Abstract
ErbB signaling regulates cell adhesion and movements during Xenopus gastrulation, but the downstream pathways involved have not been elucidated. In this study, we show that phosphatidylinositol-3 kinase (PI3K) and Erk mitogen-activated protein kinase (MAPK) mediate ErbB signaling to regulate gastrulation. Both PI3K and MAPK function sequentially in mesoderm specification and movements, and ErbB signaling is important only for the late phase activation of these pathways to control cell behaviors. Activation of either PI3K or Erk MAPK rescues gastrulation defects in ErbB4 morphant embryos, and restores convergent extension in the trunk mesoderm as well as coherent cell migration in the head mesoderm. The two signals preferentially regulate different aspects of cell behaviors, with PI3K more efficient in rescuing cell adhesion and spreading and MAPK more effective in stimulating the formation of filopodia. PI3K and MAPK also weakly activate each other, and together they modulate gastrulation movements. Our results reveal that PI3K and Erk MAPK, which have previously been considered as mesodermal inducing signals, also act downstream of ErbB signaling to participate in regulation of gastrulation morphogenesis.
Keywords: PI3K, Erk, ErbB, Xenopus, gastrulation
1. Introduction
Gastrulation is the first major morphogenetic event in vertebrate development, during which process prospective mesoderm and endoderm are internalized to produce patterned body axes. Different cell movements occur in different regions and are coordinated to generate proper tissue architecture. The behaviors of the mesodermal cells in Xenopus gastrulation are best understood. Following involution, head mesoderm migrates along the blastocoel roof (BCR) towards the animal pole, whereas trunk mesoderm undergoes mediolateral cell intercalation to converge toward the midline and extend in an anterior-posterior direction (Keller, 1991; Winklbauer et al., 1996; Keller et al., 2003). The molecular mechanisms controlling these cell movements have been analyzed, and multiple signaling pathways are implicated. Both non-canonical Wnt and fibroblast growth factor (FGF) signals are shown to regulate convergent extension (C&E) movements (Wallingford et al., 2002). Non-canonical Wnt stimulates planar cell polarity (PCP) and Ca2+ pathways to regulate polarized membrane protrusions through Rho family of small GTPases (Wallingford et al., 2000; Habas et al., 2001, 2003; Choi and Han, 2002; Penzo-Mendez et al., 2003; Tahinci and Symes, 2003; Kwan and Kirschner, 2005). By contrast, FGF affects C&E through both regulation of mesodermal cell fate and modulation of cell behaviors. FGF activates mitogen-activated protein kinase (MAPK) signal to control the expression of the transcription factor Brachyury and its downstream target Wnt11 (Amaya et al., 1991; Conlon and Smith, 1999; Tada and Smith, 2000) to influence cell fate and movements; FGF also activates protein kinase C (PKC)/Ca2+ pathway to regulate morphogenesis directly (Nutt et al., 2001; Sivak et al., 2005). It is currently unclear how non-canonical Wnt and FGF signals coordinate to modulate different facets of cell behaviors during C&E. In the head mesoderm, platelet-derived growth factor (PDGF) signaling regulates the directionality of cell migration toward the animal pole (Ataliotis et al., 1995; Nagel et al., 2004); and PDGF also stimulates phosphatidylinositol-3 kinase (PI3K) to control cell spreading on fibronectin (Symes and Mercola, 1996). As PDGF does not influence cell migration per se, other signals may exist to modulate head mesoderm migration.
Recently, we have identified that signaling through ErbB receptor tyrosine kinases (RTKs) also controls Xenopus gastrulation. Uniquely, ErbB signaling controls both C&E of the trunk mesoderm and migration of the head mesoderm (Nie and Chang, 2007). When ErbB signaling is interrupted, dorsal mesodermal cells fail to intercalate mediolaterally and C&E movements are impaired. In addition, head mesoderm shows reduced ability to migrate, and individual cells rather than a continuous cell sheet are seen to move out of the explants. ErbB signaling seems to control cell-cell and cell-matrix adhesion as well as the formation of dynamic membrane protrusions during gastrulation. However, it remains unknown whether ErbB signaling stimulates distinct downstream pathways to modulate different cell behaviors and movements.
Like other RTKs, ErbB receptors dimerize and trans-phosphorylate each other upon ligand stimulation. Phosphorylated tyrosine residues then serve as docking sites to recruit various src homology 2 (SH2) domain-containing proteins, which subsequently activate multiple downstream signaling cascades. Among the best characterized signals activated by RTKs, including ErbBs, are MAPK and PI3K pathways (Olayioye et al., 2000; Yarden and Sliwkowski, 2001; Carpenter, 2003; Citri et al., 2003). ErbBs activate the small GTPase Ras through Shc and Grb2 adaptors, which stimulates MAPKKK Raf that in turn phosphorylates the MAPK kinases MEKs and activates the extracellular signal-regulated kinases 1/2 (Erk1/2) via dual phosphorylation of the proteins (Schlessinger, 1994). By contrast, ErbBs stimulate PI3K signaling through recruitment of the p85 regulatory subunit of PI3K to the activated receptors, which helps to bring its associated catalytic subunit p110 close to the plasma membrane to phosphorylate its lipid substrate and activate downstream kinases phosphoinositide-dependent kinase 1 (PDK1) and Akt (Cantrell, 2001). Both MAPK and PI3K have been shown to regulate cell migration in different contexts, such as during cancer cell invasion and in neutrophil chemotaxis (Adam et al., 1998; Servant et al., 2000; Spenser et al., 2000; Funamoto, 2002; Holbro et al., 2003).
In Xenopus, blocking either MAPK or PI3K signals has been shown to interfere with gastrulation; however, the mesodermal fate is also disrupted in these cases (Carballada et al., 2001; Sivak et al., 2005). Activation of either MAPK or PI3K in ectodermal explants is sufficient to induce mesoderm, and the two pathways synergize in mesodermal induction (Gotoh et al., 1995; LaBonne et al., 1995; Umbhauer et al., 1995; Carballada et al., 2001). The data thus suggest that MAPK and PI3K are important signals in mesodermal fate determination, and it is unclear whether these signals can also regulate cell movements subsequently to their function in mesoderm induction.
In this study, we show that inhibition of MAPK or PI3K signals after mesoderm induction blocks C&E movements, indicating that these pathways control morphogenesis during gastrulation. We then demonstrate that ErbB signaling regulates activation of endogenous MAPK and PI3K. As blocking ErbB signaling interferes with gastrulation movements without affecting mesodermal cell fate (Nie and Chang, 2006, 2007), we propose that ErbBs are involved in activation of MAPK and PI3K at gastrula stages to control cell behaviors. Consistent with this idea, we illustrate that activation of MAPK or PI3K rescues gastrulation defects in ErbB4 morphant embryos. Our data thus establish that PI3K and MAPK mediate ErbB signaling to control cell adhesion and movements during gastrulation.
