,C DHE would partition equally between POPC or POPC-Chol bilayers, on the one hand, and SpM-Chol bilayers, on the other.
TABLE 3.
Hypothetical equilibrium partition coefficients and thermodynamic parameters for partitioning of DHE between coexisting lipid phases at 288 and 308 K
|
KP(L1/L2)
|
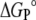 (kJ mol−1) (kJ mol−1)
|
||||
|---|---|---|---|---|---|
| Type of phase coexistence | 288 K | 308 K | 288 K | 308 K | |
| POPC/POPC-Chol (1:1) | ld / lo | 0.7 | 0.5 | 1 | 2 |
| POPC/SpM-Chol (6:4) | ld / lo | 2.2 | 0.3 | −2 | 3 |
| POPC-Chol (1:1)/SpM-Chol (6:4) | lo / lo | 3.1 | 0.5 | −3 | 2 |
DISCUSSION
The homeostasis of cholesterol in animal cells and in the whole animal is increasingly recognized as an important parameter in a large number of cholesterol-related diseases. There has been, as a consequence, a vast body of literature that addresses this question (for reviews see (1,3)). In particular, the exchange of sterols between lipid bilayer membranes and acceptor entities (usually other lipid bilayer membranes) has been examined in some detail. Most of the work reported was done under conditions in which the donor→acceptor transfer is limited by first-order desorption of the sterols from the donor membranes so that the exchange rate constants obtained are roughly comparable to the true desorption rate constants measured in our work and listed in Table 1. In Table 4, we have compiled some of the work reported in the literature. Most of the work reporting nonmediated exchange of sterols between donor and acceptor membranes used donor membranes made from egg yolk PC or POPC with sterol contents significantly lower than those that result in a pure lo phase. Those results should, therefore, be compared with our results for pure POPC ld phase membranes. Data directly comparable with our results for POPC-Chol (supposedly lo phase membranes) and lo phase SpM-Chol membranes are relatively scarce. Another aspect of the data compiled in Table 4 is that most of that work used small unilamellar vesicles (SUV) whereas our work used LUV with a 100-nm diameter. Despite all the differences, and notwithstanding the observation (22) that exchange rates from SUV donors are twice as large as exchange rates from LUV donors, it is noteworthy that the exchange rate constants reported in the literature for DHE are similar to the desorption rate constants measured by us whereas the exchange rates reported for cholesterol are somewhat slower.
TABLE 4.
Some reported rate constants for exchange of sterols between lipid bilayer (donors) and other lipid bilayers or cyclodextrin (acceptors)
| Exchanged molecule | Donor membrane | Acceptor membrane | kexchange (s−1) | T (K) | Reference |
|---|---|---|---|---|---|
| Cholesterol | Erythrocyte ghosts | eggPC, SUV | 1.3 × 10−5 | 319 | (19) |
| PC-Chol-dicetylphosphate (65:20:15); SUV | PC-Chol (80:20); SUV | 8.9 × 10−5 | 310 | (20) | |
| eggPC-Chol (90:10); SUV | eggPC-Chol (90:10); SUV | 1.6 × 10−4 | 310 | (21) | |
| eggPC-Chol (94:6); SUV | eggPC-Chol (94:6); SUV | 6.6 × 10−5 | 310 | (22) | |
| eggPC-Chol (94:6); SUV | eggPC-Chol (94:6); LUV | 7.5 × 10−5 | 310 | ||
| eggPC-Chol (94:6); LUV | eggPC-Chol (94:6); SUV | 3.5 × 10−5 | 310 | ||
| eggPC-Chol (94:6); LUV | eggPC-Chol (94:6); LUV | 3.6 × 10−5 | 310 | ||
| SpM-Chol (1:1); SUV | Rat liver microsomes | 6.8 × 10−6 | 310 | (23) | |
| PC-Chol (1:1); SUV | Rat liver microsomes | 7.5 × 10−5 | 310 | ||
| POPC-Chol (99:1); SUV | POPC-Chol (99:1); SUV | 2.5 × 10−4 | 310 | (24) | |
| POPC-Chol (90:10); SUV | POPC-Chol (90:10); SUV | 1.6 × 10−4 | 310 | ||
| POPC-Chol (80:20); SUV | POPC-Chol (80:20); SUV | 1.3 × 10−4 | 310 | ||
| eggPC-Chol-Cerebroside (75:10:15); SUV | eggPC; LUV | 1.2 × 10−4 | 310 | (12) | |
| eggPC-Chol-Cerebroside (75:10:15); LUV | eggPC; LUV | 5.0 × 10−5 | 310 | ||
| eggPC-Chol-Cerebroside (75:10:15); MLV | eggPC; LUV | 1.6 × 10−5 | 310 | ||
| DPPC-POPG-Chol (80:15:5); LUV | Cyclodextrin | 2.5 × 10−2 (78%) | 310 | (25) | |
