Abstract
We investigated the effect of small cortical ischemic lesions, produced by intracerebral injection of the vasoconstrictor endothelin-1, on neurogenesis in the adult mouse subventricular zone. Endothelin-1 (0.5–1 μg) produced infarcts restricted to the cortex, and associated neurobehavioral deficits that largely resolved by 3 d. Bromodeoxyuridine labeling of proliferating cells in the subventricular zone was elevated by about 50% in endothelin-1-treated mice, and cells reactive for doublecortin, a marker for immature neurons, were similarly increased in number. These findings indicate that small ischemic lesions restricted to adult cerebral cortex can stimulate neuroproliferation at a distance.
Keywords: doublecortin, endothelin, ischemia, neurogenesis, stroke, subventricular zone
1. Introduction
Rodent models have provided valuable information about pathophysiological features of focal cerebral ischemia, including neuroprotection, ischemic tolerance, and ischemia-induced neurogenesis. However, models that employ different species (e.g., rat versus mouse) and different techniques for producing ischemia can elicit substantially different lesions, which may vary in their suitability for studying different aspects of human stroke [Carmichael, S.T., 2005]. Small cortical strokes can be produced by intracerebral injection of the potent vasoconstrictor peptide, endothelin-1 (ET-1) [Fuxe, K., et al., 1992; Gilmour, G., et al., 2004; Hughes, P.M., et al., 2003], which has been useful for studying mechanisms of postischemic repair and recovery. Depending on the dose and site of injection, ET-1 can also produce large corticostriatal ischemic lesions [Baldauf, K. and Reymann, K.G., 2005], which have been shown to stimulate neurogenesis in the rat [Baldauf, K. and Reymann, K.G., 2005].
Understanding the relationship between characteristics of ischemic brain lesions and their propensity to stimulate neurogenesis may be important because of the proposed role of neurogenesis in brain repair after stroke and its potential as a target for stroke therapy. With these considerations in mind, we investigated the effect of small cortical ischemic lesions, produced by intracortical injection of ET-1, on neurogenesis in the adult mouse subventricular zone (SVZ).
2. Results
Intracortical administration of 0.5 or 1 μg of ET-1 had no effect on systemic blood pressure, but reduced regional cerebral blood flow (rCBF) near the site of injection by ~70–80%, consistent with the induction of focal cerebral ischemia (Table 1). At both doses, the resulting infarction at 3 d affected a columnar region of cerebral cortex, which extended to the corpus callosum, but which spared the underlying striatum (Fig. 1A). The affected region was more extensive at the higher dose of ET-1, corresponding to an increase of ~50% in infarct volume (Fig. 1B). Functional neurological impairment was evident at 1 h post-ischemia, but had almost resolved by 3d (Fig. 1C), and defects in rotarod performance followed a similar time course (Fig. 1D).
Table 1.
Effects of intracortical ET-1 on regional (frontoparietal) rCBF and mean arterial blood pressure (MABP)
| Measure | ET-1 (μg) | Basal value | Peak decrease (%) | Peak increase (%) |
|---|---|---|---|---|
| rCBF (laser-Doppler flow units) | 0 | 28 ± 3 | −9 ± 5 | — |
| 0.5 | 26 ± 3 | −68 ± 6* | — | |
| 1 | 27 ± 4 | −80 ± 7* | — | |
| MABP (mm Hg) | 0 | 81 ± 4 | −4 ± 2 | 5 ± 3 |
| 0.5 | 79 ± 4 | −5 ± 2 | 6 ± 6 | |
| 1 | 82 ± 4 | −6 ± 2 | 8 ± 4 |
p<0.01 compared to 0 μg ET-1
Figure 1.