2. Results
2.1 PI3K and Erk MAPK regulate mesoderm specification and gastrulation at different developmental stages
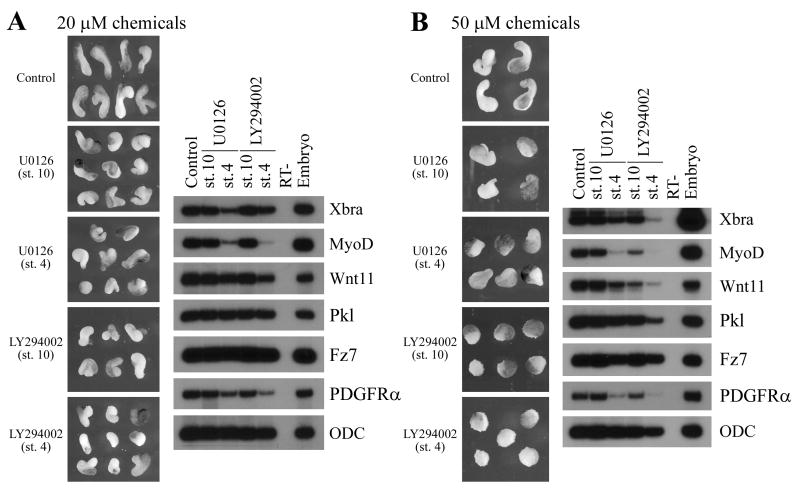
It has previously been shown that inhibition of either PI3K or MAPK pathways leads to impaired mesodermal formation and consequentially gastrulation defects (Amaya et al., 1991; Conlon and Smith, 1999; Tada and Smith, 2000; Carballada et al., 2001). Since blocking ErbB signaling interferes with gastrulation without affecting mesodermal cell fate, it implies that if PI3K and MAPK mediate ErbB action, they should function in a second phase to regulate mesodermal cell movements after proper germ layers are specified. To test this idea, we applied chemical inhibitors of PI3K and MAPK signals at different developmental stages. U0126, a potent inhibitor of MAPK kinases MEK1 and MEK2, and LY294002, a specific inhibitor of PI3K, were applied to the embryos from cleavage stages (stage 4) or early gastrula stages (stage 10) onward. Dorsal marginal zone (DMZ) explants were dissected at stage 10; mesoderm formation was assayed by RT-PCR at mid-gastrula stages and the convergent extension movements of the explants were analyzed at late neurula stages. As shown in Fig. 1, when applied at cleavage stages at 20μM concentration, both inhibitors reduced several mesodermal markers in DMZ explants, including Brachyury, MyoD and PDGF receptor. Other markers, such as Frizzled 7 and Prickled, which have been implicated in regulation of gastrulation, were not changed. As a consequence of impaired mesoderm formation, the elongation of the DMZ explants was shortened. The effect was dose-dependent, so that more severe defects were observed when the chemicals were used at 50μM (Fig. 1). If the inhibitors were added at early gastrula stages, they were incapable or inefficient in blocking mesoderm induction, but they retained the ability to block elongation of the explants (Fig. 1). The results indicate that PI3K and MAPK signals are employed sequentially in mesoderm specification and gastrulation, and that ErbBs may potentially activate only the late stage signals to control gastrulation movements.
Figure 1. MAPK and PI3K regulate mesoderm formation and gastrulation at different developmental stages in Xenopus.
Embryos were incubated with the MAPK inhibitor U0126 or the PI3K inhibitor LY294002 at 20μM (A) or 50μM (B) from stage 4 or stage 10 onward. DMZ explants were dissected at stage 10, mesodermal markers were analyzed by RT-PCR at stage 11, and the morphology of the explants was examined at stage 16. While both inhibitors reduced the elongation of the DMZ explants in a dose-dependent manner when applied from either early or late stages, only inhibitors applied from stage 4 could efficiently block expression of a panel of mesodermal markers.
2.2 ErbB signaling regulates activation of PI3K and Erk MAPK during Xenopus gastrulation
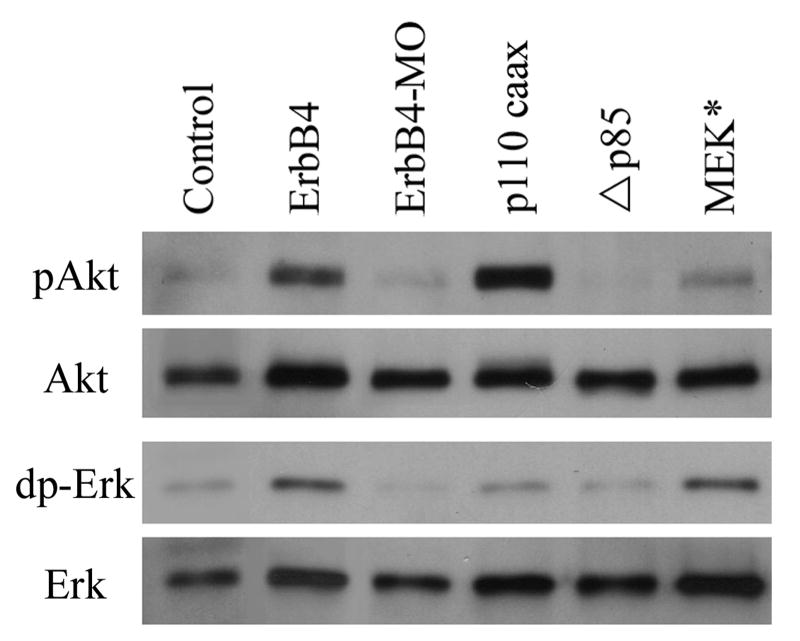
To test whether ErbB signaling indeed modulates activation of PI3K and Erk MAPK in Xenopus embryos, we examined the phosphorylation status of key components of these pathways in embryos with altered ErbB signaling levels. Dual phosphorylation of Erk1/2 (dp-Erk) and phosphorylation of Akt (pAkt) were used to assess the activation of MAPK and PI3K signals, respectively. DMZ explants from control, ErbB4-overexpressing and ErbB4-MO-injected embryos were assayed at mid-gastrula stages by Western blot. Overexpression of ErbB4 enhanced, while knockdown of ErbB4 reduced, the activation of both PI3K and MAPK signals (Fig. 2). As controls, we also examined the levels of pAkt and dp-Erk in DMZ explants from the embryos that overexpressed a constitutively active PI3K (p110caax, membrane-targeted p110 catalytic subunit of PI3K), a dominant negative PI3K (Δp85, deletion mutant of the p85 regulatory subunit of PI3K, Carballada et al., 2001), or a constitutively active MEK (MEK*, SESE mutations of MEK/MAPKK, Gotoh et al., 1995). As expected, p110caax and MEK* activated PI3K and MAPK signals, respectively, while Δp85 reduced the level of pAkt (Fig. 2). Interestingly, we observed that MEK* weakly stimulated Akt phosphorylation, and p110caax weakly activated Erk. This indicated that PI3K and MAPK signals, when strongly activated, might crosstalk with each other in frog embryos. The observation that ErbB signaling regulates the activation of PI3K and Erk MAPK in dorsal mesodermal cells during Xenopus gastrulation implies that PI3K and Erk MAPK may potentially mediate ErbB signaling in controlling morphogenetic movements.
Figure 2. ErbB signaling regulates activation of PI3K and Erk MAPK pathways in Xenopus DMZ explants.
