Abstract
Elevated levels of polyamines have long been associated with skin tumorigenesis. Tightly regulated metabolism of polyamines is critical for cell survival and normal skin homeostasis, and these controls are dysregulated in skin tumorigenesis. A key enzyme in polyamine biosynthesis, ornithine decarboxylase (ODC) is upregulated in skin tumors compared to normal skin. Use of transgenic mouse models has demonstrated that polyamines play an essential role in the early promotional phase of skin tumorigenesis. The formation of skin tumors in these transgenic mice is dependent upon polyamine biosynthesis, especially putrescine, since treatment with inhibitors of ODC activity blocks the formation of skin tumors and causes the rapid regression of existing tumors. Although the mechanism by which polyamines promote skin tumorigenesis are not well understood, elevated levels of polyamines have been shown to stimulate epidermal proliferation, alter keratinocyte differentiation status, increase neovascularization, and increase synthesis of extracellular matrix proteins in a manner similar to that seen in wound healing. It is becoming increasingly apparent that elevated polyamine levels activate not only epidermal cells but also underlying stromal cells in the skin to promote the development and progression of skin tumors. The inhibition of polyamine biosynthesis has potential to be an effective chemoprevention strategy for nonmelanoma skin cancer.
Keywords: Polyamines, skin cancer, ornithine decarboxylase
Introduction
More than half of all cancers in North America occur in the skin, and they represent a major public health concern due to the very high incidence, associated medical costs, morbidity, and cosmetic defects resulting from current treatments (Dlugosz et al., 2002). It is well documented that chronic exposure to UV radiation is the primary cause of nonmelanoma and melanoma skin cancer in humans. UV light is a complete carcinogen in that it can act as both a tumor initiator and a tumor promoter. Whereas UV light produces irreversible mutagenic damage, UV also induces epigenetic changes that promote the expansion of the initiated cell population that includes dermal inflammation, epidermal hyperplasia, and changes in the expression of multiple genes associated with proliferation, differentiation, and eicosanoid and cytokine production (Yuspa, 1986; Afaq et al., 2005). It is thought that many epidermal genetic lesions are caused by exposure to solar irradiation early in life (de Gruijl, 1999; Kennedy et al., 2003). However, cells harboring these mutations often remain dormant for many years until triggered to form tumors later in life. The induction of ornithine decarboxylase (ODC) activity with subsequent increased levels of polyamines, has been shown to play a causal role in skin tumor development in a variety of animal models. Moreover, inhibitors of polyamine synthesis have been shown to effectively suppress skin tumor incidence and severity in both UV and chemically induced experimental models and in cancer chemoprevention trials in high risk human populations.
The polyamines putrescine, spermidine and spermine are some of the major cations present in eukaryotic cells. The majority of polyamine molecules are bound to polyanionic macromolecules such as DNA, RNA, and phospholipids (Igarashi et al., 1982), resulting in far-reaching effects upon cellular processes including DNA replication, transcription, and translation. It is not surprising that numerous studies using specific inhibitors of polyamine biosynthesis have documented that these small ubiquitous molecules are absolutely required for all cell growth and differentiation. Although a vast number of studies have shown that polyamines are crucial to the growth and proliferation of cells, the cellular functions of polyamines and their interactions with cellular components that play a key role in promoting tumorigenesis remain largely unknown. This review will focus on our current understanding of the relationship between polyamines and nonmelanoma skin tumorigenesis.
Mammalian Metabolism of Polyamines
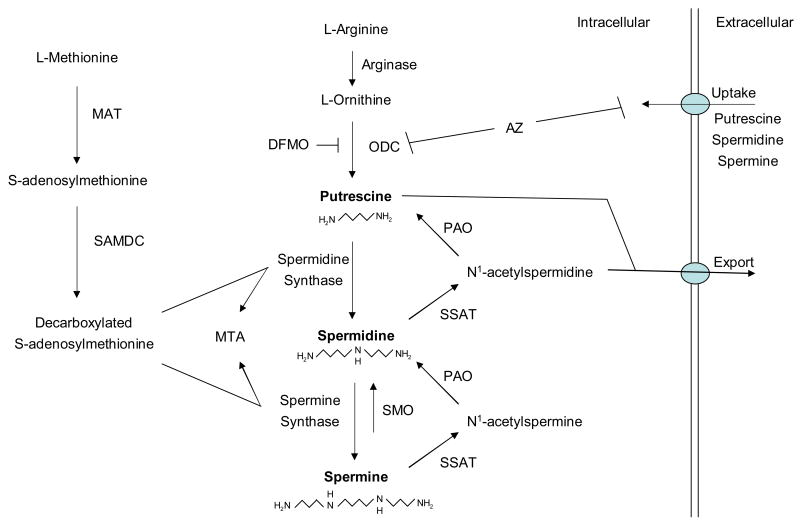
Polyamine levels are tightly controlled by a complex array of biosynthetic and catabolic pathways and a multitude of compensatory mechanisms (see Fig. 1), all of which attest to the essential role of polyamines in cell survival (Thomas and Thomas, 2003; Wallace et al., 2003; Gerner and Meyskens, 2004). In mammalian cells, polyamines are synthesized from the amino acids L-methionine and L-arginine. L-arginine is metabolized to L-ornithine through the action of arginase. Polyamine biosynthesis begins with the decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Spermidine and spermine are synthesized by the sequential additions of aminopropyl groups provided by L-methionine after its conversion into S-adenosylmethionine (SAM) by methionine adenosyltransferase (MAT). Decarboxylation of S-adenosylmethionine (SAM) by S-adenosylmethionine decarboxylase (SAMDC) generates decarboxylated SAM which donates its propyl amine moiety to the formation of spermidine and spermine by spermidine synthase and spermine synthase, respectively. Both ODC and SAMDC are rate-limiting enzymes in the biosynthesis of polyamines.