| 5.3 × 10−5 (22%) | |||||
| CHO-K1 cells | Cyclodextrin | 4.6 × 10−2 (25%) | 310 | (26) | |
| 5.5 × 10−4 (75%) | |||||
| DHE | POPC-DHE (65:35); SUV | POPC-DHE (65:35) | 3.6 × 10−4 (12.5%) | 297 | (6) |
| 4.9 × 10−5 (87.5%) | |||||
| POPC-DHE (99:1); SUV | POPC-POPG (85:15); SUV | 7.7 × 10−4 | 298 | (27) | |
| 4.3 × 10−4 | |||||
| POPC-DHE (65:35); SUV | POPC-DHE (65:35); SUV | 3.9 × 10−4 (36%) | 310 | (8) | |
| 1.0 × 10−4 (64%) | |||||
| POPC; SUV | Cyclodextrin | 2.6 × 10−1 | 283 | (45) | |
| POPC; LUV | Cyclodextrin | 1.7 × 10−1 | 283 |
Exchange rate constants reported in the literature for membrane→cyclodextrin sterol exchange have also been included in Table 4. The kinetics for exchange of sterols between membrane donors and the cyclodextrin acceptor are generally bimodal. One of the exchange rate constants is comparable to and the other is approximately three orders-of-magnitude larger than the desorption rate constants that we measure. It would appear that cyclodextrin-mediated depletion of sterols from membranes occurs via two mechanisms one of which involves capture of a desorbed sterol in the aqueous phase and the other a process in which nonassisted sterol desorption from the donor membrane into the aqueous phase is not a rate-limiting step.
Considering that the insertion rate constants reported in Table 1 are not values expected for a diffusion-limited process, we have to understand what property of the outer monolayer of the LUV limits the insertion of DHE. The amphiphile is being driven into the monolayer by the hydrophobic effect and its insertion is resisted by the elasticity of the monolayer. Thus, k+ is related to the membrane elasticity, lower values for k+ being expected for a more elastic membrane. When we compare the values of k+ (see Table 1) for insertion of DHE into ld phase POPC bilayers and those for insertion into (supposedly) lo phase bilayers of POPC-Chol, we observe that the values are almost identical for the two membranes but are four and eight times lower for SpM-Chol membranes than they are for POPC membranes. This suggests that the in-plane elasticities of POPC and POPC-Chol membranes, seen by the DHE probe, are very similar but are both very different from the elasticity of SpM-Chol membranes. Once the DHE is incorporated into the membrane, its desorption to the membrane surface is determined by the van der Waals interactions between the DHE and the membrane interior. The magnitude of these van der Waals interactions is reflected in the value of the desorption rate constant, k−. Going back to Table 1, we note that k− for DHE desorption from POPC and POPC-Chol membranes are very similar to each other but significantly different from k− for DHE desorption from SpM-Chol membranes. Similar, although considerably more accentuated, results for the insertion and desorption rate constants of other amphiphiles (NBD-DMPE and NBD-lysoMPE) have been reported previously by us (28,29). On the basis of these cumulative results, we argue that the POPC-Chol membrane is more comparable to the POPC membrane than it is to the SpM-Chol membrane. However, micropipette measurements on 1-stearoyl-2-oleoyl phosphatidylcholine giant unilamellar vesicles show that the macroscopic in-plane elasticity of these membranes increases linearly with cholesterol concentration (47). Also, the condensing effect of cholesterol on POPC monolayers is significantly larger than it is on DPPC or SpM monolayers (48). On the other hand, recent volumetric studies (49) indicate that this condensing effect of cholesterol in membranes is not comparable for membranes of fully saturated PCs and SpMs on the one hand and PCs with one or two unsaturated chains on the other. The activation energies for lateral diffusion in bilayers prepared from binary mixtures of POPC and cholesterol compared to bilayers of saturated chain lipids and cholesterol ((50), W. L. C. Vaz, unpublished results) also indicates that POPC does not form an lo phase with cholesterol.