DMZ explants from embryos overexpressing ErbB4 (2ng mRNA/embryo), ErbB4-MO (20ng MO/embryo), p110caax (1ng mRNA/embryo), Δp85 (1ng mRNA/embryo) or MEK* (1ng mRNA/embryo) were examined at gastrula stages for activation of PI3K (pAkt) or Erk MAPK (dpErk) pathways. Overexpression of ErbB4 enhanced, while depletion of ErbB4 reduced, the levels of both pAkt and dpErk. p110caax and MEK* stimulated pAkt and dpErk, respectively; but they also weakly increased phosphorylation of the other pathway component, suggesting a crosstalk between the two pathways.
2.3 Activation of PI3K or Erk MAPK rescues gastrulation defects in ErbB4 morphant embryos
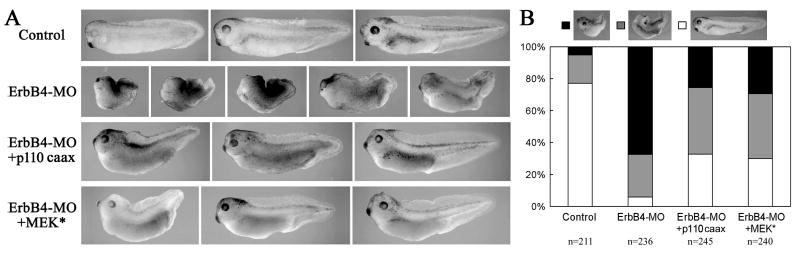
If PI3K and Erk MAPK acted downstream of ErbB signaling, we would expect that activation of these pathways might rescue the gastrulation defects induced by inhibition of ErbB signaling at the receptor level. To test this idea, we coexpressed p110caax or MEK* with ErbB4-MO and observed the phenotypes of the embryos at tadpole stages. As reported before (Nie and Chang, 2007), ErbB4 morphant embryos displayed failure of blastopore closure (67%, n=236) or short and curved body axis; coexpression of ErbB4-MO with p110caax or MEK* decreased the number of the embryos with open blastopore phenotype (25-29%) and reduced the severity of axial defects in the embryos (Fig. 3). An increase in the number of the embryos with normal morphology was also observed (30% versus 6% in ErbB4 morphants). When p110caax and MEK* were coexpressed together, we detected a similar level of rescue (not shown). These results demonstrate that both PI3K and Erk MAPK may contribute to the actions of ErbB4 during Xenopus gastrulation.
Figure 3. Activation of PI3K or Erk MAPK rescued gastrulation defects in ErbB4 morphant embryos.
(A) Coinjection of 5-10pg of p110caax or MEK* RNA rescued the open blastopore and axial defects induced by depletion of ErbB4 (20ng ErbB4-MO/embryo). (B) Embryos with different phenotypes were counted and summarized in the bar graph.
2.4 PI3K and Erk MAPK act downstream of ErbB4 to regulate convergent extension and mediolateral cell intercalation in dorsal mesoderm
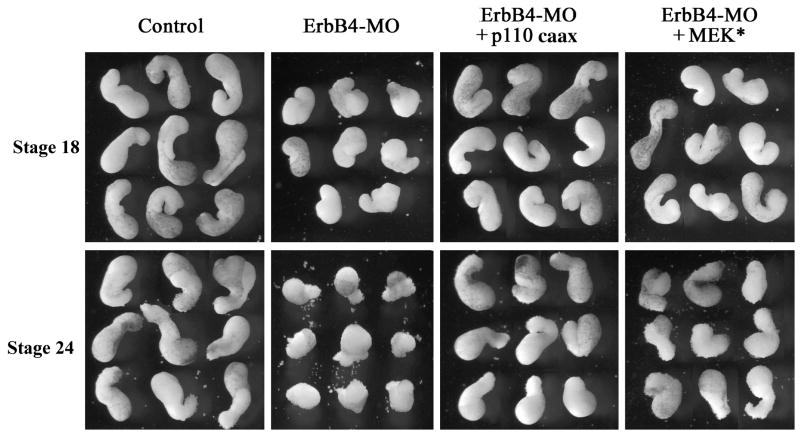
ErbB signaling modulates both convergent extension of the trunk mesoderm and migration of the head mesoderm (Nie and Chang, 2007). It is conceivable that the downstream signals PI3K and Erk MAPK may act in different mesodermal regions to regulate distinct movements. Alternatively, both pathways may be required for different gastrulation movements. To distinguish whether PI3K and Erk MAPK act similarly during gastrulation, we first determined whether they were both capable of rescuing C&E in DMZ explants from ErbB4 morphant embryos. Depletion of ErbB4 resulted in impaired C&E and reduced elongation of DMZ explants; coexpression of either p110caax or MEK* sufficiently rescued the defects, so that elongation of the dorsal mesoderm was restored in these explants at neurula stages (Fig. 4). At tailbud stages, explants from the ErbB4 morphants started to dissociate and shed cells around them (Nie and Chang, 2007); this defect was also rescued by both p110caax and MEK*, though MEK* was less efficient in preventing cell dissociation so that the explants assumed a rough surface with exposed or shed loose cells (Fig. 4). The data reveal that while both PI3K and Erk MAPK can compensate for the loss of ErbB signaling during early C&E, only PI3K can efficiently maintain cell adhesion in the absence of ErbB4 at late stages.
Figure 4. Activation of PI3K or Erk MAPK rescued convergent extension of DMZ explants from ErbB4 morphant embryos.
Coexpression of 5-10pg mRNA/embryo of p110caax or MEK* rescued elongation of the DMZ explants from ErbB4 morphant embryos (20ng ErbB4-MO/embryo) at mid-neurula (stage 18) and tailbud (stage 24) stages. At tailbud stages, explants from ErbB4-MO injected embryos started to dissociate, and p110caax prevented cell shedding more efficiently than MEK* did.
