Abstract
The present study was designed to examine whether life-long exposure to standard or enriched housing affects the ability of estrogen to improve spatial and object memory throughout the lifespan. Three week-old female mice were maintained in standard or enriched housing up to and through ovariectomy and behavioral testing at 5, 17, or 22 months of age. Spatial memory was tested in the Morris water maze and object memory was tested using an object recognition task. Immediately after training each day, mice were injected intraperitoneally with vehicle or 0.2 mg/kg 17β-estradiol. Among young females, object recognition was enhanced by estradiol alone, an effect that was reduced by enrichment. In contrast, spatial water maze performance was impaired by estradiol alone, but improved by the combination of both estradiol and enrichment. At middle-age, object recognition was enhanced by estradiol or enrichment alone, and the combination of both treatments. Spatial memory in the water maze was also improved by both treatments at middle-age, but the beneficial effects of estradiol were limited to standard-housed females. Finally, whereas enrichment in aged females significantly enhanced performance in both tasks, estradiol had no effect at this age in either task. In total, the data indicate that life-long enrichment can significantly alter the extent to which estradiol affects memory in mice throughout the lifespan. Importantly, the interaction between these treatments is highly dependent on age and type of memory tested.
Keywords: Spatial memory, object memory, aging, estradiol, Morris water maze
1. Introduction
The question of whether estrogen treatment can reduce age-related memory decline in females has been the subject of much recent debate. Estrogen is thought to be beneficial for memory because it enhances the structure and function of mnemonic brain regions such as the hippocampus that deteriorate in normal aging and Alzheimer's disease (Foy, Xu, Xie, Brinton, Thompson & Berger, 1999; Warren, Humphreys, Juraska & Greenough, 1995; Woolley & McEwen, 1992, 1993), and because hippocampal-dependent memory (e.g., spatial memory, object recognition) is typically improved by exogenous estrogen administration in young ovariectomized female rats (Bimonte & Denenberg, 1999; Daniel, Fader, Spencer & Dohanich, 1997; Gibbs, 1999; O'Neal, Means, Poole & Hamm, 1996; Sandstrom & Williams, 2001) and mice (Gresack & Frick, 2004, 2006b; Heikkinen, Puoliväli, Liu, Rissanen & Tanila, 2002; Rissanen, Puoliväli, van Groen & Riekkinen, 1999). Estrogen is also a trophic factor for the adult hippocampus (Brinton, 2001), and therefore, estrogen deficiency during aging may render the hippocampus more vulnerable to deterioration and exacerbate emerging age-related memory deficits. Indeed, the loss of estrous cycling in middle-age has been associated with the onset of spatial memory decline studies in female rats (Markowska, 1999) and mice (Frick, Burlingame, Arters & Berger-Sweeney, 2000). Accordingly, chronic estrogen treatment administered prior to training can improve various types of learning and memory in middle-aged (Daniel, Hulst & Berbling, 2006; Fernandez & Frick, 2004; Foster, Sharrow, Kumar & Masse, 2003; Markham, Pych & Juraska, 2002) and aged (Frick, Fernandez & Bulinski, 2002; Gibbs, 2000; Heikkinen et al., 2002; Markowska & Savonenko, 2002; Vaucher, Reymond, Najaffe, Kar, Quirion, Miller & Franklin, 2002) female rodents.
Although some studies in menopausal women indicate that estrogen treatment reduces verbal (Maki, Zonderman & Resnick, 2001; Sherwin, 1999), object (Duka, Tasker & McGowan, 2000), and spatial memory decline (Duff & Hampson, 2000; Duka et al., 2000), recent clinical data indicate that estrogen therapy, either alone or in combination with progestin, increases a woman's risk of cognitive decline and dementia (Shumaker, Legault, Kuller, Rapp, Thal, Lane, Fillit, Stefanick, Hendrix, Lewis, Masaki & Coker, 2004; Shumaker, Legault, Thal, Wallace, Ockene, Hendrix, Jones, Assaf, Jackson, Kotchen, Wassertheil-Smoller & Wactawski-Wende, 2003). These findings may be due to several factors, including the possibility that women may have been too old or well educated to benefit from treatment (Maki, 2006). Women who initiate hormone therapy are generally more educated (Keating, Cleary, Rossi, Zaslavsky & Ayanian, 1999) and healthier (Matthews, Kuller, Wing, Meilahn & Plantinga, 1996) than women who do not elect treatment. This selection bias may skew perceptions about the effectiveness of hormone therapy because women who are well educated (> 11 yrs) are less likely to develop dementia than poorly educated (< 8 yrs) women (Launer, Andersen, Dewey, Letenneur, Ott, Amaducci, Brayne, Copeland, Dartigues, Kragh-Sorensen, Lobo, Martinez-Lage, Stijnen & Hofman, 1999). Interestingly, some evidence suggests that estrogen may more effectively improve cognition in women with less education than in those with greater education (Matthews, Cauley, Yaffe & Zmuda, 1999). This situation could result if differences in baseline neural and cognitive function produce discrepant treatment windows for estrogen, allowing more room for improvement in women with less education. Alternatively, rich cognitive experience may alter neural function such that memory is less amenable to modulation by ovarian hormones.