Figure 1.
Mammalian polyamine metabolic pathways. Abbreviations include: MAT, methionine adenosyltransferase; SAMDC, S-adenosylmethionine decarboxylase; ODC, ornithine decarboxylase; DFMO, α-difluoromethylornithine; MTA, 5′-methylthioadenosine; SSAT, spermidine/spermine acetyltransferase; PAO, polyamine oxidase; SMO, spermine oxidase; AZ, antizyme.
Intracellular levels of polyamines are also controlled by catabolic pathways that permit the conversion of spermine back to putrescine. In this retroconversion process, spermine and spermidine are acetylated by spermidine/spermine acetyltransferase (SSAT) using acetyl-CoA to form N1-acetylspermine and N1-acetylspermidine. These acetylated polyamines are substrates for a peroxisomal, FAD-dependent polyamine oxidase (PAO) which catalyzes their conversion back to spermidine and putrescine. In a second pathway, putrescine and N1-acetylspermidine can be exported by the transporter, diamine exporter (DAX) (Xie et al., 1997) and then eliminated in the urine. As a consequence of this export system, acetylated polyamines are rarely found in normal cells, but are found in high concentrations in some cancer cells that demonstrate altered polyamine catabolism (Wallace et al., 2000; Wallace et al., 2003). In addition, a second polyamine catabolic pathway has recently been characterized. The inducible, cytosolic spermine oxidase (SMO) oxidizes non-acetylated spermine to spermidine, H2O2, and aldehyde 3-aminopropanol (Pledgie et al., 2005). Polyamine levels are controlled not only by these highly regulated biosynthetic and catabolic pathways, but are also fine-tuned by an energy-dependent and carrier-mediated polyamine uptake system that is important in maintaining cellular polyamine homeostasis. This alternative supply of polyamines is upregulated in response to polyamine depletion, and the polyamine uptake system can provide a major method of resistance to chemotherapeutic treatments that rely on inhibition of polyamine biosynthesis.
Additional regulation of polyamine homeostasis is provided by the protein antizyme (AZ) that binds noncovalently to the ODC monomer to inhibit ODC activity and to target ODC for proteasomal degradation in an ubiquitin-independent manner (Coffino, 2001; Mangold, 2005). In addition, AZ inhibits the uptake of exogenous polyamines and promotes polyamine excretion. Thus, whereas AZ is upregulated by high levels of polyamines, it functions to suppress polyamine accumulation via multiple mechanisms.
NonMelanoma Skin Tumorigenesis in Humans and Animals
Malignant skin tumors in humans include malignant melanoma, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) (Klein-Szanto, 1989; Dlugosz et al., 2002). BCCs are the most common human cutaneous malignancy and they are typically slow-growing, locally invasive tumors that rarely metastasize. Although SCCs only represent about 20% of nonmelanoma skin cancers in humans, SCCs are generally more aggressive than the more common basal cell carcinomas (BCC) and can be lethal. SCCs are usually very well differentiated tumors with massive production of horny material, which progressively invade surrounding tissue and can eventually metastasize to distant sites. Approximately 60% of SCCs arise from a preexisting benign actinic keratoses (Marks et al., 1988). While most actinic keratoses do not progress to SCC, actinic keratoses represent SCC in situ at its earliest stages (Cockerell, 2000).
One of the most frequently used animal models for studying carcinogenesis in a lining epithelium is the initiation-promotion model of tumorigenesis in mouse skin. Whereas actinic keratoses are thought to be the benign precursor for human SCCs, papillomas have been identified as benign precursors for murine SCCs. Papillomas are benign epidermal tumors seen very frequently after chemical carcinogen exposure, especially in two-stage carcinogenesis protocols in mouse skin (Klein-Szanto, 1989). Murine papillomas are cauliflower-like structures with a series of folds consisting of a central vascularized, connective tissue core covered by a proliferative, stratified squamous epithelium and an abundant, orthokeratotic horny layer. Some papillomas regress, but others can progress to malignancy. Squamous cell carcinomas (SCC) can be induced in animals using UV light, ionizing radiation, or chemical carcinogens. In contrast to humans, mice have a strong predisposition to developing squamous cell carcinomas and appear to be relatively resistant to the development of BCCs. Following exposure to UV light or complete carcinogens, mice can also develop keratoacanthomas (KA), which have a cup-shaped form with a horny central crater and epidermal edges that appear to be a continuation of the upper third of hair follicles. Unlike human KA, murine KA rarely regress and often convert to squamous carcinomas (Klein-Szanto, 1989).
ODC and NonMelanoma Skin Tumorigenesis
Polyamines have long been known to be associated with cell proliferation in both normal and neoplastic tissues (Tabor and Tabor, 1984; Pegg, 1986; Pegg, 1988). Elevated levels of ODC and increased polyamines were initially suspected to play a causal role in skin tumorigenesis largely due to the early induction of ODC by tumor promoters (O'Brien, 1976; Gilmour et al., 1986);(Gilmour et al., 1987)}, and to studies using inhibitors of ODC (Bollag, 1972; Verma et al., 1980; Weeks et al., 1982; Takigawa et al., 1983). For instance, α-difluoromethylornithine (DFMO), which is a specific and irreversible inhibitor of ODC enzyme activity, inhibits the development of skin tumors in carcinogen-treated mice when it is given during the promotion phase (Weeks et al., 1982; Takigawa et al., 1983). Although cellular mechanisms exist to tightly control the expression of ODC in normal cells, the regulation of ODC is altered in tumor cells, yielding constitutively high levels of ODC expression (O'Brien, 1976; Gilmour et al., 1986; Gilmour et al., 1987; Pegg et al., 1998) and subsequent increased levels of polyamines (Koza et al., 1991). ODC activity and polyamine levels are dramatically elevated in human squamous cell carcinomas compared to adjacent normal skin tissue (Scalabrino et al., 1980; Hietala et al., 1988). This can result from the upregulation of ODC expression by oncogenes such as c-myc (Bello-Fernandez et al., 1993; Ben-Yosef et al., 1998), v-src (Höltta et al., 1993), v-raf (Shantz and Pegg, 1998), or an activated Ras or RhoA (Shantz and Pegg, 1998). In fact, c-Myc has been shown to transactivate ODC (Bello-Fernandez et al., 1993; Ben-Yosef et al., 1998). However, although some oncogenes can increase ODC activity, ODC is also transiently induced in the skin by a variety of stimuli including mitogens, tumor promoters such as 12-O-tetradecanoylphorbol ester (TPA), and hormones.