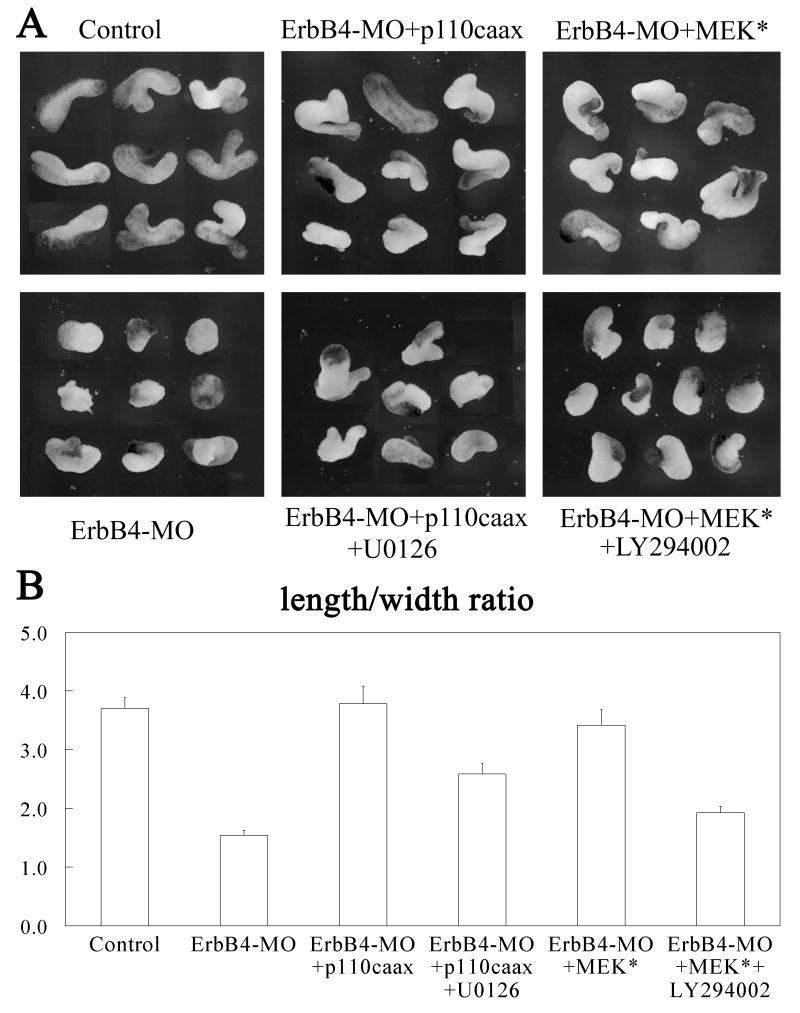
ErbB signaling activates different downstream pathways that crosstalk with each other. In Xenopus, PI3K and Erk MAPK may also crosstalk when strongly activated, as seen in our Western blot analyses (Fig. 2). Though it is unclear whether weak activation of each pathway by using a low dose of RNA could still stimulate another pathway, it is conceivable that p110caax and MEK* might activate each other, and that the rescue of the gastrulation defects in ErbB4 morphant embryos only requires the activation of one pathway stimulated by either molecule. To test this possibility, we treated the DMZ explants that were rescued with p110caax with the MEK inhibitor U0126 at early gastrula stages, and treated MEK*-expressing explants with LY294002 in a similar way. As shown in Fig. 5, while p110caax nicely rescued C&E of DMZ explants from ErbB4 morphant embryos, incubation with U1026 reduced the extent of explant elongation. Similarly, treatment of MEK*-expressing explants with LY294002 led to decreased C&E, and the effect was more severe than that in U0126-treated explants (Fig. 5). The results indicate that indeed both PI3K and Erk MAPK contribute to the regulation of C&E by ErbB signaling, and PI3K may be more critical in this process.
Figure 5. Both PI3K and MAPK are required for optimal rescue of convergent extension in ErbB4-depleted explants.
A) 20μM of U0126 or LY294002 were applied at stage 10 to DMZ explants from ErbB4-depleted (20ng ErbB4-MO/embryo) and p110caax or MEK* (5-10pg mRNA/embryo) rescued embryos respectively. While both p110caax and MEK* rescued convergent extension of the explants, treatment with either U0126 or LY294002 reduced the elongation of the explants. B) Morphometric analyses of the elongation of the DMZ explants (15 explants for each sample). The length-to-width (L/W) ratios of the DMZ explants were measured and calculated, and the statistical significance of the differences was analyzed using student t-test. The difference between control and ErbB4-morphant was significant (P-value less than 0.0005), but the control and the rescued samples were not significantly different (P-values of 0.81 and 0.37 for p110caax and MEK* rescued samples respectively compared with the control explants). Treatment of the rescued samples with the chemical inhibitors significantly decreased the L/W ratios of the explants (P-values less than 0.003 compared with the control or the rescued explants).
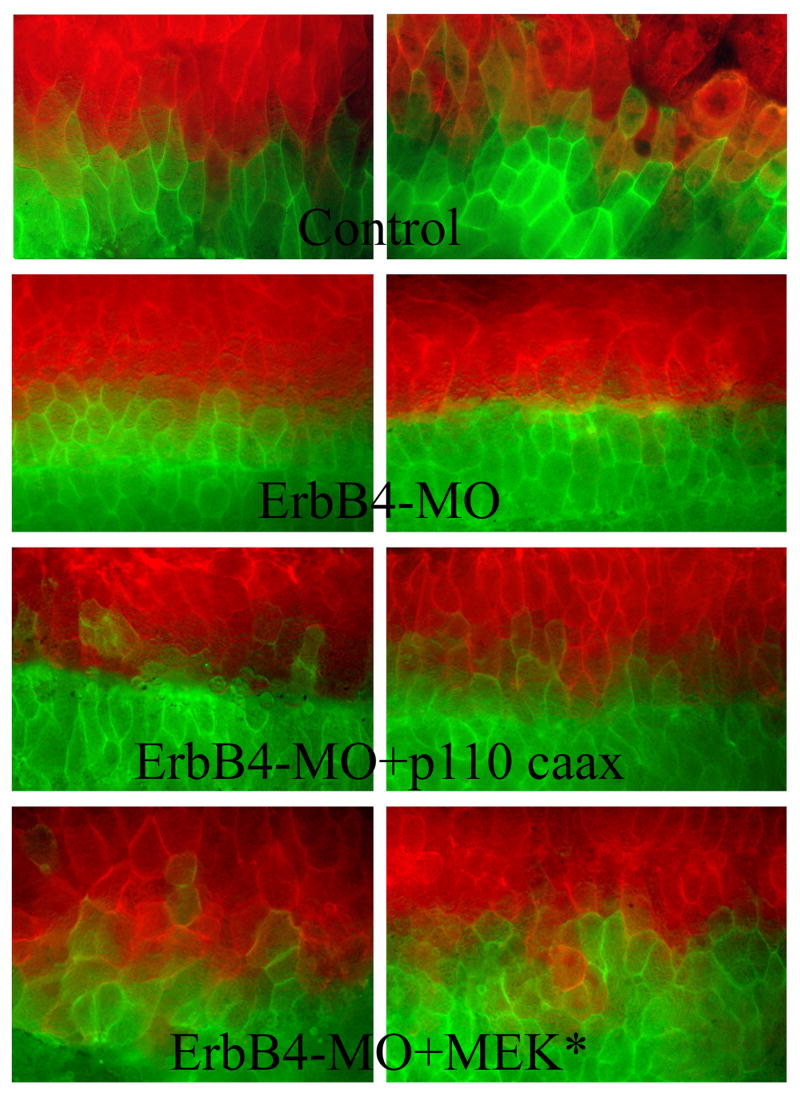
One main reason underlying impaired C&E in ErbB4 morphant embryos is that dorsal cells fail to intercalate in the mediolateral direction efficiently (Nie and Chang, 2007). To see how activation of PI3K and MAPK may affect this process, we examined cell behaviors in the open-face explants taken from the embryos co-expressing p110caax or MEK* with ErbB4-MO. Dorsal cells in these explants were labeled with two different membrane-tethered fluorescent proteins at the 4-cell stage, so that the two halves of the embryos were marked by different fluorescent signals. Explants from ErbB4 morphant embryos showed that the cells did not intermingle efficiently so that a clear division of the fluorescent signals at the midline was detected. However, when p110caax or MEK* were coexpressed with ErbB4-MO, the cells with different labels invaded the other domain and mixed with each other (Fig. 6). This result suggests that ErbB signaling may stimulate PI3K and Erk MAPK to modulate mediolateral cell intercalation behaviors during C&E.