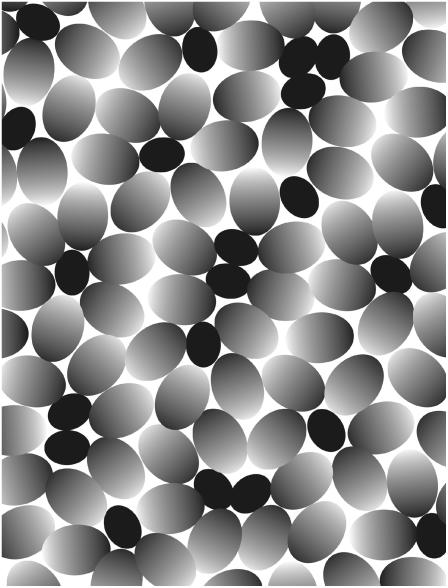
In light of the preceding, our results on the insertion and desorption rate constants of DHE (this work), NBD-DMPE (28), and NBD-lysoMPE (29) in their association with the POPC-Chol bilayer need to be explained on the basis of the properties of this membrane compared with the ld phase POPC membrane and the lo phase SpM-Chol membrane. A uniform condensing effect of cholesterol in the POPC-Chol membrane would not explain the close similarity of the observed k+ and k− values for these and the cholesterol-free POPC bilayers. It is also incompatible with the similarities of long-range diffusion in POPC-Chol membranes compared to membranes of pure POPC (50). On the other hand, the condensing effect of cholesterol cannot be denied. An intuitively appealing model that would be compatible with the observed results is based on a microheterogeneous structure for the POPC-Chol membrane (Fig. 4). It may be argued that, in a fluid phase membrane, the conformational entropy of the chains is so large that there is no preferential interaction between two fully saturated chains compared to the interaction between a fully saturated chain and an unsaturated chain. They are, therefore, completely miscible with each other. However, when a flat and rigid surface (such as presented by the cholesterol molecule) is incorporated into the membrane, the interaction of the fully saturated chains with that surface will be more exothermic than that of the cis-unsaturated chains. We may, therefore, expect a preferential solvation of the cholesterol surface by the saturated chains leading to a two-dimensional micellelike structure in the monolayer. The exothermicity of the interaction between the saturated chain and the flat rigid surface would, of course, have to compensate the decrease in entropy of the system. It must be emphasized that this micellar structure is a result of the relative cosmophilicity (order-loving) of a fully saturated acyl chain compared to a cis-unsaturated chain and is not related to the hydrophilicity/hydrophobicity that leads to formation of micelles in water. The concept is not new. Mitchell and Litman (51) proposed the formation of similar microheterogeneous structures in lipid bilayers prepared from lipids with one polyunsaturated acyl chain. In the context of this model for the microscopic structure of the POPC-Chol bilayer the insertion and desorption of amphiphiles will take place preferentially in the more disordered areas between micelles and will, therefore, not distinguish itself from the same processes in POPC membranes. In fact, the preferential orientation of the POPC molecules will generate regions of higher disorder (free area) and the insertion/desorption from the POPC-Chol bilayer could even be expected to be faster than from the pure POPC bilayer. This behavior is actually observed for the case of the interaction of NBD-DMPE with POPC and POPC-Chol bilayers (28). The proposed model is not antagonistic to the observations of Needham and Nunn (47) because those authors observe a macroscopic effect of cholesterol on the elasticity of the 1-stearoyl-2-oleoyl phosphatidylcholine bilayer and, within the context of our model (Fig. 4), this would be the mean over the entire microheterogeneous system. Our results on the insertion of amphiphiles into membranes, on the other hand, have a spatial resolution on the molecular dimension and the inserting probe only reports the elasticity of the membrane at its weakest point—the intermicellar space in the case of the microheterogeneous POPC-Chol bilayer.