Use of transgenic mouse models has demonstrated that polyamines play an essential role in the early promotional phase of skin tumorigenesis (Pegg et al., 2003; Gerner and Meyskens, 2004). Elevated ODC activity in K6/ODC and K5/ODC transgenic mice has been constitutively targeted to either the outer root sheath of hair follicles or the basal epidermal layer in the skin with a keratin 6 or keratin 5 promoter, respectively, to yield increases in polyamine pools, especially putrescine levels (Megosh et al., 1995; O'Brien et al., 1997). These sustained high levels of putrescine lead to alopecia, the development of follicular dermal cysts, increased nail growth, and skin wrinkling. With regard to tumor development, the targeted expression of ODC to the skin also increases the susceptibility of these mice to skin tumor formation following a variety of initiating events including carcinogens (O'Brien et al., 1997; Chen et al., 2000), UV irradiation (Ahmad et al., 2001), and oncogenes (Smith et al., 1998; Tang et al., 2004; Lan et al., 2005). The treatment of newborn or adult K6/ODC transgenic mice with a single topical application of the carcinogen 7, 12-dimethylbenz[a]anthracene (DMBA) yields papilloma formation six to eight weeks later without the use of tumor promoting agents (O'Brien et al., 1997; Peralta Soler et al., 1998). Likewise, single treatment with other carcinogens from different chemical classes also induces skin tumors in K6/ODC transgenic mice (Chen et al., 2000). With particular relevance to human skin tumorigenesis, K6/ODC transgenic mice develop more papillomas following UVB irradiation compared to wildtype littermates (Ahmad et al., 2001).
Expression of specific oncogenes in the skin of K6/ODC transgenic mice is sufficient to lead to spontaneous and differential skin tumor outcomes without the need of additional chemical carcinogens or tumor promoters. Bi-transgenic mice expressing both skin-targeted ODC and the v-Ha-ras oncogene develop spontaneous keratoacanthomas and squamous cell carcinomas, whereas no tumors develop in the mono-transgenic mice (Smith et al., 1998; Lan et al., 2005). On the other hand, basal cell carcinomas develop in mice that are both heterozygous null for the patched tumor suppressor gene and overexpressing ODC in follicular cells (Tang et al., 2004).
The formation of skin tumors in these transgenic mice is dependent upon polyamine biosynthesis, especially putrescine, since treatment with the specific inhibitor of ODC activity, α-difluoromethylornithine (DFMO) blocks the formation of skin tumors and causes the rapid regression of existing tumors (Smith et al., 1998). Furthermore, overexpression of AZ in the skin of transgenic mice leads to decreased ODC activity following tumor promoter treatment and also suppresses tumor growth in the classic DMBA/TPA skin tumorigenesis model (Feith et al., 2001) and in the UV carcinogenesis model using the Ptch+/- heterozygous mouse model (Tang et al., 2004). Both DFMO and AZ inhibition of skin tumor development primarily inhibit the accumulation of putrescine, whose levels correlate very closely with tumor development in the skin (Peralta Soler et al., 1998). Even modest reductions in ODC expression reduce skin tumor susceptibility as demonstrated by the reduced skin tumor yield in Odc+/- haploinsufficient mice subjected to a two-stage initiation-promotion protocol (Guo et al., 2005).
The association between increased levels of putrescine and skin tumorigenesis is also seen in transgenic mice in which SSAT is targeted to the skin with a keratin 6 promoter (K6/SSAT) (Coleman et al., 2002). Although increased expression of a key regulatory enzyme in the catabolism of polyamines would be expected to suppress skin tumorigenesis, expression of SSAT in K6/SSAT transgenic mice increases the incidence of skin papillomas and their progression to carcinomas in response to a two-stage carcinogenesis protocol (Coleman et al., 2002). On the other hand, transgenic mice that express SSAT under the control of its own promoter (line UKU 165b) or a metallothionein I promoter (line UKU 181) have been reported to be resistant to the development of papillomas during two-stage skin carcinogenesis (Pietila et al., 2001). UKU 165b transgenic mice overaccumulate putrescine in their skin, lose their hair by 3 weeks of age, and develop large dermal cysts as do transgenic mice in which ODC is constitutively targeted to the skin. In contrast, K6/SSAT transgenic mice have a normal skin phenotype and normal hair cycle since the keratin 6-driven SSAT expression is only increased in skin tumors (Coleman et al., 2002). It is likely that the different skin tumor responses of the UKU 165b and the K6/SSAT transgenic mice to a two-stage carcinogenesis protocol is due to the use of different transgene promoters which have dissimilar regulation in different subpopulations of the skin. Moreover, unlike the normal skin phenotype of K6/SSAT transgenic mice, UKU 165b transgenic mice exhibit dramatic changes in their skin morphology which may alter their responses to carcinogens and tumor promoters. Indeed, ODC activity and spermidine levels are higher in nontransgenic littermates following tumor promoter treatment and in subsequent skin tumors compared to that seen in UKU 165b transgenic mice (Pietila et al., 2001). It has been hypothesized that the changes in tumor development may arise from a greatly accelerated polyamine metabolic flux which is driven by decreased spermidine and spermine pools that in turn trigger a sustained increase in polyamine biosynthetic activity and a sustained release of reactive by-products (Tucker et al., 2005; Janne et al., 2006). Further studies are obviously needed to better understand the role of SSAT in skin carcinogenesis.