Figure 6. Activation of PI3K and Erk MAPK rescued mediolateral intercalation of dorsal mesodermal cells.
Membrane-tethered fluorescence proteins (EGFP and Cherry) were injected separately into two dorsal blastomeres of 4-cell embryos, together with ErbB4-MO (20ng/embryo) and RNAs encoding p110caax or MEK* (5-10pg/embryo). Cells from ErbB4 morphant embryos respected the midline and did not intermingle, but cells co-expressing the MO and p110caax or MEK* intercalated with each other, so that cells with different fluorescent signals mixed across the midline.
2.5 PI3K and Erk MAPK mediate ErbB signaling in head mesoderm migration
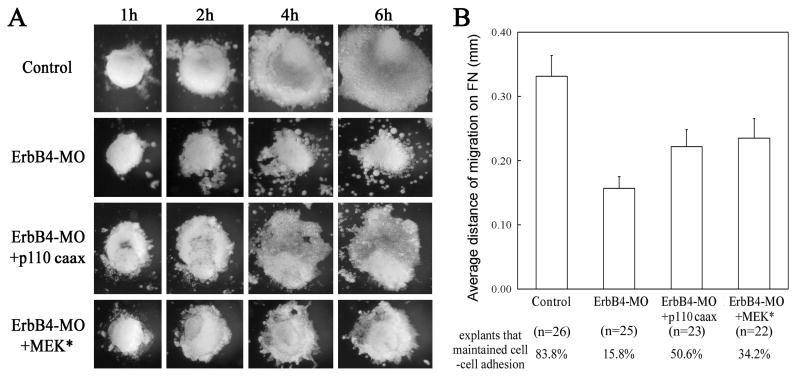
To further determine whether PI3K and Erk MAPK have distinct activities in regulation of cell movements in the anterior mesoderm, we assayed for their function in head mesoderm migration. Head mesodermal explants were dissected from early gastrula embryos and plated on fibronectin (FN)-coated dishes. The migratory behaviors of these explants were examined at one-hour intervals for six hours. As shown before (Nie and Chang, 2007), explants from control embryos migrated effectively as a continuous sheet of cells; however, explants from ErbB4 morphants did not migrate efficiently and many cells moved individually without interconnection (15.8% explants with adherent cell sheet, versus 83.8% in control explants). The distance of mesodermal migration was also reduced (Fig. 7). When p110caax or MEK* were coexpressed with ErbB4-MO, cells from the explants maintained their association during migration, and the rescue was more evident in p110caax-expressing explants (50.6% explants with continuous cell sheet, which was not statistically different from that of control explants, Fig. 7). PI3K and MEK* also significantly improved, though not completely rescued, the distance of head mesoderm migration (Fig. 7). Our results illustrate that both PI3K and Erk MAPK can function downstream of ErbB4 to regulate head mesoderm migration on FN substratum, but PI3K signal is more efficient in maintaining cell adhesion during migration.
Figure 7. PI3K and MAPK rescued head mesoderm migration on fibronectin.
(A) Migration of head mesoderm was impaired in explants from ErbB4 morphant embryos (20ng ErbB4-MO), and both p110caax and MEK* (5-10pg mRNA) rescued the migration. The distance of migration was enhanced, and the cell-cell contact between migratory cells was restored. (B) Activation of either p110caax or MEK* rescued the distance of head mesoderm migration partially but significantly (with p-values of 0.03 from student t-test). The percentage of the explants that maintained cell-cell adhesion was also greatly improved, with p110caax rescued the adhesion more efficiently to a level with no statistical difference from that of control (p=0.16; for MEK*, p=0.03).
2.6 PI3K and Erk MAPK regulate cell adhesion and cell spreading with different efficiencies
ErbB signaling modulates cell adhesion in both the trunk and the head mesoderm, and activation of PI3K seems to restore adhesion in mesodermal explants more efficiently than that of Erk MAPK does. This suggests that the two downstream signals may have differential ability to regulate cell adhesion. We therefore tested this possibility more directly using dissociated cells. We first examined the roles of the two pathways in ErbB-regulated cell-cell adhesion by cell dissociation and reaggregation assay. DMZ explants from early gastrula embryos were dissociated in calcium- and magnesium-free buffer (CMFB) for an hour; cells were then reaggregated on orbital horizontal shaker in a buffer containing calcium and magnesium (MBSH). Control cells formed a single large aggregate after one hour and a very tight cluster after 3 hours. In contrast, cells from ErbB4 morphants only aggregated into many loose pieces with variable sizes at one hour and loose clusters after 3 hours (Fig. 8). Coexpression of p110caax or MEK* enhanced cell reaggregation so that cells formed a main cluster after one hour. After 3 hours, cells expressing p110caax formed tight aggregates, whereas cells expressing MEK* showed less efficient reaggregation and existed as relatively loose cell clusters (Fig. 8). These results confirm our explant assays and indicate that while both PI3K and MAPK regulate cell-cell adhesion downstream of ErbB4, PI3K is more efficient in this process.
Figure 8. PI3K rescued cell-cell adhesion more efficiently than Erk MAPK.
Dorsal mesodermal cells were dissociated for an hour before reaggregated on agarose-coated dish for 3 hours. Cells from ErbB4 morphant embryos (20ng ErbB4-MO) did not reaggregate well, so that loose clusters formed. Coexpression with p110caax (5-10pg mRNA) effectively rescued cell adhesion to a level similar to that of control, while MEK* (5-10pg mRNA) was less efficient in rescue, so that aggregates were looser in structure.
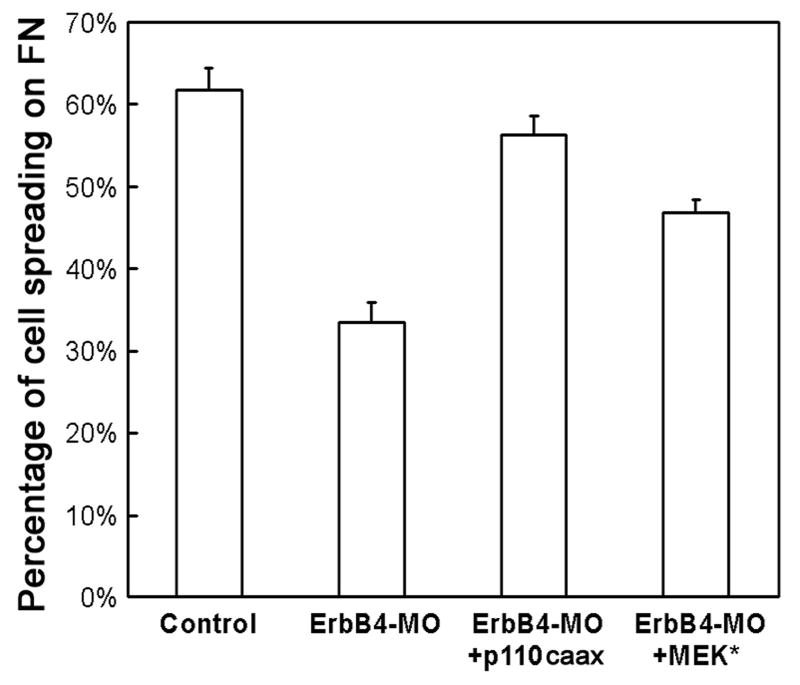
To analyze whether PI3K and MAPK also differentially regulate cell-matrix adhesion, we next examined the binding of the mesodermal cells to the FN matrix. Dissociated dorsal mesodermal cells were plated on FN-coated cover slip in MBSH for an hour before unattached cells were washed away gently. Comparison of the number of the cells on the cover slip before and after the washes showed that depletion of ErbB4 decreased the adherent cells from 94% to 84%; coexpression of p110caax restored cell adhesion to 91%, while MEK* was less efficient that increased the adherent cells to 89% (not shown). Close examination of the morphologies of the cells remaining on the cover slip showed that 62% of control cells were flattened and spread on FN, but only 34% of cells from ErbB4 morphants spread. Coexpression of either p110caax or MEK* significantly increased the percentage of the spreading cells to 56% or 47%, with p110caax rescued cell spreading more fully to a level comparable to that of control cells (Fig. 9). The data demonstrate that PI3K and Erk MAPK act differentially downstream of ErbB4 to regulate cell adhesion and cell spreading on FN.