FIGURE 4.
Model for the two-dimensional micellelike microheterogeneous structure proposed for a POPC-Chol membrane formed at low cholesterol concentrations. Cholesterol is represented by a solid ellipse, while POPC is represented by the ellipse with a gradient ranging from dark (representing the fully saturated palmitoyl chain of POPC) to light (representing the cis-unsaturated oleoyl chain of POPC). The preferential solvation of the (flat and rigid) cholesterol molecule by the fully saturated chains of POPC orients this side of the POPC molecules toward the cholesterol molecule while the cis-unsaturated chains are oriented away from it forming a two-dimensional micellelike structure.
The thermodynamics of association of DHE with the three types of bilayers examined is strongly entropic as may be expected from the hydrophobic effect. The association of DHE with SpM-Chol bilayers has one particularity that distinguishes it from the association of NBD-DMPE and NBD-lysoMPE with the same membranes: It has a very large endothermic component. It is also interesting to note that the enthalpy of association of DHE with POPC-Chol bilayers is approximately half as exothermic as the enthalpy of DHE association with POPC membranes, an observation similar to that reported by Tsamaloukas et al. (52). In the case of association of NBD-DMPE (28) and NBD-lysoMPE (29), however, the enthalpic and entropic contributions to the association free energy are identical for the association of each of these probes with POPC and POPC-Chol bilayers. The differences must have to do with the solvation of these probes (NBD-DMPE, NBD-lysoMPE, and DHE) by the different membrane phases but any further conclusions at this stage would be speculative.
We may also compare the lipid-phase/aqueous-phase partition coefficient for partitioning of DHE between the aqueous phase and the three membrane phases studied in this work. The endothermicity of DHE association with SpM-Chol bilayers results in a better partitioning of DHE into these bilayers at higher temperatures, the situation being the opposite in POPC and POPC-Chol membranes. KP(L/W) for the partitioning of DHE between POPC or POPC-Chol bilayers and the aqueous phase is not distinguishable within the limits of experimental error.
We have used KP(L/W) for the three lipid phases examined to calculate a hypothetical value for the partition coefficient for DHE between any two of these phases if they coexisted in the same membrane. The results shown, in Table 3, indicate that at 35°C, DHE would partition into the SpM-Chol phase in preference to the pure POPC or the POPC-Chol phase. At 15°C, however, the opposite is true. At room temperature (25°C), there would be no partitioning preference. Lindblom and colleagues (53) have recently reached a similar conclusion based upon long-range translational diffusion in these membranes. Earlier, McIntosh and co-workers (54) had reported values of KP for partitioning of cholesterol between SpM-poor, detergent-soluble membranes and SpM-rich, detergent-resistant membranes that, at 35°C, are similar to the values we report in Table 3. We may caution, however, that this result only implies a phase solubility preference and does not contradict the fact, well substantiated in the literature (16,55), that sterols have a preferred interaction with certain lipid chemical species, the physico-chemical basis for which is not yet completely clear. The hypothetical transfer of DHE from POPC or POPC-Chol bilayers to SpM-Chol bilayers would be an endothermic process with a large positive entropy of transfer (see Table 2). This, at first surprising, result is in agreement with the observation (56) that the tie lines in the lo–ld phase coexistence region of the phase diagram for the DOPC/DPPC/Chol ternary mixture become progressively horizontal as the temperature is decreased from 30 to 20°C.