Accumulating reports in the literature suggest a strong link between oncogene activation and increased polyamine biosynthesis in skin tumorigenesis. ODC is induced by activation of Ras and its downstream effector pathways Raf/MEK/ERK and PI 3-kinase in NIH 3T3 cells (Shantz, 2004) and in skin tumors that spontaneously develop in transgenic mice that express an activated MEK protein in the basal layer of the epidermis (Feith et al., 2005). Moreover, skin tumorigenesis in response to Ras activation requires increased polyamine biosynthesis since DFMO and AZ expression can block the spontaneous development of skin papillomas in transgenic mice that express an activated MEK mutant in the basal layer of the epidermis (Feith et al., 2005; Feith et al., 2006). However, activation of Raf or MEK in normal transgenic mouse skin or in primary cultures of keratinocytes does not increase ODC activity and is not sufficient to convert normal keratinocytes to an invasive phenotype (Feith et al., 2005; Hayes et al., 2006). Indeed, conversion of normal keratinocytes to invasive, malignant cells minimally requires activation of the Raf/MEK/ERK signaling pathway and a threshold level of increased ODC activity (Smith et al., 1997; Hayes et al., 2006). Another requirement may involve a selective susceptibility of a targeted subpopulation of keratinocytes or stem cells within the skin. In order to promote invasiveness in keratinocytes, increased ODC activity may cooperate with Raf/ERK signaling by activation of the Akt/mTOR and/or Rho/Rac signaling pathways (Hayes et al., 2006).
Effects of Polyamines in Skin
High levels of polyamines are usually correlated with rapid proliferation, and induction of ODC enzyme activity is one of the classic characteristics of tumor promoter activity in the skin (Slaga et al., 1980). Studies using transgenic mice have shown that proliferation and differentiation of keratinocytes are regulated by changes in their cellular polyamine content. The epithelial cells lining the follicular cysts of K6/ODC transgenic mice express high levels of ODC and demonstrate a high proliferative index (Gilmour et al., 1999). Since it is not known how the abnormal skin phenotype of the K6/ODC transgenic mouse may contribute to changes in proliferation and tumorigenesis, a transgenic mouse model with a normal skin phenotype in which an inducible form of ODC is targeted to suprabasal epidermal cells by an involucrin promoter was generated (Lan et al., 2005). De novo induction of suprabasal epidermal ODC activity in ODCER transgenic mice increases both proliferation in the basal layer of the epidermis as well as epidermal differentiation (Lan et al., 2005). Suprabasal cells in intact, normal adult skin are no longer proliferating and are committed to terminally differentiate. This proliferation control is disrupted in skin tumorigenesis, and cycling cells that express high levels of ODC can be found also in suprabasal layers of skin tumors (Gilmour et al., 1986; Smith et al., 1998). However, induction of only ODC activity without any oncogene activation does not rescue primary epidermal keratinocyte cultures isolated from ODCER mice from a calcium-triggered DNA synthesis block in cells committed to terminally differentiate (Lan et al., 2005). Altered keratinocyte differentiation and an increased proliferation index has also been reported with SSAT overexpression and increased putrescine levels in UKU 165b transgenic mice (Pietila et al., 2005).
One mechanism by which elevated levels of polyamines affect cell cycling and differentiation status in the skin is through their effects on gene expression (Celano et al., 1989; Wang et al., 1993; Bryans et al., 1996; Veress et al., 2000). Polyamines regulate gene expression by altering DNA structure (Peng and Jackson, 2000) and by modulating the binding of transcription factors to response elements in target genes (Thomas and Thomas, 2003). In addition, polyamines have been shown to affect chromatin remodeling in the skin, in part, via elevated intrinsic histone acetyltransferase (HAT) activity that has a pronounced specificity preference for histone H4 (Hobbs et al., 2002; Hobbs et al., 2003). Both p300/CBP-associated HAT activity and the Tip60 HAT enzyme are elevated following ODC overexpression in transgenic mouse skin (Hobbs et al., 2006). The polyamine-stimulation of these HAT enzymes in the skin may alter the recruitment of a subset of transcription factors to the regulatory regions of genes to influence their expression.
Interestingly, ODC overexpression targeted to the suprabasal layer of nontumor-bearing ODCER transgenic epidermis appears to affect other cell subpopulations in the skin, resulting in increased proliferation in the basal cells of the epidermis and activation of the underlying stromal layer with neovascularization and increased synthesis of extracellular matrix proteins in a manner similar to wound repair in skin (Lan et al., 2005). Increased vascularization of the skin has been reported in K6/ODC transgenic mice as well (Lan et al., 2000). Moreover, there is a positive correlation of DFMO-induced regression of ODC/Ras tumors with decreased vascularization and increased apoptosis of both tumor epithelial and stromal cells (Lan et al., 2000). In this study, there was no effect of DFMO inhibition on the epithelial proliferation index in the regressed ODC/Ras skin tumors (Lan et al., 2000). However, DFMO-induced tumor regression in DMBA-initiated papillomas in K6/ODC transgenic mice is associated with a selective decreased proliferation in tumor epithelial cells and no effect on the proliferation index in normal keratinocytes (Peralta Soler et al., 1998).