Figure 9. PI3K rescued mesodermal cell spreading on fibronectin more efficiently than MAPK.
The morphology of the mesodermal cells attached to fibronectin was examined and the number of the spreading cells was quantified in the bar graph. While ErbB4-MO (20ng/embryo) reduced cell spreading on FN matrix, coinjection of p110caax or MEK* (5-10pg mRNA/embryo) increased the percentage of the spreading cells significantly (p<0.0001 compared with ErbB4-MO alone). Expression of p110caax, but not MEK*, rescued cell spreading to the level comparable to that of the control cells (p=0.14 and 3E-05 for p110caax and MEK*, respectively, compared with the control).
2.7 PI3K and Erk MAPK regulate dynamic membrane protrusions downstream of ErbB4
ErbB signaling is required for formation of proper membrane protrusions, so that in its absence, membrane blebs instead of filopodia and lamellipodia form predominantly (Nie and Chang, 2007). To test whether activation of PI3K and Erk MAPK is sufficient to overcome the defects induced by the lack of ErbB receptor, we coexpressed p110caax or MEK* with ErbB4-MO and analyzed membrane protrusions of dorsal mesodermal cells by time-lapse microscopy. Cells from ErbB4 morphant embryos had dramatic reduction of filopodia and lamellipodia, but displayed increased and dynamic circular-moving blebs at the cell periphery. Overexpression of either p110caax or MEK* effectively suppressed the blebs and restored the filopodia and lamellipodia (Fig. 10; supplementary movies). Interestingly, we observed that cells expressing MEK* showed enhanced formation of filopodia, which was distinct from the behaviors of the cells expressing p110caax (Fig. 10, supplementary movies). Our results imply that both PI3K and Erk MAPK can act downstream of ErbB4 to regulate membrane protrusions, but they may preferentially modulate distinct aspect of the protrusions, with Erk MAPK associated more closely with filopodia formation.
Figure 10. Activation of PI3K and MAPK restored the formation of membrane protrusions in cells from ErbB4 morphant embryos.
Membrane-tethered EGFP was used to label cell membrane, and formation of the cell protrusions in dissociated DMZ cells on FN matrix were observed by time lapse movies. A) Cells from ErbB4 morphant embryos (20ng ErbB4-MO/embryo) showed reduced filopodia and lamellipodia, but with increased membrane blebs. Coexpression of p110caax or MEK* (5-10pg mRNA/embryo) restored the formation of lamellipodia and filopodia. B) Quantification of the membrane protrusions. Lamellipodia, filopodia and membrane blebs were counted separately and summarized in the bar graph. ErbB4-MO reduced the average lamellipodia and filopodia from 1.7 and 3.7 to 0.3 and 0.7 per cell per minute, respectively, while increased the formation of the membrane blebs from 0.2 to 1.4 per cell per minute (p-values less than E-10 for all type of protrusions, as assayed by student t-test). Coexpression of p110caax and MEK* restored the frequencies of lamellipodia and filopodia (p>0.05 for all protrusions compared with the control cells), with MEK* stimulating filopodia more efficiently. The total numbers of the cells counted were given under the bar graph.
3. Discussion
Gastrulation in Xenopus has previously been shown to be under the control of non-canonical Wnt, FGF, PDGF and ErbB signals. These signals regulate different aspects of cell behaviors. PDGF signaling orients head mesodermal cells toward the animal pole for directional migration, but does not affect convergent extension movements. PI3K pathway is utilized downstream of PDGFR to modulate directionality of cell migration (Ataliotis et al., 1995; Symes and Mercola, 1996; Nagel et al., 2004). By contrast, non-canonical Wnt and FGF control C&E movements. Both signals employ the PKC/Ca2+ pathway to influence mediolateral cell intercalation; but additionally, non-canonical Wnt also activates Dishevelled and Rho/Rac GTPases to control polarized membrane protrusions (Wallingford et al., 2002). In comparison, ErbB signaling regulates both head mesoderm migration and trunk mesoderm convergent extension (Nie and Chang, 2007). ErbBs modulate cell-cell and cell-matrix adhesion as well as membrane protrusions to control cell movements. However, it is unclear whether the effects of ErbB signaling on different cell behaviors in different mesodermal regions are mediated by the same or distinct downstream signals. Our current study shows that PI3K and MAPK both act downstream of ErbB receptors to regulate gastrulation, and they have overlapping as well as distinct activities.
3.1 PI3K and MAPK in mesoderm induction and gastrulation
Both PI3K and MAPK have been implicated in mesoderm induction. Interference with these signals with dominant negative components, natural negative regulators or chemical inhibitors all leads to impaired mesoderm formation (Gotoh et al., 1995; LaBonne et al., 1995; Umbhauer et al., 1995; Carballada et al., 2001; Sivak et al., 2005). Though gastrulation is also impeded in the absence of these signals, it has been considered as a secondary effect. The direct involvement of PI3K and MAPK in gastrulation morphogenesis has not been firmly established. By applying the chemical inhibitors of PI3K and MEK at different developmental stages, we show that these signals are required sequentially during mesoderm specification and gastrulation. Blocking PI3K and MAPK at gastrula stages does not inhibit mesoderm formation efficiently, but it suffices to hinder the C&E movements. During development, MAPK is activated in the marginal zone at the blastula stages and is later detected at high levels in the involuting mesoderm during gastrulation (LaBonne and Whitman, 1997; Christen and Slack, 1999; Curran and Grainger, 2000; Schohl and Fagotto, 2002). The expression pattern is thus consistent with a function of the activated MAPK in gastrulation. The distribution of activated PI3K signaling components during early Xenopus development has not been described. ErbB receptors, as well as the neuregulin 1 (NRG1) ligand, are expressed maternally and throughout early frog embryogenesis (Yang et al., 1998, 1999; Nie and Chang, 2006). Since inhibition of ErbB signaling does not block mesoderm formation and patterning but interferes with gastrulation (Nie and Chang, 2006, 2007), we propose that ErbB signaling mainly regulates the second phase of PI3K and MAPK activation during gastrulation, but is dispensable for PI3K- and MAPK-dependent mesodermal induction. Depletion of ErbB4 leads to decreased activation of both PI3K and Erk MAPK in the dorsal mesoderm of Xenopus gastrulae, suggesting that ErbB signaling is indeed important to stimulate these pathways endogenously. However, low level residual activation of PI3K and Erk MAPK can still be detected in ErbB4 morphant embryos, indicating that other signals, such as those of FGF and PDGF, may also participate in activation of PI3K and MAPK during gastrulation. Apparently the strength of these signals is not sufficient for proper morphogenetic movements in the absence of ErbB4. Our results thus imply that ErbB, FGF and PDGF signals cooperate in vivo to regulate gastrulation movements through activation of the PI3K and the Erk MAPK pathways.