Acknowledgments
This work was supported by the Fundação para a Ciência e a Tecnologia (FCT) of the Portuguese Ministry for Higher Education and Scientific Research through the POCTI program. L.M.B.B.E. acknowledges support through grant No. SFRH/BD/6746/2001 from the FCT.
Editor: Antoinette Killian.
References
- 1.Maxfield, F. R., and I. Tabas. 2005. Role of cholesterol and lipid organization in disease. Nature. 438:612–621. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield, F. R., and A. K. Menon. 2006. Intracellular sterol transport and distribution. Curr. Opin. Cell Biol. 18:379–385. [DOI] [PubMed] [Google Scholar]
- 3.Pennings, M., I. Meurs, D. Ye, R. Out, M. Hoekstra, T. J. C. Van Berkel, and M. Van Eck. 2006. Regulation of cholesterol homeostasis in macrophages and consequences for atherosclerotic lesion development. FEBS Lett. 580:5588–5596. [DOI] [PubMed] [Google Scholar]
- 4.Kalaany, N. Y., and D. J. Mangelsdorf. 2006. LXRs and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 68:159–191. [DOI] [PubMed] [Google Scholar]
- 5.Phillips, M. C., W. J. Johnson, and G. H. Rothblat. 1987. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim. Biophys. Acta. 906:223–276. [DOI] [PubMed] [Google Scholar]
- 6.Nemecz, G., R. N. Fontaine, and F. Schroeder. 1988. A fluorescence and radiolabel study of sterol exchange between membranes. Biochim. Biophys. Acta. 943:511–521. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder, F., and G. Nemecz. 1989. Interaction of sphingomyelins and phosphatidylcholines with fluorescent dehydroergosterol. Biochemistry. 28:5992–6000. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder, F., P. Butko, G. Nemecz, and T. J. Scallen. 1990. Interaction of fluorescent Δ5,7,9(11),22-ergostatetraen-3β-ol with sterol carrier protein-2. J. Biol. Chem. 265:151–157. [PubMed] [Google Scholar]
- 9.Miida, T., C. J. Fielding, and P. E. Fielding. 1990. Mechanism of transfer pf LDL-derived free cholesterol to HDL subfractions in human plasma. Biochemistry. 29:10469–10474. [DOI] [PubMed] [Google Scholar]
- 10.Kan, C. C., and R. Bittman. 1991. Spontaneous rates of sitosterol and cholesterol exchange between phospholipid vesicles and between lysophospholipid dispersions: evidence that desorption rate is impeded by the 24α-ethyl group of sitosterol. J. Am. Chem. Soc. 113:6650–6656. [Google Scholar]
- 11.Johnson, W. J., F. H. Mahlberg, G. H. Rothblat, and M. C. Phillips. 1991. Cholesterol transport between cells and high-density lipoproteins. Biochim. Biophys. Acta. 1085:273–298. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigueza, W. V., J. J. Wheeler, S. K. Klimuk, C. N. Kitson, and M. J. Hope. 1995. Transbilayer movement and net flux of cholesterol and cholesterol sulfate between liposomal membranes. Biochemistry. 34:6208–6217. [DOI] [PubMed] [Google Scholar]
- 13.Davidson, W. S., W. V. Rodrigueza, S. Lund-Katz, W. J. Johnson, G. H. Rothblat, and M. C. Phillips. 1995. Effects of acceptor particle size on the efflux of cellular free cholesterol. J. Biol. Chem. 270:17106–17113. [DOI] [PubMed] [Google Scholar]
- 14.Ohvo-Rekilä, H., B. Akerlund, and J. P. Slotte. 2000. Cyclodextrin-catalyzed extraction of fluorescent sterols from monolayer membranes and small unilamellar vesicles. Chem. Phys. Lipids. 105:167–178. [DOI] [PubMed] [Google Scholar]
- 15.Leventis, R., and J. R. Silvius. 2001. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 81:2257–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu, S. L., and B. J. Litman. 2002. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 83:3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steck, T. L., J. Ye, and Y. Lange. 2002. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys. J. 83:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann, N. A., D. P. Sullivan, H. Ohvo-Rekilä, C. Simonot, A. Pottekat, Z. Klaassen, C. T. Beh, and A. K. Menon. 2005. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 44:5816–5826. [DOI] [PubMed] [Google Scholar]