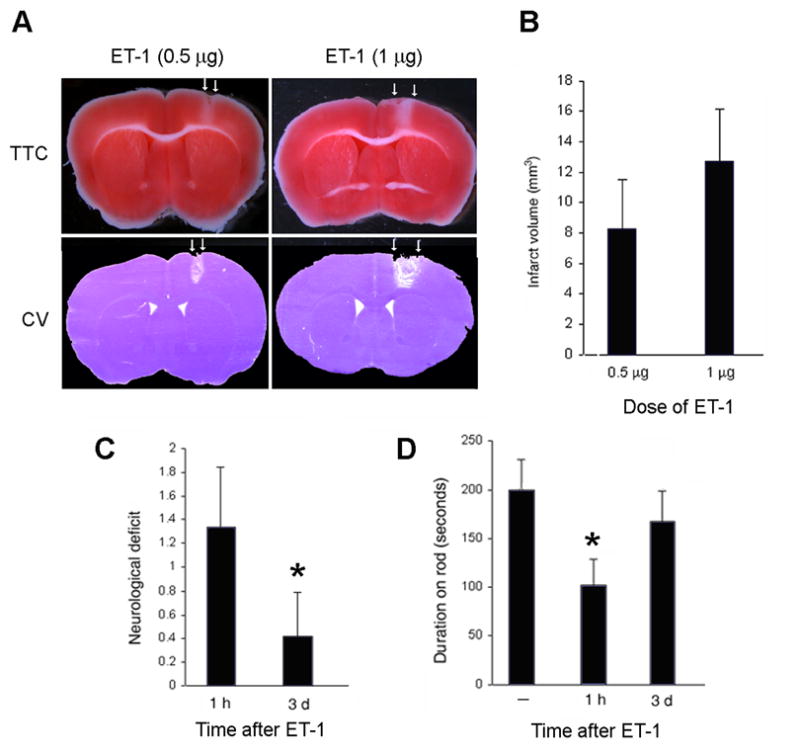
Effects of intracortical ET-1 on infarct size and neurobehavioral function. (A) ET-1 at the doses shown was injected into mouse cortex and, 3 d later, brians were stained with TTC or cresyl violet (CV). Arrows show margins of infarcts. (B) Effect of ET-1 dose on infarct volume. (C) Neurological deficit score (0, no deficit; 4, no spontaneous movement on affected side) after intracortical ET-1 (1 μg). *, p<0.05 compared to 1 h. (D) Rotarod performance after intracortical ET-1 (1 μg). *, p<0.05 compared to baseline (—). N=4 per condition per assay.
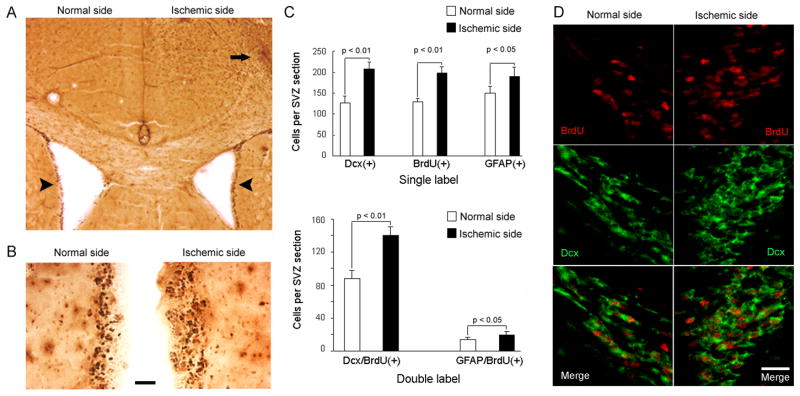
To examine the effects of ET-1-induced ischemia on neurogenesis in SVZ, which is the principal source of new neurons induced by larger ischemic lesions, mice were given bromodeoxyuridine (BrdU) twice daily for 3 d, beginning at the time of ET-1 administration. Coronal sections through the anterior SVZ showed increased numbers of BrdU-immunoreactive cells on the ET-1-treated side compared to the contralateral side, associated with widening of the SVZ (Fig. 2A–B). BrdU-positive cells were also observed in cerebral cortex on the ischemic side. Cell counting revealed an increase of ~50% in BrdU-positive nuclei on the injected side (Fig. 2C). Because BrdU labeling is not lineage-specific, some sections through SVZ were stained for both BrdU and the immature neuronal marker doublecortin (Dcx), or the astroglial marker glial fibrillary acidic protein (GFAP). These experiments demonstrated that Dcx—and, to a lesser extent, GFAP— immunoreactivity was also increased ipsilateral to ET-1 injection, and that most BrdU-positive nuclei were surrounded by Dcx-positive cytoplasm (Fig. 2D).
