Abstract
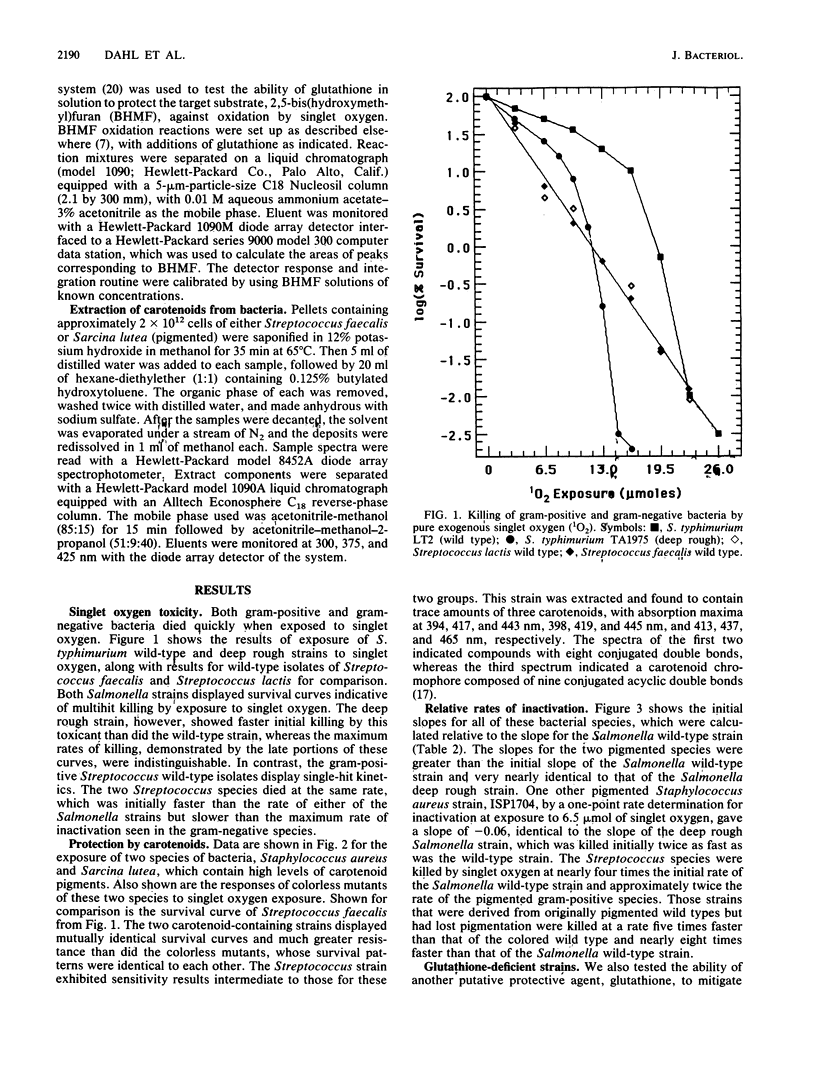
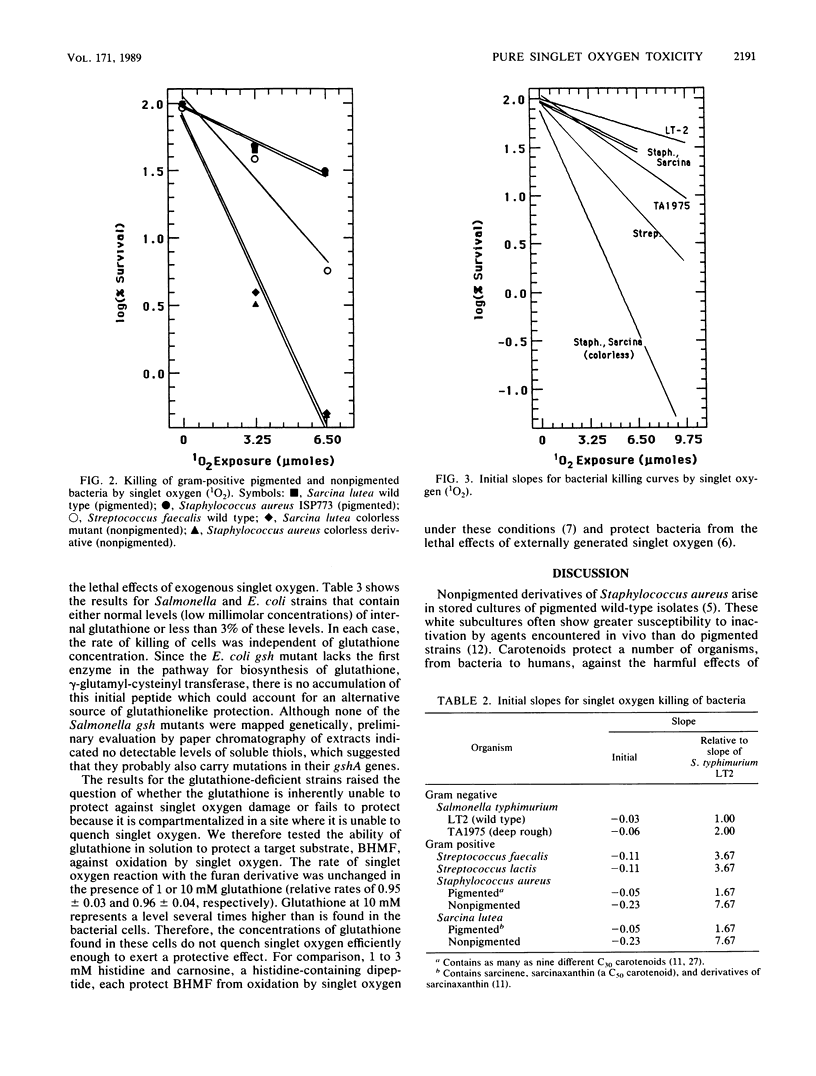
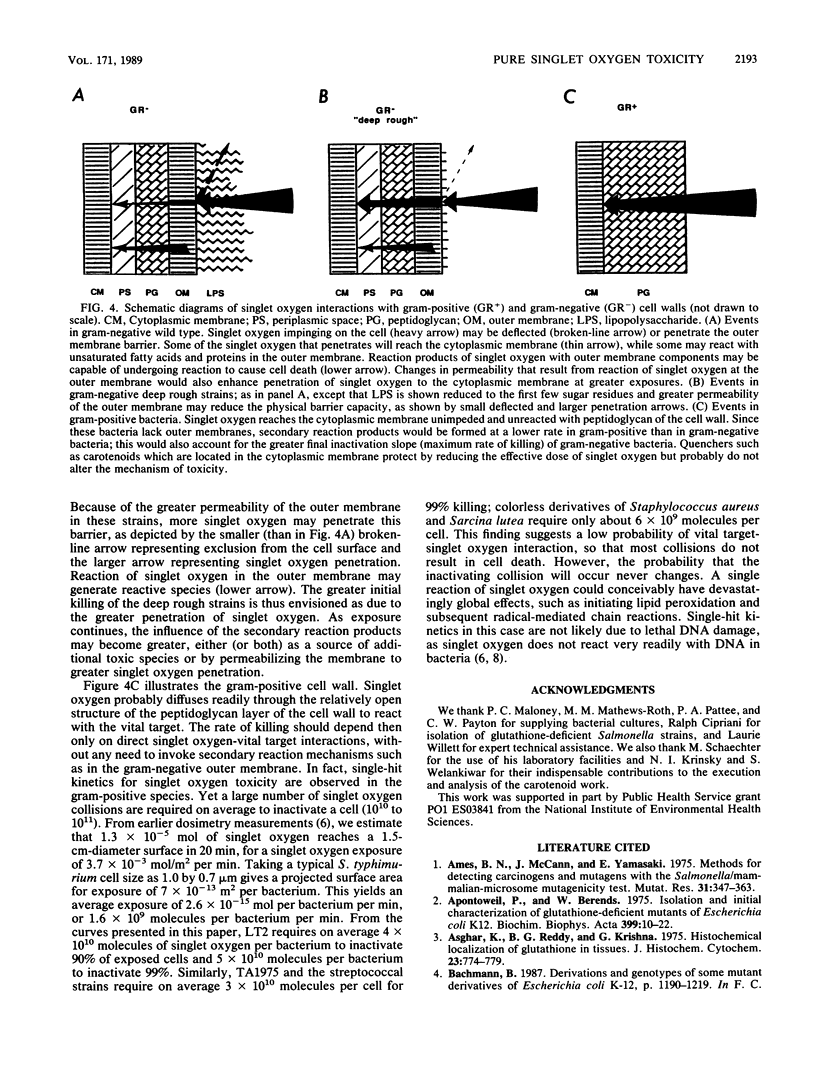
Gram-negative and gram-positive bacteria were found to display different sensitivities to pure singlet oxygen generated outside of cells. Killing curves for Salmonella typhimurium and Escherichia coli strains were indicative of multihit killing, whereas curves for Sarcina lutea, Staphylococcus aureus, Streptococcus lactis, and Streptococcus faecalis exhibited single-hit kinetics. The S. typhimurium deep rough strain TA1975, which lacks nearly all of the cell wall lipopolysaccharide coat and manifests concomitant enhancement of penetration by some exogenous substances, responded to singlet oxygen with initially faster inactivation than did the S. typhimurium wild-type strain, although the maximum rates of killing appeared to be quite similar. The structure of the cell wall thus plays an important role in susceptibility to singlet oxygen. The outer membrane-lipopolysaccharide portion of the gram-negative cell wall initially protects the bacteria from extracellular singlet oxygen, although it may also serve as a source for secondary reaction products which accentuate the rates of cell killing. S. typhimurium and E. coli strains lacking the cellular antioxidant, glutathione, showed no difference from strains containing glutathione in response to the toxic effects of singlet oxygen. Strains of Sarcina lutea and Staphylococcus aureus that contained carotenoids, however, were far more resistant to singlet oxygen lethality than were both carotenoidless mutants of the same species and other gram-positive species lacking high levels of protective carotenoids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Asghar K., Reddy B. G., Krishna G. Histochemical localization of glutathione in tissues. J Histochem Cytochem. 