It remains to be determined what essential survival factors and/or angiogenic factors are regulated by polyamines that play a key role in the maintenance of these skin tumors. These studies suggest that polyamine-activation of keratinocytes and underlying stromal cells is an early event in the tumor process that creates a more permissive microenvironment for tumor development. An under-explored area of research in this field is the molecular pathways impinged upon by elevated levels of polyamines that are responsible for promoting skin tumorigenesis.
Polyamine-Based Therapy in Skin Cancer
The dysregulation of polyamine metabolism in skin cancer provides a rational target for therapeutic intervention in human patients. The premier drug used to target polyamines in human skin cancer is DFMO since it has been shown to inhibit skin tumor incidence in animals following induction with either chemical carcinogens (Weeks et al., 1982; Takigawa et al., 1983) or UVB irradiation (Fischer et al., 2001). In addition, DFMO causes rapid regression of murine squamous cell carcinomas, with the exception of resistant spindle cell carcinomas (Chen et al., 2004). Currently DFMO has been used in skin cancer chemoprevention trials that focus on the patients with precancerous lesions such as actinic keratoses. Topical DFMO ointment (10% w/w) significantly reduces the numbers of actinic keratoses, spermidine levels, and epidermal p53 expression with no change in the proliferation or apoptotic rate (Alberts et al., 2000; Einspahr et al., 2002). These trials appear promising since topical application of DFMO has shown no adverse side effects (Alberts et al., 2000), and systemic DFMO treatment is associated with reversible ototoxicity only at high doses (Alberts et al., 2000).
O'Brien et al. have reported a positive association between an ODC polymorphism (associated with higher ODC activity) and increased prostate cancer risk in men who smoke or have high risk alleles of the androgen receptor gene (Visvanathan et al., 2004). Although there is no data in humans to suggest that some individuals may be at an elevated risk for nonmelanoma skin cancer based on the presence of ODC polymorphic variants, an area for future studies is whether chemoprevention using DFMO is more efficacious if combined with screening for ODC polymorphic variants that may be associated with greater potential for ODC induction and increased cancer risk (Guo et al., 2000). In addition, future studies using mouse strains with varying tumor responses may identify human genetic loci that modify ODC-dependent enhanced susceptibility to skin tumorigenesis (Megosh et al., 2002; George et al., 2005).
Despite these promising results in clinical trials for cancer prevention, there have been no published reports of polyamine-based chemotherapeutic trials for basal cell carcinomas or squamous cell carcinomas. In part, this may be due to the lack of success of DFMO as a global antitumor agent in other tissue types since ODC inhibition leads to a compensatory increased cellular uptake of polyamines from the circulation which neutralizes the cytostatic effect of DFMO (Meyskens and Gerner, 1999). However, murine squamous cell carcinomas, with the exception of aggressive spindle cell carcinomas, show rapid tumor regression following treatment with high doses of DFMO (Chen et al., 2004). In addition, an attempt to achieve sufficient depletion of polyamines by inhibiting not only polyamine biosynthesis but also polyamine uptake has recently been reported to be significantly more effective in causing regression of murine squamous cell carcinomas compared to DFMO alone (Chen et al., 2006). Use of a polyamine uptake inhibitor in this study also permitted the use of lower doses of DFMO to achieve tumor regression and led to a greater decline in tumor polyamine levels compared to that seen using higher doses of DFMO alone (Chen et al., 2006). Thus, the effects of DFMO in combination chemotherapy for squamous cell carcinomas remain to be more fully investigated, especially in recurrent or inoperable skin cancer.
Skin tumor development involves not only genetic alterations in the epithelium that are well documented in the literature, but also less-well-understood epigenetic contributions from surrounding supportive stromal cells. Recent studies suggest that elevated ODC activity and polyamines stimulate modifier genes acting in critical metabolic pathways that ultimately determine the fate of genetically altered epithelial cells and the actual development of a tumor. However, the molecular pathways modulated by polyamines that play a key role in promoting tumorigenesis are not well characterized and is an understudied area of research. In addition, polyamines themselves are strong biological modifiers that promote skin tumorigenesis by altering the regulation of multiple genes. Since elevated levels of ODC and polyamines are common to skin tumors, it is important to characterize cells that are targeted by polyamines as well as nodal signaling pathways that mediate the tumor promoting effects of polyamines. The identification of mechanisms by which elevated levels of polyamines alter environmental signals to trigger the proliferation of latent epidermal stem cells possessing genetic lesions will lead to the development of better chemopreventive and chemotherapeutic strategies for cutaneous neoplasia.