- 19.Lange, Y., J. S. D'Alessandro, and D. M. Small. 1979. The affinity of cholesterol for phosphatidylcholine and sphingomyelin. Biochim. Biophys. Acta. 556:388–398. [DOI] [PubMed] [Google Scholar]
- 20.McLean, L. R., and M. C. Phillips. 1981. Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles. Biochemistry. 20:2893–2900. [DOI] [PubMed] [Google Scholar]
- 21.McLean, L. R., and M. C. Phillips. 1984. Kinetics of phosphatidylcholine and lysophosphatidylcholine exchange between unilamellar vesicles. Biochemistry. 23:4624–4630. [DOI] [PubMed] [Google Scholar]
- 22.Fugler, L., S. Clejan, and R. Bittman. 1985. Movement of cholesterol between vesicles prepared with different phospholipids or sizes. J. Biol. Chem. 260:4098–4102. [PubMed] [Google Scholar]
- 23.Bhuvaneswaran, C., and K. A. Mitropoulos. 1986. Effect of liposomal phospholipid composition on cholesterol transfer between microsomal and liposomal vesicles. Biochem. J. 238:647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar, L. K., Y. Barenholz, and T. E. Thompson. 1986. Fraction of cholesterol undergoing spontaneous exchange between small unilamellar phosphatidylcholine vesicles. Biochemistry. 25:6701–6705. [DOI] [PubMed] [Google Scholar]
- 25.Yancey, P. G., W. V. Rodrigueza, E. P. C. Kilsdonk, G. W. Stoudt, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1996. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 271:16026–16034. [DOI] [PubMed] [Google Scholar]
- 26.Haynes, M. P., M. C. Phillips, and G. H. Rothblat. 2000. Efflux of cholesterol from different cellular pools. Biochemistry. 39:4508–4517. [DOI] [PubMed] [Google Scholar]
- 27.Bar, L. K., P. L. G. Chong, Y. Barenholz, and T. E. Thompson. 1989. Spontaneous transfer between phospholipid bilayers of dehydroergosterol, a fluorescent cholesterol analog. Biochim. Biophys. Acta. 983:109–112. [DOI] [PubMed] [Google Scholar]
- 28.Abreu, M. S. C., M. J. Moreno, and W. L. C. Vaz. 2004. Kinetics and thermodynamics of association of a phospholipid derivative with lipid bilayers in liquid-disordered and liquid-ordered phases. Biophys. J. 87:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampaio, J. L., M. J. Moreno, and W. L. C. Vaz. 2005. Kinetics and thermodynamics of association of a fluorescent lysophospholipid derivative with lipid bilayers in liquid-ordered and liquid-disordered phases. Biophys. J. 88:4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estronca, L. M. B. B., M. J. Moreno, J. A. N. Laranjinha, L. M. Almeida, and W. L. C. Vaz. 2005. Kinetics and thermodynamics of lipid amphiphile exchange between lipoproteins and albumin in serum. Biophys. J. 88:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaz, W. L. C., and E. C. C. Melo. 2001. Fluorescence spectroscopic studies on phase heterogeneity in lipid bilayer membranes. J. Fluor. 11:255–271. [Google Scholar]
- 32.Abreu, M. S. C., L. M. B. B. Estronca, M. J. Moreno, and W. L. C. Vaz. 2003. Binding of a fluorescent lipid amphiphile to albumin and its transfer to lipid bilayer membranes. Biophys. J. 84:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaz, W. L. C., M. J. Moreno, J. L. Sampaio, M. S. C. Abreu, and C. T. A. Vaz. 2004. Kinetics of the association of amphiphiles with lipid bilayers as a probe of membrane physical state. In Biophysical Society Discussions, 2004 Studybook. SP12A–SP12J.