1975 Oct;23(10):774–779. doi: 10.1177/23.10.53246. [DOI] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Pure exogenous singlet oxygen: nonmutagenicity in bacteria. Mutat Res. 1988 Sep;201(1):127–136. doi: 10.1016/0027-5107(88)90119-4. [DOI] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Pure singlet oxygen cytotoxicity for bacteria. Photochem Photobiol. 1987 Sep;46(3):345–352. doi: 10.1111/j.1751-1097.1987.tb04779.x. [DOI] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Some prevalent biomolecules as defenses against singlet oxygen damage. Photochem Photobiol. 1988 Mar;47(3):357–362. doi: 10.1111/j.1751-1097.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Deml E., Oesterle D. Histochemical demonstration of enhanced glutathione content in enzyme-altered islands induced by carcinogens in rat liver. Cancer Res. 1980 Feb;40(2):490–491. [PubMed] [Google Scholar]
- Grinsted J., Lacey R. W. Ecological and genetic implications of pigmentation in Staphylococcus aureus. J Gen Microbiol. 1973 Apr;75(2):259–267. doi: 10.1099/00221287-75-2-259. [DOI] [PubMed] [Google Scholar]
- KLOOS W. E., PATTEE P. A. A BIOCHEMICAL CHARACTERIZATION OF HISTIDINE-DEPENDENT MUTANTS OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1965 May;39:185–194. doi: 10.1099/00221287-39-2-185. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. The function of the carotenoid pigments of Sarcina lutea. Arch Mikrobiol. 1960;35:139–146. doi: 10.1007/BF00425002. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Krinsky N. I. Failure of conjugated octaene carotenoids to protect a mutant of Sarcina lutea against lethal photosensitization. Photochem Photobiol. 