Figure 2.
Effects of intracortical ET-1 (1 μg) on cell proliferation and neurogenesis in SVZ. (A) Coronal brain section through SVZ shows increased BrdU incorporation (dark brown) on ET-1 treated (right) relative to control (left) side (compare lateral margins of lateral ventricles, arrowheads). BrdU-immunopositive cells are also seen in the ischemic (arrow), but not contralateral, cerebral cortex. (B) Higher-magnification views of SVZ show increased BrdU incorporation (dark brown) and widening of SVZ on right. Bar, 50 μm. (C) Dcx-, BrdU-, and GFAP-positive (top) and Dcx/BrdU- and Dcx/GFAP- doubly positive (bottom) cell counts in SVZ of ischemic (ET-1-treated) versus nonischemic hemisphere. (D) Dual-label fluorescence immunohistochemistry in brain slices through SVZ show increased BrdU incorporation (red, top panels), increased Dcx immunoreactivity (green, middle panels) and increased numbers of BrdU/Dcx-coexpressing cells (red nuclei surrounded by green cytoplasm, bottom panels) on ET-1-treated (ischemic) compared to nonischemic side. The lateral ventricle is at lower left in sections from normal side and lower right in sections from ischemic side. Bar, 30 μm.
3. Discussion
ET-1 is a 21-amino acid vasoconstrictor peptide that was isolated originally from aortic endothelial cells [Yanagisawa, M., et al., 1988]. ET-1 is not directly neurotoxic [Lustig, H.S., et al., 1992], but its topical application on the brain’s surface [Fuxe, K., et al., 1997], perivascular administration in the vicinity of the middle cerebral artery [Sharkey, J., et al., 1993], or direct injection into cerebral cortex [Gilmour, G., et al., 2004; Hughes, P.M., et al., 2003; Luke, L.M., et al., 2004], striatum [Fuxe, K., et al., 1992; Hughes, P.M., et al., 2003], subcortical white matter [Frost, S.B., et al., 2006; Hughes, P.M., et al., 2003] or hippocampus [Mateffyova, A., et al., 2006] results in decreased rCBF and brain infarction in rodents.
The major finding of this study is that small cortical infarcts produced by intracerebral injection of ET-1 enhance SVZ neurogenesis in adult mice. We and others have reported focal ischemia-induced adult neurogenesis using different models in the past [Arvidsson, A., et al., 2002; Jin, K., et al., 2001; Parent, J.M., et al., 2002; Zhang, R.L., et al., 2001], and Baldauf and Reymann have described increased SVZ neurogenesis after ET-1-induced lesion in rats that affected both cortex and striatum [Baldauf, K. and Reymann, K.G., 2005]. However, questions remain regarding the relationship between the sites of ischemia and neuroproliferation. One such question is how remote from the brain’s neuroproliferative zones (SVZ and hippocampal dentate gyrus) an ischemic lesion can be and still stimulate neurogenesis. In global cerebral ischemia, which triggers dentate neurogenesis [Liu, J., et al., 1998], the hippocampus is itself a major site of ischemic damage. In contrast, ET-1-induced focal ischemia affecting the cortex and striatum did not enhance dentate neurogenesis [Baldauf, K. and Reymann, K.G., 2005]. The hippocampus is spared in standard middle cerebral artery occlusion models of stroke in rodents, and although dentate neurogenesis is increased in these models, it is affected less prominently than SVZ. Gu and colleagues [Gu, W., et al., 2000] reported cortical neurogenesis after small photothromotic strokes, but the source of new neurons was thought to be local. Our results suggest that comparatively small ischemic lesions affecting remote brain regions, in this case cerebral cortex, can nevertheless signal to SVZ to elicit a neuroproliferative response. That neurons were among the cell types induced to proliferate in this study is supported by the finding that not only BrdU labeling, but also the number of Dcx immunopositive cells, was increased after ET-1 administration.