3.2 Overlapping and distinct activities of PI3K and Erk MAPK
Depletion of ErbB4 in early frog embryos results in gastrulation defects, which can be rescued by overexpression of either human ErbB4 (Nie and Chang, 2007) or the activated components of PI3K or Erk MAPK pathways (this study). Combined with the Western blot analyses (see above), the data suggest that PI3K and Erk MAPK mediate ErbB signaling in regulation of gastrulation. Detailed analyses of cell behaviors and movements indicate that PI3K and MAPK do not always function equivalently. Activation of PI3K rescues cell-cell and cell –matrix adhesion more efficiently, both in explants and in dissociated mesodermal cells, and allows cells to spread on fibronectin more effectively. By contrast, activation of MAPK stimulates the formation of animated filopodia in isolated mesodermal cells more effectively. The differential activities of PI3K and MAPK likely reflect distinct cytoplasmic effectors of these kinase pathways. In mammalian cells, PI3K can be recruited into cadherin-catenin complexes at the plasma membrane to influence cell properties, and the Akt effector of PI3K activates Rho/Rac family of GTPases to control cytoskeleton reorganization (Reif et al., 1996; Han et al., 1998; Kotelevets et al., 2005; Barber and Welch, 2006; Xie and Bikle, 2007). Erk MAPK controls cell movement by phosphorylating myosin light chain kinase (MLCK), focal adhesion kinase (FAK) and paxillin, thus influencing cell adhesion and membrane protrusions (Huang et al., 2004). Though the two signals crosstalk with each other in various contexts, including in Xenopus embryos (Fig. 2), the strength and the kinetics of the effector activation may be quite different with each primary signals. As a result, the two signals preferentially regulate different cell behaviors with different efficiencies and both pathways are needed for optimal convergent extension to occur (Fig. 5). Because of the functional distinction, it is expected that the two signals may synergize in rescuing gastrulation defects in ErbB4 morphant embryos. However, we did not observe such synergy over a range of RNA doses used for p110caax and MEK* (not shown). There may be at least two explanations for this result. First, we always used 1:1 ratio of p110caax and MEK*, and this might not reflect the endogenous ratio of the activation of the two pathways in the dorsal mesoderm, and thus we could not get significant improvement in phenotypic rescue by the two components over that when a single active component was used. Second, dynamic regulation of both signals may be important for proper gastrulation, and activation followed by inactivation of one or both signals may be required. For example, it has been documented in mammalian cells that transient and prolong MAPK signals may have differential effects on cell proliferation or differentiation (Nguyen et al., 1993). It is thus possible that continued activation of these pathways may prevent optimal movements of the dorsal mesoderm and thus underlie the lack of synergy between the two signals.
3.3 Other downstream signals in ErbB-mediated gastrulation process
In addition to PI3K and MAPK signals, other cytoplasmic pathways may be stimulated downstream of the activated ErbB receptors to regulate gastrulation. One prominent signal that is activated in mammalian cells is the phospholipase C gamma (PLCγ)/PKC pathway. During neurite outgrowth and cancer cell migration and invasion, PLCγ/PKC signaling is stimulated downstream of ErbBs to regulate actin remodeling (Kassis et al., 1999; Vaskovsky et al., 2000; Dittmar et al., 2002; Gerecke et al., 2004; Sewell et al., 2005). Since PKC has been shown to act downstream of both non-canonical Wnt and FGF signals (Kinoshita et al., 2003; Sivak et al., 2005), it is possible that the three signals converge on PKC to influence gastrulation movements. In addition, Src family of non-receptor tyrosine kinases may be activated downstream of ErbBs, as they are in mammalian cells, to control Rho/Rac GTPases and cellular protrusions (Yarden and Sliwkowski, 2001; Bromann et al., 2004; Playford and Schaller, 2004). In Xenopus, inhibition of Src activity blocks elongation of activin-treated animal caps and induces open blastopore phenotype (Denoyelle et al., 2001). In zebrafish, Src family kinases Fyn and Yes converge with non-canonical Wnt signaling on RhoA to modulate convergent extension movements (Jopling and den Hertog, 2005). Furthermore, ErbB signaling may stimulate p38 and Jun N-terminal kinase (JNK) MAPK cascades to influence cell migratory behaviors. In the future, it will be important to determine whether these cytoplasmic pathways play a role in mediating ErbB-signaling to regulate distinct cell behaviors during gastrulation.
4. Experimental Procedures
4.1 Embryo manipulations
Embryos were obtained, maintained and microinjected with capped RNAs or ErbB4-MO as described (Chang et al., 1997). PI3K inhibitor LY294002 (Calbiochem) and MAPK inhibitor U0126 (Calbiochem) were applied at 20μM or 50μM doses at stages indicated in the text. The ErbB4-MO was the same as reported previously (Nie and Chang, 2007). MEK* (SESE-MAPKK), p110caax and Δp85 constructs were kindly provided by Dr. Asashima and Dr. Kodjabachian, respectively. RNAs were synthesized using mMessage mMachine in vitro transcription kit (Ambion). For rescue experiments, ErbB4-MO (total 20ng/embryo) and p110caax and Δp85 mRNA (0.5-10pg/embryo) were injected into the dorsal marginal zone region of 4-cell stage embryos; for Western blot analyses, 20ng/embryo of ErbB4-MO and 1ng/embryo of the above RNAs were used.
4.2 Western blot analyses
DMZ explants were dissected from early gastrula stage (stage10) embryos and incubated to mid-gastrula stages (stage11+) before total protein was extracted. Lysate equivalent to one explant per lane was separated on 10% PAGE and transferred to Immobilon P membrane (Millipore). Western blot was performed with 1:2000 dilutions of anti-activated MAPK (pTEpY; Promega) or anti-phospho-Akt (Ser473; Cell Signaling) antibodies. Total Erk and Akt levels were examined with anti-ERK2 (BD Bioscience) and anti-PKBα/Akt1 (clone PKB-175; Sigma) antibodies at 1:3000 dilutions.
4.3 Cell intercalation, head mesoderm migration and cell adhesion assays
These assays were performed as described (Nie and Chang, 2007).
