Abstract
Neuroglobin (Ngb) is a recently discovered vertebrate globin expressed primarily in neurons. Ngb expression is induced by hypoxia and ischemia, and Ngb protects neurons from these insults. However, its normal physiological role and the mechanism underlying its neuroprotective action are uncertain. We report production of a transgenic mouse in which Ngb is overexpressed under the control of the chicken β-actin promoter. This mouse should prove helpful for studying Ngb-mediated effects in vitro and in vivo.
Keywords: oxygen, globin, hypoxia, ischemia, brain, neuron
1. Introduction
Since its discovery in the 1770s by Scheele, Priestly and Lavoisier (Jackson, 2005), O2 has been a focal point of biological investigation. O2-binding proteins were the first proteins for which the three-dimensional crystal structure was determined (myoglobin) (Kendrew et al., 1958) and the first in which a single amino acid substitution was shown to be the cause of a disease (hemoglobin in sickle-cell disease) (Ingram, 1956).
Globins are proteins that contain a heme prosthetic group. They are found in bacteria, archaea and eukaryotic plants and animals, and over 300 have been identified to date (Vinogradov et al., 2006). Within the past few years, two new vertebrate globins — neuroglobin (Ngb) (Burmester et al., 2000) and cytoglobin (Cgb) (Burmester et al., 2002) — were described. Ngb is a monomeric globin of Mr~17 kDa, which is expressed primarily in neurons, but also in some endocrine cells (Burmester et al., 2000). In the retina, the cellular distribution of Ngb parallels O2 demand (Schmidt et al., 2002). Ngb binds O2, NO and CO with high affinity, although its physiological role is poorly understood. Possible functions of Ngb include those ascribed to other vertebrate globins, which include O2 transport, storage, facilitated diffusion, sensing and scavenging, and NO scavenging (Suzuki and Imai, 1998; Weber and Vinogradov, 2001). However, some of these seem unlikely because of the low intracellular concentrations at which Ngb occurs (Brunori and Vallone, 2006) and its unfavorable O2-binding affinity under physiological conditions (Fago et al., 2004). On the other hand, Ngb could serve as a sensor of local O2 and NO concentrations, as proposed by Brunori (Brunori et al., 2005). Finally, Ngb has been reported to act as a guanine nucleotide dissociation inhibitor, binding to Gαi subunits of G proteins and thereby increasing levels of free Gβγ (Wakasugi et al., 2003).
The neuronal localization of Ngb and its ability to bind O2 suggests a possible role in neuronal adaptation to hypoxic conditions, including ischemia. Consistent with this idea, hypoxia increases Ngb expression in primary neuronal cultures prepared from mouse brain and focal cerebral ischemia increases expression in rat brain (Sun et al., 2001). Knockdown of Ngb rendered these cultures more vulnerable to hypoxia, whereas forced overexpression of Ngb in a cell line derived from hippocampal neurons conferred relative protection from hypoxia. In another study, cultured human neuroblastoma cells transfected with a Ngb- (or Cgb-) expressing plasmid were resistant to oxidative injury induced by H2O2 (Fordel et al., 2006). In vivo studies yielded similar results. Thus, the volume of cerebral infarcts caused by occlusion of the middle cerebral artery in the rat was increased by intracerebroventricular administration of a Ngb antisense oligodeoxynucleotide and reduced by intracerebral injection of a Ngb-expressing adeno-associated virus vector (Sun et al., 2003).
Here we describe the production of transgenic (Tg) mice that overexpress Ngb. As reported recently (Khan et al., 2006), these mice are resistant to ischemia in tissues with elevated Ngb levels. These include not only brain, but also tissues like heart, where Ngb is not normally found. The availability of genetically modified mice with altered Ngb expression may facilitate studies on Ngb function under normal and pathological conditions.
2. Materials and methods
2.1. Production of transgenic mouse
Ngb-overexpressing Tg (Ngb-Tg) mice were produced through the Buck Institute’s Knockout-Transgenic Core with the approval of the institutional Animal Care and Use Committee.