1970 Jun;11(6):555–557. doi: 10.1111/j.1751-1097.1970.tb06027.x. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M. Photoprotection by carotenoids. Fed Proc. 1987 Apr;46(5):1890–1893. [PubMed] [Google Scholar]
- Mathews-Roth M. M., Wilson T., Fujimori E., Krinsky N. I. Carotenoid chromophore length and protection against photosensitization. Photochem Photobiol. 1974 Mar;19(3):217–222. doi: 10.1111/j.1751-1097.1974.tb06501.x. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Hartman P. E. Export of glutathione by some widely used Salmonella typhimurium and Escherichia coli strains. J Bacteriol. 1986 Oct;168(1):109–114. doi: 10.1128/jb.168.1.109-114.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTEE P. A., BALDWIN J. N. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J Bacteriol. 1961 Dec;82:875–881. doi: 10.1128/jb.82.6.875-881.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougee M., Bensasson R. V., Land E. J., Pariente R. Deactivation of singlet molecular oxygen by thiols and related compounds, possible protectors against skin photosensitivity. Photochem Photobiol. 1988 Apr;47(4):485–489. doi: 10.1111/j.1751-1097.1988.tb08835.x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Robins P. Isolation of mutants of Escherichia coli with increased resistance to alkylating agents: mutants deficient in thiols and mutants constitutive for the adaptive response. Mol Gen Genet. 1980;180(1):85–90. doi: 10.1007/BF00267355. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Valenzeno D. P. Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol. 1987 Jul;46(1):147–160. doi: 10.1111/j.1751-1097.1987.tb04749.x. [DOI] [PubMed] [Google Scholar]