- 34.Sankaram, M. B., and T. E. Thompson. 1990. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 29:10670–10675. [DOI] [PubMed] [Google Scholar]
- 35.Mateo, C. R., A. U. Acuña, and J.-C. Brochon. 1995. Liquid-crystalline phases of cholesterol/lipid bilayers as revealed by the fluorescence of trans-parinaric acid. Biophys. J. 68:978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Almeida, R. F. M., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartlett, G. R. 1959. Phosphorous assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- 38.Taylor, R. P., A. V. Broccoli, and C. M. Grisham. 1978. Enzymatic and colorimetric determination of total serum cholesterol. J. Chem. Educ. 55:63–64. [DOI] [PubMed] [Google Scholar]
- 39.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- 40.Peters, T., Jr. 1997. All About Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press, San Diego, CA.
- 41.Hope, M. J., M. B. Bally, G. Webb, and P. R. Cullis. 1985. Production of large unilamellar vesicles by a rapid extrusion procedure—characterization of size distribution, trapped volume, and ability to maintain a membrane potential. Biochim. Biophys. Acta. 812:55–65. [DOI] [PubMed] [Google Scholar]
- 42.Sanger, F. 1945. The free amino groups of insulin. Biochem. J. 39:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer, R. T., M. S. Cowlen, M. E. Dempsey, and F. Schroeder. 1985. Fluorescence of Δ5,7,9(11),22-ergostatetraen-3β-ol in micelles, sterol carrier protein complexes, and plasma membranes. Biochemistry. 24:3322–3331. [DOI] [PubMed] [Google Scholar]
- 44.Haberland, M. E., and J. A. Reynolds. 1973. Self-association of cholesterol in aqueous solution. Proc. Natl. Acad. Sci. USA. 70:2313–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John, K., S. Schreiber, J. Kubelt, A. Herrmann, and P. Müller. 2002. Transbilayer movement of phospholipids at the main phase transition of lipid membranes: implications for rapid flip-flop in biological membranes. Biophys. J. 83:3315–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinfeld, J. I., J. S. Francisco, and W. L. Hase. 1999. Chemical Kinetics and Dynamics, 2nd Ed. Prentice Hall, Upper Saddle River, NJ.
- 47.Needham, D., and R. S. Nunn. 1990. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 58:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smaby, J. M., H. L. Brockman, and R. E. Brown. 1994. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: Hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 33:9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwood, A. I., S. Tristram-Nagle, and J. F. Nagle. 2006. Partial molecular volumes of lipids and cholesterol. Chem. Phys. Lipids. 143:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filippov, A., G. Orädd, and G. Lindblom. 2003. The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys. J. 84:3079–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell, D. C., and B. J. Litman. 1998. Effect of cholesterol on molecular order and dynamics in highly polyunsaturated phospholipid bilayers. Biophys. J. 75:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsamaloukas, A., H. Szadkowska, P. J. Slotte, and H. Heerklotz. 2005. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 89:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindblom, G., G. Orädd, and A. Filippov. 2006. Lipid lateral diffusion in bilayers with phosphatidylcholine, sphingomyelin and cholesterol. An NMR study of dynamics and lateral phase separation. Chem. Phys. Lipids. 141:179–184. [DOI] [PubMed] [Google Scholar]
- 54.McIntosh, T. J., A. Vidal, and S. A. Simon. 2003. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophys. J. 85:1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohvo-Rekilä, H., B. Ramstedt, P. Leppimäki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipid in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- 56.Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia, W., and M. F. Thorpe. 1988. Percolation properties of random ellipses. Phys. Rev. A. 38:2650–2656. [DOI] [PubMed] [Google Scholar]
- 58.Vaz, W. L. C., and P. F. F. Almeida. 2003. Phase topology and percolation in multi-phase lipid bilayers: is the biological membrane a domain mosaic? Curr. Opin. Struct. Biol. 3:482–488. [Google Scholar]