Full-length murine Ngb cDNA (a gift from Drs. Thomas Hankeln and Thorsten Burmster, Institute of Molecular Genetics, Johannes Gutenberg University Mainz, Mainz, Germany), was subcloned into the Spe1 and EcoRV sites of pTR-UF12d, downstream of the chicken β-actin promoter and CMV enhancer downstream and upstream of Renilla reniformis green fluorescent protein (GFP) (Figure 1A). Final plasmids were verified in human embryonic kidney (HEK 293) cells (Figure 1B) and confirmed by western blotting. Plasmids were digested with restriction enzymes, purified using QIAEX II gel extraction kits (QIAGEN), and microinjected into fertilized eggs of BDF × CD1 mice [Hogan, 1986 #5270]. Transgenes were detected by PCR of mouse-tail DNA.
Figure 1.
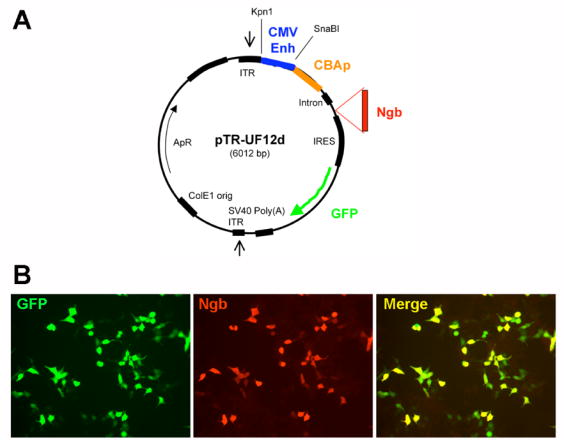
(A) Vector used to produce Ngb-Tg mice. (B) GFP (green) and Ngb (red) coexpression in vector-transfected HEK 293 cells.
A total of five BDF × CD1 founder mice expressed GFP, and in three of these, RT-PCR and western blotting confirmed Ngb overexpression in cerebral cortex and striatum. These brain regions were chosen for screening because of our interest in possible effects of Ngb overexpression on focal cerebral ischemia in the middle cerebral artery distribution (Sun et al., 2003). However, Ngb overexpression was also observed in other brain areas, including olfactory bulb, hippocampus, cerebellum and brainstem.
The offspring of matings between heterozygous Ngb-Tg (Ngb+/−) mice showed genotypes in a ratio consistent with mendelian transmission without disproportionate pre- or perinatal lethality. Intersibling matings over ≥6 generations were used to generate experimental subjects. The experiments reported here employed homozygous Ngb-Tg (Ngb+/+) mice and, where applicable, wild type littermate controls. The Ngb- and GFP-specific PCR primers used for genotyping mouse-tail DNA are described elsewhere (Khan et al., 2006).
2.2. Reverse transcriptase polymerase chain reaction (RT-PCR)
Total mouse RNA was isolated with RNeasy Mini kits (QIAGEN) and reverse-transcribed into first strand cDNA using the Reverse Transcription System and Oligo dT12–18, according to instructions. Reverse-transcribed samples (2 ng RNA) were added to 20 mM Tris-HCl (pH. 8.4), 50 mM KCl, 2 mM MgCl2, 0.5 mM dNTPs, 1 unit taq polymerase and 0.5 nM each of Ngb or GFP primers, in a total volume of 25 μl. Conditions for PCR amplification were: 94°C × 5 min, 30 cycles at 94°C × 30 s, 57°C × 30 s, 72°C × 45 s, 72°C × 5 min. PCR products were separated on 1% agarose gels.