Acknowledgments
I apologize to my many colleagues in the polyamine field whose work was not cited due to space limitations. I particularly thank Dr. Tom O'Brien for helpful discussions and critical reading of this manuscript. I also thank Loretta Rossino for editorial assistance. The research that was performed in the laboratory of S.K.G. and summarized in this review was supported by NIH grants CA070739 and CA95592.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Gilliam AC, Katiyar SK, O'Brien TG, Mukhtar H. A definitive role of ornithine decarboxylase in photocarcinogenesis. Am J Pathol. 2001;159:885–892. doi: 10.1016/S0002-9440(10)61764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts DS, Dorr RT, Einspahr JG, Aickin M, Saboda K, Xu MJ, Peng YM, Goldman R, Foote JA, Warneke JA, Salasche S, Roe DJ, Bowden GT. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol Biomarkers Prev. 2000;9:1281–1286. [PubMed] [Google Scholar]
- Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci USA. 1993;90:7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yosef T, Yanuka O, Halle D, Benvenisty N. Involvement of Myc targets in c-myc and N-myc induced human tumors. Oncogene. 1998;17:165–171. doi: 10.1038/sj.onc.1201939. [DOI] [PubMed] [Google Scholar]
- Bollag W. Prophylaxis of chemically induced benign and malignant epithelial tumors by vitamin A acid (retinoic acid) Eur J Cancer. 1972;8:689–693. doi: 10.1016/0014-2964(72)90153-3. [DOI] [PubMed] [Google Scholar]
- Bryans M, Harley E, Gilmour SK. Elevated cellular polyamine levels enhance promoter activity in vivo. Biochem Biophys Res Commun. 1996;226:618–625. doi: 10.1006/bbrc.1996.1405. [DOI] [PubMed] [Google Scholar]
- Celano P, Baylin SB, Casero RA. Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Bio Chem. 1989;264:8922–8927. [PubMed] [Google Scholar]
- Chen Y, Hu J, Boorman D, Klein-Szanto A, O'Brien TG. Therapy of murine squamous cell carcinomas with 2-difluoromethylornithine. J Carcinog. 2004;3:10. doi: 10.1186/1477-3163-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Megosh LC, Gilmour SK, Sawicki JA, O'Brien TG. K6/ODC transgenic mice as a sensitive model for carcinogen identification. Toxicol Lett. 2000;116:27–35. doi: 10.1016/s0378-4274(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Weeks RS, Burns MR, Boorman DW, Klein-Szanto A, O'Brien TG. Combination therapy with 2-difluoromethylornithine and a polyamine transport inhibitor against murine squamous cell carcinoma. Int J Cancer. 2006;118:2344–2349. doi: 10.1002/ijc.21621. [DOI] [PubMed] [Google Scholar]
- Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”) J Am Acad Dermatol. 2000;42:11–17. doi: 10.1067/mjd.2000.103344. [DOI] [PubMed] [Google Scholar]
- Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- Coleman CS, Pegg AE, Megosh LC, Guo Y, Sawicki JA, O'Brien TG. Targeted expression of spermidine/spermine N1-acetyltransferase increases susceptibility to chemically induced skin carcinogenesis. Carcinogenesis. 2002;23:359–364. doi: 10.1093/carcin/23.2.359. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35:2003–2009. doi: 10.1016/s0959-8049(99)00283-x. [DOI] [PubMed] [Google Scholar]
- Dlugosz A, Merlino G, Yuspa SH. Progress in cutaneous cancer research. J Investig Dermatol Symp Proc. 2002;7:17–26. doi: 10.1046/j.1523-1747.2002.19631.x. [DOI] [PubMed] [Google Scholar]
- Einspahr JG, Nelson MA, Saboda K, Warneke J, Bowden GT, Alberts DS. Modulation of biologic endpoints by topical difluoromethylornithine (DFMO), in subjects at high-risk for nonmelanoma skin cancer. Clin Cancer Res. 2002;8:149–155. [PubMed] [Google Scholar]
- Feith DJ, Bol DK, Carboni JM, Lynch MJ, Sass-Kuhn S, Shoop PL, Shantz LM. Induction of ornithine decarboxylase activity is a necessary step for mitogen-activated protein kinase kinase-induced skin tumorigenesis. Cancer Res. 2005;65:572–578. [PubMed] [Google Scholar]
- Feith DJ, Origanti S, Shoop PL, Sass-Kuhn S, Shantz LM. Tumor suppressor activity of ODC antizyme in MEK-driven skin tumorigenesis. Carcinogenesis. 2006;27:1090–1098. doi: 10.1093/carcin/bgi343. [DOI] [PubMed] [Google Scholar]
- Feith DJ, Shantz LM, Pegg AE. Targeted antizyme expression in the skin of transgenic mice reduces tumor promoter induction of ornithine decarboxylase and decreases sensitivity to chemical carcinogenesis. Cancer Res. 2001;61:6073–6081. [PubMed] [Google Scholar]
- Fischer SM, Lee M, Lubet RA. Difluoromethylornithine is effective as both a preventive and therapeutic agent against the development of UV carcinogenesis in SKH hairless mice. Carcinogenesis. 2001;22:83–88. doi: 10.1093/carcin/22.1.83. [DOI] [PubMed] [Google Scholar]
- George K, Iacobucci A, Uitto J, O'Brien TG. Identification of an X-linked locus modifying mouse skin tumor susceptibility. Mol Carcinog. 2005;44:212–218. doi: 10.1002/mc.20130. [DOI] [PubMed] [Google Scholar]
- Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- Gilmour SK, Aglow E, O'Brien TG. Heterogeneity of ornithine decarboxylase expression in 12-O-tetradecanoylphorbol-13-acetate-treated mouse skin and in epidermal tumors. Carcinogenesis. 1986;7:943–947. doi: 10.1093/carcin/7.6.943. [DOI] [PubMed] [Google Scholar]
- Gilmour SK, Birchler M, Smith MK, Rayca K, Mostochuk J. Effect of elevated levels of ornithine decarboxylase on cell cycle progression in skin. Cell Growth Differ. 1999;10:739–748. [PubMed] [Google Scholar]