Although our study focused on the acute postischemic interval, prior work indicates that neurobehavioral deficits resulting from ET-1 induced ischemia can persist for longer intervals. Abnormalities in forelimb placing and foot faults were present for at least 2 wks after injection of ET-1 into the internal capsule of rats [Frost, S.B., et al., 2006]. Following topical application of ET-1 on the surface of sensorimotor cortex, rats showed defects in a skilled reaching task that lasted at least 20 days [Luke, L.M., et al., 2004]. Intracortical ET-1 impaired accuracy on a reaching task in rats when measured for up to 12 wks post-ischemia [Gilmour, G., et al., 2004]. Finally, intrahippocampal injection of ET-1 in 12-day-old rats prolonged escape latencies in the Morris water maze over 3 mos [Mateffyova, A., et al., 2006]. These observations suggest that depending on the sensitivity of the neurobehavioral tests used, ET-1-induced lesions may be suitable for studying subacaute or chronic features of brain repair after stroke, such as the relationship between neurogenesis and functional recovery.
In previous studies, ischemia-induced neurogenesis in rodents was associated with migration of newborn cells of neuronal lineage to sites of ischemia in striatum or cerebral cortex [Arvidsson, A., et al., 2002; Jin, K., et al., 2003; Parent, J.M., et al., 2002; Zhang, R., et al., 2004]. We did not follow our mice over a period sufficiently long to characterize the ultimate destinations of new neurons, but Baldauf and Reymann found increased numbers of BrdU- and Dcx-positive cells in striatum of ET-1-lesioned rats [Baldauf, K. and Reymann, K.G., 2005] at 14 d post-ischemia. In future work it will be important to establish whether small, remote, ET-1-induced ischemic lesions can not only trigger neurogenesis, but also direct neuromigration to affected brain areas, as well as the extent to which these new neurons survive and function in neuronal circuits.
4. Experimental Procedures
Experiments employed 8-week-old (20–25 g) male C57BL/6J mice and were approved by the Institutional Animal Care and Use Committee. Measures were taken to minimize pain and discomfort. Focal cerebral ischemia was induced by intracortical microinjection of ET-1 (AnaSpec, Inc., San Jose, CA), placed 1.0 mm anterior to the bregma, 1.2 mm lateral to the midline and 1.6 mm below the pia. A 5-μl Hamilton syringe was used to inject 0.5 or 1 μg of ET-1 in 1μl of artificial cerebrospinal fluid at a rate of 2 μl/h, using a syringe pump. Blood pressure was measured via a heparinized plastic catheter in the left femoral artery. rCBF in the vicinity of ET-1 injection was measured by laser Doppler flowmetry and expressed as a percentage of mean basal flow prior to ET-1 application. In some cases, BrdU (Sigma, St. Louis, MO), 50 mg/kg in saline by the intraperitoneal route, was given at 8-hr intervals twice daily for 3 d following ET-1 administration.
For neurobehavioral testing, mice were trained on an accelerating (5–15 rpm) rotarod for 3 d prior to ET-1 injection, and only those mice able to remain on the rod for 200 sec at 15 rpm were given ET-1. Test sessions, consisting of four trials at 15 rpm, were conducted immediately prior to ET-1 injection and 1 hr and 3 d later. The final score was expressed as the mean time that a mouse was able to remain on the rod over four trials. Neurological deficits were evaluated using a 5-point neurological scale ranging from 0=normal function to 4=absence of spontaneous motor activity in the limbs conralateral to ET-1 injection.