Supplementary Material
Supplementary movies
Time-lapse microscopy was performed to record the dynamic formation of the membrane protrusions of DMZ cells on fibronectin. Cells from control, ErbB4 morphant embryos (20ng ErbB4-MO), or ErbB4 morphants coinjected with p110caax or MEK* (5-10pg mRNA/embryo) were examined. Two characteristic movies were shown for each type of the cells.
Acknowledgments
We thank Dr. Eisuke Nishida and Dr. Laurent Kodjabachian for kindly providing MEK*, p110caax and Δp85 constructs. This study is supported by the NIH grant HD43345.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Ataliotis P, Symes K, Chou MM, Ho L, Mercola M. PDGF signalling is required for gastrulation of Xenopus laevis. Development. 1995;121:3099–3110. doi: 10.1242/dev.121.9.3099. [DOI] [PubMed] [Google Scholar]
- Barber MA, Welch HCE. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44–E52. [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- Carballada R, Yasuo H, Lemaire P. Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development. 2001;128:35–44. doi: 10.1242/dev.128.1.35. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB4: mechanism of action ad biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol. 2002;244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- Christen B, Slack JMW. Spatial response to fibroblast growth factor signalling in Xenopus embryos. Development. 1999;126:119–125. doi: 10.1242/dev.126.1.119. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Smith JC. Interference with Brachyury function inhibits convergent extension, causes apoptosis, and reveals separate requirements in the FGF and activin signalling pathways. Dev Biol. 1999;213:85–100. doi: 10.1006/dbio.1999.9330. [DOI] [PubMed] [Google Scholar]
- Curran KL, Grainger RM. Expression of activated MAP kinase in Xenopus laevis embryos: evaluating the roles of FGF and other signaling pathways in early induction and patterning. Dev Biol. 2000;228:41–56. doi: 10.1006/dbio.2000.9917. [DOI] [PubMed] [Google Scholar]
- Denoyelle M, Valles AM, Lentz D, Thiery JP, Boyer B. Mesoderm-independent regulation of gastrulation movements by the Src tyrosine kinase in Xenopus embryo. Differentiation. 2001;69:38–48. doi: 10.1046/j.1432-0436.2001.690104.x. [DOI] [PubMed] [Google Scholar]
- Dittmar T, Husemann A, Schewe Y, Nofer JR, Niggemann B, Zanker KS, Brandt BH. Induction of cancer cell migration by epidermal growth factor is initiated by specific phosphorylation of tyrosine 1248 of c-erbB-2 receptor via EGFR. FASEB J. 2002;16:1823–1825. doi: 10.1096/fj.02-0096fje. [DOI] [PubMed] [Google Scholar]
- Elenius K, Choi CJ, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene. 1999;18:2607–2615. doi: 10.1038/sj.onc.1202612. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1β induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- Gotoh Y, Masuyama N, Suzuki A, Ueno N, Nishida E. Involvement of the MAP kinase cascade in Xenopus mesoderm induction. EMBO J. 1995;14:2491–2498. doi: 10.1002/j.1460-2075.1995.tb07246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Jones NP, Peak J, Brader S, Eccles SA, Katan M. PLCgamma1 is essential for early events in integrin signalling required for cell motility. J Cell Sci. 2005;118:2695–2706. doi: 10.1242/jcs.02374. [DOI] [PubMed] [Google Scholar]
- Jopling C, den Hertog J. Fyn/Yes and non-canonical Wnt signaling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 2005;6:426–431. doi: 10.1038/sj.embor.7400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J, Moellinger J, Lo H, Greenberg NM, Kim HG, Wells A. A role for phospholipase C-γ-mediated signaling in tumor cell invasion. Clin Cancer Res. 1999;5:2251–2260. [PubMed] [Google Scholar]
- Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991;36:61–113. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKCδ is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 2005;19:115–117. doi: 10.1096/fj.04-1942fje. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis. Development. 2005;132:4599–4610. doi: 10.1242/dev.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C, Burke B, Whitman M. Role of MAP kinase in mesoderm induction and axial patterning during Xenopus development. Development. 1995;121:475–486. doi: 10.1242/dev.121.5.1475. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Localization of MAP kinase activity in early Xenopus embryos: implications for endogenous FGF signaling. Dev Biol. 1997;183:9–20. doi: 10.1006/dbio.1996.8497. [DOI] [PubMed] [Google Scholar]
- MacNicol AM, Muslin AJ, Howard EL, Kikuchi A, MacNicol MC, Williams LT. Regulation of Raf-1-dependent signaling during early Xenopus development. Mol Cell Biol. 1995;15:6686–6693. doi: 10.1128/mcb.15.12.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JA, Kilian B, Chan J, Bayliss PE, Heisenberg CP. Phosphoinositide 3-kinase is required for process outgrowth and cell polarization of gastrulating mesendodermal cells. Curr Biol. 2003;13:1279–1289. doi: 10.1016/s0960-9822(03)00505-0. [DOI] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Scimeca JC, Filloux C, Peraldij P, Carpentier JL, van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem. 1993;268:9803–9810. [PubMed] [Google Scholar]
- Nie S, Chang C. Regulation of early Xenopus development by ErbB signaling. Dev Dyn. 2006;235:301–314. doi: 10.1002/dvdy.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie S, Chang C. Regulation of Xenopus gastrulation by ErbB signaling. Dev Biol. 2006;303:93–107. doi: 10.1016/j.ydbio.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF. Activation of Gβγ signaling downstream of Wnt-11/Xfz-7 regulates Cdc42 activity during Xenopus gastrulation. Dev Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrin in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3- kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. β-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell JM, Smyth JF, Langdon SP. Role of TGFα stimulation of the ERK, PI3 kinase and PLCγ pathways in ovarian cancer growth and migration. Exp Cell Res. 2005;304:305–316. doi: 10.1016/j.yexcr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbamcr.2006.10.005. In press. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Spencer KSR, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1996;93:9641–9644. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Tsang M, Maegawa S, Kiang A, Habas R, Weinberg E, Dawid IB. A role for MKP3 in axial patterning of the zebrafish embryo. Development. 2004;131:2769–2779. doi: 10.1242/dev.01157. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kono R, Iwao Y. PTEN is required for the normal progression of gastrulation by repressing cell proliferation after MBT in Xenopus embryos. Dev Biol. 2006;297:274–283. doi: 10.1016/j.ydbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature. 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. ErbB-4 activation promotes neurite outgrowth in PC12 cells. J Neurochem. 2000;74:979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Keller RE. Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev Biol. 1996;177:413–426. doi: 10.1006/dbio.1996.0174. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-γ1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the noncanonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Zhou H, Pun S, Ip NY, Peng HB, Tsim KWK. Cloning of cDNAs encoding Xenopus neuregulins: expression in myotomal muscle during embryo development. Mol Brain Res. 1998;58:59–73. doi: 10.1016/s0169-328x(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Yang JF, Zhou H, Choi RC, Ip NY, Peng HB, Tsim KWK. A cysteine-rich form of Xenopus neuregulin induces the expression of acetylcholine receptors in cultured myotubes. Mol Cell Neurosci. 1999;13:415–429. doi: 10.1006/mcne.1999.0759. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-γ1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.