- Gilmour SK, Verma AK, Madara T, O'Brien TG. Regulation of ornithine decarboxylase gene expression in mouse epidermis and epidermal tumors during two-stage tumorigenesis. Cancer Res. 1987;47:1221–1225. [PubMed] [Google Scholar]
- Guo Y, Cleveland JL, O'Brien TG. Haploinsufficiency for odc modifies mouse skin tumor susceptibility. Cancer Res. 2005;65:1146–1149. doi: 10.1158/0008-5472.CAN-04-3244. [DOI] [PubMed] [Google Scholar]
- Guo Y, Harris RB, Rosson D, Boorman D, O'Brien TG. Functional analysis of human ornithine decarboxylase alleles. Cancer Res. 2000;60:6314–6317. [PubMed] [Google Scholar]
- Hayes CS, DeFeo K, Lan L, Paul B, Sell C, Gilmour SK. Elevated levels of ornithine decarboxylase cooperate with Raf/ERK activation to convert normal keratinocytes into invasive malignant cells. Oncogene. 2006;25:1543–1553. doi: 10.1038/sj.onc.1209198. [DOI] [PubMed] [Google Scholar]
- Hietala O, Dzubow L, Dlugosz AA, Pyle JA, Jenney F, Gilmour SK, O'Brien TG. Activation of human squamous cell carcinoma ornithine decarboxylase activity by guanosine triphosphate. Cancer Res. 1988;48:1252–1257. [PubMed] [Google Scholar]
- Hobbs CA, Paul BA, Gilmour SK. Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors. Cancer Res. 2002;62:67–74. [PubMed] [Google Scholar]
- Hobbs CA, Paul BA, Gilmour SK. Elevated levels of polyamines alter chromatin in murine skin and tumors without global changes in nucleosome acetylation. Exp Cell Res. 2003;290:427–436. doi: 10.1016/s0014-4827(03)00352-5. [DOI] [PubMed] [Google Scholar]
- Hobbs CA, Wei G, Defeo K, Paul B, Hayes CS, Gilmour SK. Tip60 Protein Isoforms and Altered Function in Skin and Tumors that Overexpress Ornithine Decarboxylase. Cancer Res. 2006;66:8116–8122. doi: 10.1158/0008-5472.CAN-06-0359. [DOI] [PubMed] [Google Scholar]
- Höltta E, Auvinen M, Andersson LC. Polyamines are essential for cell transformation by pp60v-src delineation of molecular events relevant for the transformed phenotype. J Cell Biol. 1993;122:903–914. doi: 10.1083/jcb.122.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Sakamoto l, Goto N, Kashiwagi K, Honma R, Hirose S. Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys. 1982;219:438–443. doi: 10.1016/0003-9861(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Janne J, Alhonen L, Pietila M, Keinanen TA, Uimari A, Hyvonen MT, Pirinen E, Jarvinen A. Genetic manipulation of polyamine catabolism in rodents. J Biochem (Tokyo) 2006;139:155–160. doi: 10.1093/jb/mvj035. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–1093. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto AJ. Pathology of human and experimental skin tumors. Carcinog Compr Surv. 1989;11:19–53. [PubMed] [Google Scholar]
- Koza RA, Megosh LC, Palmieri M, O'Brien TG. Constitutively elevated levels of ornithine and polyamines in mouse epidermal papillomas. Carcinogenesis. 1991;12:1619–1625. doi: 10.1093/carcin/12.9.1619. [DOI] [PubMed] [Google Scholar]
- Lan L, Hayes CS, Laury-Kleintop L, Gilmour S. Suprabasal induction of ornithine decarboxylase in adult mouse skin is sufficient to activate keratinocytes. J Invest Dermatol. 2005;124:602–614. doi: 10.1111/j.0022-202X.2005.23620.x. [DOI] [PubMed] [Google Scholar]
- Lan L, Trempus C, Gilmour SK. Inhibition of ornithine decarboxylase (ODC) decreases tumor vascularization and reverses spontaneous tumors in ODC/Ras transgenic mice. Cancer Res. 2000;60:5696–5703. [PubMed] [Google Scholar]
- Mangold U. The antizyme family: polyamines and beyond. IUBMB Life. 2005;57:671–676. doi: 10.1080/15216540500307031. [DOI] [PubMed] [Google Scholar]
- Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1:795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- Megosh L, Gilmour SK, Rosson D, Soler AP, Blessing M, Sawicki JA, O'Brien TG. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res. 1995;55:4205–4209. [PubMed] [Google Scholar]
- Megosh LC, Hu J, George K, O'Brien TG. Genetic control of polyamine-dependent susceptibility to skin tumorigenesis. Genomics. 2002;79:505–512. doi: 10.1006/geno.2002.6736. [DOI] [PubMed] [Google Scholar]
- Meyskens FL, Gerner EW. Development of a-difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–951. [PubMed] [Google Scholar]
- O'Brien TG. The induction of ornithine decarboxylase as an early, possibly obligatory event in mouse skin carcinogenesis. Cancer Res. 1976;36:2644–2653. [PubMed] [Google Scholar]
- O'Brien TG, Megosh LC, Gilliard G, Soler AP. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- Pegg AE. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986;234:249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Polyamine metabolism and its importance in neoplastic growth as a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- Pegg AE, Feith DJ, Fong LY, Coleman CS, O'Brien TG, Shantz LM. Transgenic mouse models for studies of the role of polyamines in normal, hypertrophic and neoplastic growth. Biochem Soc Trans. 2003;31:356–360. doi: 10.1042/bst0310356. [DOI] [PubMed] [Google Scholar]
- Pegg AE, Xiong H, Feith DJ, Shantz LM. S-adenosylmethionine decarboxylase: structure, function and regulation by polyamines. Biochem Soc Trans. 1998;26:580–586. doi: 10.1042/bst0260580. [DOI] [PubMed] [Google Scholar]
- Peng HF, Jackson V. In vitro studies on the maintenance of transcription-induced stress by histones and polyamines. J Biol Chem. 2000;275:657–668. doi: 10.1074/jbc.275.1.657. [DOI] [PubMed] [Google Scholar]