2.3. Western blotting
Protein was extracted in 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 μg/ml of aprotinin and 100 μg/ml of phenylmethylsulfonyl fluoride in PBS. A Bio-Rad protein assay was used to determine protein concentration, and 50 μg of protein per sample was boiled in SDS sample buffer (100°C × 5 min), then separated by 12% SDS-PAGE and transferred to polyvinyldifluoridine membranes. Membranes were incubated with affinity-purified goat anti-Ngb (1:200; Santa Cruz Biotechnology), washed 0.1% Tween-20 in PBS, incubated at room temperature for 60 min with horseradish peroxidase-conjugated anti-goat IgG (Santa Cruz Biotechnology; 1:3,000) and washed (3 × 15 min) in the same manner. A chemiluminescence substrate system (NEN Life Science Products Inc.) was used to visualize peroxidase activity.
2.4. Imunohistochemistry
Fluorescence immunohistochemistry was performed as described in detail elsewhere (Sun et al., 2001; Sun et al., 2003). Primary antibodies were goat polyclonal anti-Ngb (1:200; Santa Cruz Biotechnology), mouse monoclonal anti-NeuN (1:200; Chemicon) and mouse monoclonal anti-GFAP (1:150; Sigma), and secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated rat-absorbed donkey anti-mouse and rhodamine-conjugated donkey anti-goat immunoglobulin G (Jackson ImmunoResearch; 1:200). Fluorescence was detected with a Nikon E800 microscope at λexcitation/λemission = 535/565 nm (rhodamine, red), 470/505 (FITC, green) and 360/400 (4′-6-diamidino-2-phenylindole (DAPI), blue). Controls involved preadsorbing the primary antibody or omitting the primary or secondary antibody. Results were recorded with a Magnifire digital camera (ChipCoolers, Warwick, RI). Selected images were viewed at high magnification using a Nikon PCM-2000 laser-scanning confocal microscope and Simple PCI imaging software (Compix, Cranberry Township, PA).
3. Results
3.1. Production of trangenic mouse
RT-PCR of tissue from several brain regions showed that wild-type controls expressed Ngb diffusely throughout the brain (Figure 2A), consistent with previous reports (Burmester et al., 2000; Reuss et al., 2002). F1 founders showed increased Ngb signal, as well as GFP signal consistent with the transgenic origin of this increase. Moreover, the intensity of the Ngb signal in Ngb-Tg mice was relatively uniform over the brain areas tested. Western blots showed increased Ngb protein expression in three of five founder lines (Figure 2B), as noted in Materials and Methods. The extent of Ngb overexpression varied across founder lines, as did the relative extent of expression in cortex compared to striatum. As observed previously for whole-brain protein samples (Khan et al., 2006), Ngb protein expression in samples from cortex and striatum of wild-type control mice was too low to give a detectable signal on western blots with the protein concventration and antibody we used.
Figure 2.
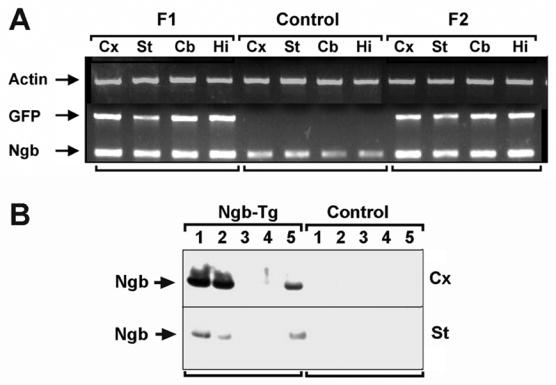
RT-PCR (A) shows increased expression of Ngb (and GFP) mRNA in brain regions from Ngb-Tg but not wild type mice. Cx, cerebral cortex; St, striatum; Cb, cerebellum; Hi, hippocampus. Western blots (B) showed increased expression of Ngb protein in cerebral cortex (Cx) and striatum (St) from 3/5 Ngb-Tg founder lines, compared to wild type controls.
3.2. Overt phenotype
There was no grossly discernable abnormality in the appearance or behavior of newborn, juvenile or young adult mice. Body size and weight were comparable to those of wild-type littermates, and there were no morphological abnormalities affecting the teeth, eyes, head, pinnae, digits, genitals or tail. Necropsy revealed no gross dysmorphogenesis of brain or viscera, and histological sections through the brain were normal.