For infarct measurement and immunohistochemistry, mice were killed 3 d after ET-1 injection. Infarct size was measured after staining with 2,3,5-triphenyl-tetrazolium chloride (TTC) or cresyl violet. For TTC staining, mice were deeply anesthetized with isoflurane, and brains were removed and sliced into 1-mm sections with the aid of brain matrices. Sections were placed into 2% TTC in PBS and incubated at 37°C for 10 min. TTC solution was replaced with 4% paraformaldehyde for overnight fixation at 4°C. For cresyl violet staining, coronal sections were collected with a cryostat and mounted on Superfrost slides. Staining was performed on 20-μm sections using standard protocols. Stained sections were scanned on a desktop scanner and infarct area was measured using NIH Image J.
In some experiments, brain sections were stained with mouse monoclonal anti-BrdU (2 μg/ml; Roche, Indianapolis, IN) and biotinylated goat-anti-mouse IgG (Vector Laboratories, Burlingame, CA; 1:200), and staining was visualized with diaminobenzidine and H2O2. To count immunolabeled cells, paraffin-embedded sections from brains perfused with 4% paraformaldehyde were stained with mouse monoclonal anti-BrdU (2 μg/ml; Roche, Indianapolis, IN), goat polyclonal anti-Dcx (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-GFAP (1:500; Sigma, St. Louis, MO) and then with FITC- or rhodamine-conjugated, species-specific anti-IgG (Jackson Immuno-Research Laboratories, Inc.; 1:200). Fluorescence signals were detected by Nikon PCM-2000 laser-scanning confocal microscopy. Controls included omitting primary or secondary antibody. Immunolabeled cells in SVZ were counted blindly in an average of nine sections per animal. Results were expressed as the average number of single- or double-immunopositive cells counted per animal.
Values are given as means ± SD and differences between groups were determined using ANOVA and Student’s t test, with p<0.05 considered significant.
Acknowledgments
Supported in part by NIH grant NS44921.
Abbreviations
- TTC
2,3,5-triphenyl-tetrazolium chloride
- BrdU
bromodeoxyuridine
- Dcx
doublecortin
- ET-1
endothelin-1
- rCBF
regional cerebral blood flow
- SVZ
subventricular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Baldauf K, Reymann KG. Influence of EGF/bFGF treatment on proliferation, early neurogenesis and infarct volume after transient focal ischemia. Brain Res. 2005;1056:158–167. doi: 10.1016/j.brainres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav Brain Res. 2006;169:206–211. doi: 10.1016/j.bbr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. Neuroreport. 1997;8:2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Kurosawa N, Cintra A, Hallstrom A, Goiny M, Rosen L, Agnati LF, Ungerstedt U. Involvement of local ischemia in endothelin-1 induced lesions of the neostriatum of the anaesthetized rat. Exp Brain Res. 1992;88:131–139. doi: 10.1007/BF02259134. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Iversen SD, O’Neill MF, Bannerman DM. The effects of intracortical endothelin-1 injections on skilled forelimb use: implications for modelling recovery of function after stroke. Behav Brain Res. 2004;150:171–183. doi: 10.1016/j.bbr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Anthony DC, Ruddin M, Botham MS, Rankine EL, Sablone M, Baumann D, Mir AK, Perry VH. Focal lesions in the rat central nervous system induced by endothelin-1. J Neuropathol Exp Neurol. 2003;62:1276–1286. doi: 10.1093/jnen/62.12.1276. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- Lustig HS, Chan J, Greenberg DA. Comparative neurotoxic potential of glutamate, endothelins, and platelet-activating factor in cerebral cortical cultures. Neurosci Lett. 1992;139:15–18. doi: 10.1016/0304-3940(92)90847-z. [DOI] [PubMed] [Google Scholar]
- Mateffyova A, Otahal J, Tsenov G, Mares P, Kubova H. Intrahippocampal injection of endothelin-1 in immature rats results in neuronal death, development of epilepsy and behavioral abnormalities later in life. Eur J Neurosci. 2006;24:351–360. doi: 10.1111/j.1460-9568.2006.04910.x. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Ritchie IM, Kelly PA. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1993;13:865–871. doi: 10.1038/jcbfm.1993.108. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]