- Peralta Soler A, Gilliard G, Megosh L, George K, O'Brien TG. Polyamines regulate expression of the neoplastic phenotype in mouse skin. Cancer Res. 1998;58:1654–1659. [PubMed] [Google Scholar]
- Pietila M, Parkkinen JJ, Alhonen L, Janne J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. J Invest Dermatol. 2001;116:801–805. doi: 10.1046/j.1523-1747.2001.01330.x. [DOI] [PubMed] [Google Scholar]
- Pietila M, Pirinen E, Keskitalo S, Juutinen S, Pasonen-Seppanen S, Keinanen T, Alhonen L, Janne J. Disturbed keratinocyte differentiation in transgenic mice and organotypic keratinocyte cultures as a result of spermidine/spermine N-acetyltransferase overexpression. J Invest Dermatol. 2005;124:596–601. doi: 10.1111/j.0022-202X.2005.23636.x. [DOI] [PubMed] [Google Scholar]
- Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA., Jr Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem. 2005;280:39843–39851. doi: 10.1074/jbc.M508177200. [DOI] [PubMed] [Google Scholar]
- Scalabrino G, Pigatto P, Ferioli ME, Modena D, Puerari M, Caru A. Levels of activity of the polyamine biosynthetic decarboxylases as indicators of degree of malignancy of human cutaneous epitheliomas. J Invest Dermatol. 1980;74:122–124. doi: 10.1111/1523-1747.ep12535012. [DOI] [PubMed] [Google Scholar]
- Shantz L, Pegg AE. Ornithine decarboxylase induction in transformation by H-Ras and RhoA. Cancer Res. 1998;58:2748–2753. [PubMed] [Google Scholar]
- Shantz LM. Transcriptional and translational control of ornithine decarboxylase during Ras transformation. Biochem J. 2004;377:257–264. doi: 10.1042/BJ20030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga TJ, Fischer SM, Weeks CE, Klein-Szanto AJ. Multistage chemical carcinogenesis in mouse skin. Curr Probl Dermatol. 1980;10:193–218. doi: 10.1159/000396290. [DOI] [PubMed] [Google Scholar]
- Smith MK, Goral MA, Wright JH, Matrisian LM, Morris RJ, Klein-Szanto AJP, Gilmour SK. Ornithine decarboxylase overexpression leads to increased epithelial tumor invasiveness. Cancer Res. 1997;57:2104–2108. [PubMed] [Google Scholar]
- Smith MK, Trempus CS, Gilmour SK. Co-operation between follicular ornithine decarboxylase and v-Ha-ras induces spontaneous papillomas and malignant conversion in transgenic skin. Carcinogenesis. 1998;19:1409–1415. doi: 10.1093/carcin/19.8.1409. [DOI] [PubMed] [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Ann Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Takigawa M, Verma AK, Simsiman RC, Boutwell RK. Inhibition of mouse skin tumor promotion and of promoter-stimulated epidermal polyamine biosynthesis by alpha-difluoromethylornithine. Cancer Res. 1983;43:3732–3738. [PubMed] [Google Scholar]
- Tang X, Kim AL, Feith DJ, Pegg AE, Russo J, Zhang H, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Bickers DR, Athar M. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/- mice. J Clin Invest. 2004;113:867–875. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JM, Murphy JT, Kisiel N, Diegelman P, Barbour KW, Davis C, Medda M, Alhonen L, Janne J, Kramer DL, Porter CW, Berger FG. Potent modulation of intestinal tumorigenesis in Apcmin/+ mice by the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase. Cancer Res. 2005;65:5390–5398. doi: 10.1158/0008-5472.CAN-05-0229. [DOI] [PubMed] [Google Scholar]
- Veress I, Haghighi S, OPulkka A, Pajunen A. Changes in gene expression in response to polyamine depletion indicates selective stabilization of mRNAs. Biochem J. 2000;346:185–191. [PMC free article] [PubMed] [Google Scholar]
- Verma AK, Ashendel CL, Boutwell RK. Inhibition by prostaglandin synthesis inhibitors of the induction of epidermal ornithine decarboxylase activity, the accumulation of prostaglandins and tumor promotion caused by 12-0-tetradecanoylphorbol-13-acetate. Cancer Res. 1980;40:308–315. [PubMed] [Google Scholar]
- Visvanathan K, Helzlsouer KJ, Boorman DW, Strickland PT, Hoffman SC, Comstock GW, O'Brien TG, Guo Y. Association among an ornithine decarboxylase polymorphism, androgen receptor gene (CAG) repeat length and prostate cancer risk. J Urol. 2004;171:652–655. doi: 10.1097/01.ju.0000108384.74718.73. [DOI] [PubMed] [Google Scholar]
- Wallace HM, Duthie J, Evans DM, Lamond S, Nicoll KM, Heys SD. Alterations in polyamine catabolic enzymes in human breast cancer tissue. Clin Cancer Res. 2000;6:3657–3661. [PubMed] [Google Scholar]
- Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JYJ, McCormack SA, Viar MJ, Wang H, Tzen CY, Scott RE, Johnson LR. Decreased expresion of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am J Physiol. 1993;265:G331–G338. doi: 10.1152/ajpgi.1993.265.2.G331. [DOI] [PubMed] [Google Scholar]
- Weeks CE, Hermann AL, Nelson FR, Slaga TS. Alpha-Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits tumor promoter-induced polyamine accumulation and carcinogenesis in mouse skin. Proc Natl Acad Sci USA. 1982;79:6028–6032. doi: 10.1073/pnas.79.19.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Gillies RJ, Gerner EW. Characterization of a diamine exporter in Chinese hamster ovary cells and identification of specific polyamine substrates. J Biol Chem. 1997;272:20484–20489. doi: 10.1074/jbc.272.33.20484. [DOI] [PubMed] [Google Scholar]
- Yuspa SH. Cellular and molecular mechanisms of carcinogenesis in lining epithelia. Symp Fundam Cancer Res. 1986;39:3–15. [PubMed] [Google Scholar]