Ngb-Tg mice and wild-type littermates both weaned at ~3 weeks of age and gained weight to an equal extent. There were no abnormailities in feeding or drinking, and developmental milestones were achieved simultaneously with wild type mice. Neither seizures nor abnormal socialization were observed. Male and female Ngb-Tg mice were fertile by 4–6 weeks of age.
3.3. Immunohistochemistry
Brain sections showed widespread expression of Ngb in multiple regions. Ngb was expressed in cells expressing neuronal markers, as observed previously in non-Tg rodents (Sun et al., 2001), but also in cells that expressed astroglial markers (Figure 3). As reported previously (Khan et al., 2006), additonal cell types, including endothleium, also expressed Ngb ectopically in Ngb-Tg mice, and Ngb was found in non-neural tissues from which it is normally absent or present in amounts too small to detect. This is not unexpected based on the use of a tissue-nonspecific (chicken β-actin) promoter.
Figure 3.
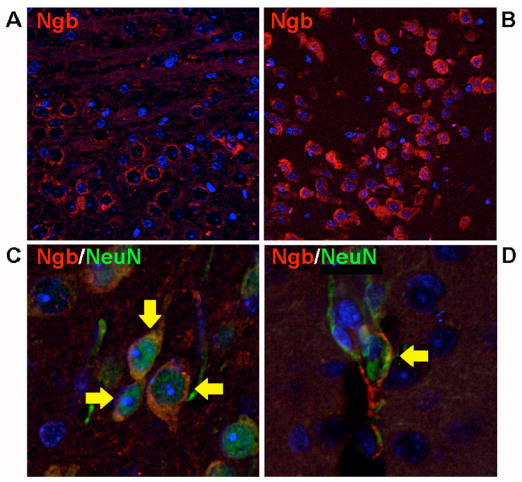
Fluorescence immunohistochemistry on brain sections from Ngb-Tg mice shows widespread Ngb expression (red) in cerebral cortex (A) and olfactory bulb (B), as well as localization (arrows) of Ngb to cells expressing both neuronal (NeuN, C) and astroglial (GFAP, D) markers (green). Note that Ngb and GFAP are cytoplasmic and NeuN nuclear. DAPI (blue) was used to stain nuclei.
4. Discussion
The main finding reported here is the production of a Ngb-overexpressing transgenic mouse. The relative resistance of this mouse to cerebral and myocardial ischemia has been described elsewhere (Khan et al., 2006). Ngb-Tg mice represent a new reagent with which to study the physiological and pathophysiological roles of Ngb, mechanisms for which remain obscure.
The observation that Ngb, an O2-binding heme protein expressed in cerebral neurons (Burmester et al., 2000), is induced by neuronal hypoxia and cerebral ischemia and protects neurons from hypoxia and ischemia (Sun et al., 2001; Sun et al., 2003), suggests that this protein may have a role in sensing or responding to neuronal hypoxia. Because O2 is critical for cellular functions like mitochondrial oxidative phosphorylation, cells require mechanisms for sensing hypoxia, transducing this signal, and eliciting adaptive cellular responses (Bunn and Poyton, 1996). The molecular oxygen sensor in most cells has not been identified with certainty, but a prevalent view is that hypoxia sensing in mammalian cells involves one or more proteins containing an iron-porphyrin (heme) group (Wenger, 2000). Candidate hypoxia sensors include AMP-activated kinase, heme oxygenase 2, NADPH oxidase, mitochondrial complexes I, III, and IV, von Hippel-Lindau protein, hypoxia-inducible factor-1 (HIF-1) prolyl hydroxylase-2, and succinate dehydrogenase (Baysal, 2006; Kemp, 2006).
Having sensed hypoxia, the sensor is next presumed to undergo a conformational change that initiates transduction of the hypoxia signal. Two major classes of hypoxia signaling pathways are activated: fast (seconds to minutes) responses mediated through O2-sensitive ion channels and slow (hours to days) responses that involve transcriptional activation of hypoxia-inducible genes (Lopez-Barneo et al., 2001). In mammalian forebrain neurons, for example, fast responses to hypoxia lead to hyperpolarization by activating KCa and KATP currents and reducing voltage-gated Na currents (Lopez-Barneo et al., 2001). The best characterized slow responses are those orchestrated through the basic helix-loop-helix Per-Arnt-Sim transcription factor, HIF-1. HIF-1-inducible genes include vascular endothelial growth factor, erythropoietin, and a variety of glycolytic enzymes.
Where Ngb might fit in this hypoxia-sensing/responding scheme is uncertain. One possibility is that Ngb is a hypoxia sensor or, as suggested recently (Brunori and Vallone, 2006), a sensor of the ratio of O2 and NO levels. The observation that Ngb expression is induced by hypoxia, cobalt and deferoxamine (Sun et al., 2001) is consistent with, but not proof of, induction via HIF-1, which would suggest a more downstream, hypoxia-responding, role for Ngb. The ability of Ngb overexpression to protect neurons from hypoxia (Sun et al., 2001) and ischemia (Sun et al., 2003; Khan et al., 2006) could be explained by enhancement of either hypoxia sensing or hypoxia responding. The transgenic model described here may be useful for resolving these and other controversies about Ngb.
Acknowledgments
Supported by National Institutes of Health grant NS35965 (D.A.G.) and by the Buck Institute for Age Research.
Abbreviations
- Cgb
cytoglobin
- DAPI
4′-6-diamidino-2-phenylindole
- FITC
fluorescein isothiocyanate
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- HIF-1
hypoxia-inducible factor-1
- Ngb
neuroglobin
- Ngb-Tg
neuroglobin-overexpressing transgenic
- RT-PCR
reverse transcriptase polymerase chain reaction
- SDS
sodium dodecyl sulfate
- Tg
transgenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baysal BE. A phenotypic perspective on mammalian oxygen sensor candidates. Ann N Y Acad Sci. 2006;1073:221–33. doi: 10.1196/annals.1353.024. [DOI] [PubMed] [Google Scholar]
- Brunori M, Giuffre A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci U S A. 2005;102:8483–8. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M, Vallone B. A globin for the brain. Faseb J. 2006;20:2192–7. doi: 10.1096/fj.06-6643rev. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed invertebrate tissues. Mol Biol Evol. 2002;19:416–21. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber RE. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J Biol Chem. 2004;279:44417–44426. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L, Dewilde S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett. 2006;410:146–151. doi: 10.1016/j.neulet.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1986. [Google Scholar]
- Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956;178:792–4. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- Jackson J. A World on Fire: A Heretic, an Aristocrat, and the Race to Discover Oxygen. Viking; New York: 2005. [Google Scholar]
- Kemp PJ. Detecting acute changes in oxygen: will the real sensor please stand up? Exp Physiol. 2006;91:829–34. doi: 10.1113/expphysiol.2006.034587. [DOI] [PubMed] [Google Scholar]
- Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958;181:662–6. doi: 10.1038/181662a0. [DOI] [PubMed] [Google Scholar]
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–8. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanisms of oxygen sensing. Annu Rev Physiol. 2001;63:259–87. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–56. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–5. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is upregulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Imai K. Evolution of myoglobin. Cell Mol Life Sci. 1998;54:979–1004. doi: 10.1007/s000180050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov SN, Hoogewijs D, Bailly X, Arredondo-Peter R, Gough J, Dewilde S, Moens L, Vanfleteren JR. A phylogenomic profile of globins. BMC Evol Biol. 2006;6:31. doi: 10.1186/1471-2148-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi K, Nakano T, Morishima I. Oxidized human neuroglobin acts as a heterotrimeric Galpha protein guanine nucleotide dissociation inhibitor. J Biol Chem. 2003;278:36505–12. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]
- Weber RE, Vinogradov SN. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203(Pt 8):1253–63. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]