Because the influence of environmental factors on the mnemonic response to estrogen is difficult to assess in humans, the environmental enrichment paradigm in rodents may provide an effective model in which to address this issue. Environmental enrichment involves housing rodents together in cages with cognitively and physically stimulating objects. Controls are group (social) or singly (isolated) housed and not exposed to enriching objects. Recent work has shown that enrichment improves hippocampal-dependent memory and enhances hippocampal morphology and plasticity in young-adult (Davis, Jones & Derrick, 2004; Duffy, Craddock & Nguyen, 2001; Kempermann, Kuhn & Gage, 1997; Rampon, Tang, Goodhouse, Shimizu, Kyin & Tsien, 2000), middle-aged (Frick, Stearns, Pan & Berger-Sweeney, 2003; Kempermann, Kuhn & Gage, 1998), and aged (Frick et al., 2002; Nakamura, Kobayashi, Ohashi & Ando, 1999; Soffié, Hahn, Terao & Eclancher, 1999; Winocur, 1998) rats and mice.
Despite the fact that enrichment has robust effects on hippocampal-dependent memory throughout the lifespan, no study has examined if the environment influences the mnemonic response of aging females to exogenous administration of hormones such as estrogen. One previous study showed that spatial memory in the Morris water maze was impaired in intact mice relative to ovariectomized mice, and that this impairment was eliminated by exposure to environmental enrichment (Daniel, Roberts & Dohanich, 1999), suggesting an interaction between enrichment and endogenous ovarian hormone levels. Our laboratory subsequently showed that exposing female mice to an enriched environment from weaning to adulthood influences their mnemonic response to exogenous administration of the potent estrogen, 17β-estradiol (Gresack & Frick, 2004). Mice were exposed for 3 hrs/day to enriched or control environments from 3 weeks to 7 months of age, at which point they were given intraperitoneal injections of 0.2 mg/kg 17β-estradiol immediately after each training session in spatial radial arm maze and object recognition tasks. Estradiol enhanced spatial working memory and object recognition only in mice raised in standard environments but had no effect on those raised in enriched environments (Gresack & Frick, 2004), suggesting that enrichment may prevent estradiol from improving memory. Although these findings are consistent with suggestions from the clinical literature (Matthews et al., 1999), the extent to which they can be applied to older females is limited because this study did not include middle-aged and aged mice.
As such, the present study examined whether life-long exposure to an enriched environment influences the extent to which estradiol can improve hippocampal-dependent spatial and object memory in young, middle-aged, and aged female mice. Spatial memory was tested using a 5-day spatial Morris water maze task that we have previously employed successfully with aging rodents (Frick, Baxter, Markowska, Olton & Price, 1995; Frick et al., 2000; Frick et al., 2002). A 2-day object recognition task with 24- and 48-hr delays was used to test object memory. Both tasks critically involve the hippocampus (Baker & Kim, 2002; Clark, Zola & Squire, 2000; Morris, Garrud, Rawlins & O'Keefe, 1982), and afford the advantages of being learned by aging mice within a few days and of avoiding food or water restriction, which can endanger the health of older animals. Selecting an estradiol treatment for use with aging rodents can pose a challenge, given that the timing, dose, and duration of administration may significantly influence whether estradiol affects memory (Frick et al., 2002; Gibbs, 2000; Gresack & Frick, 2006a; Markowska & Savonenko, 2002; Sandstrom & Williams, 2001). The present study utilized the same treatment as our previous study (Gresack & Frick, 2004), in which we administered a water-soluble form of 17β-estradiol immediately after training (post-training) each day and tested memory 24- or 48-hrs later, in order to provide a direct comparison with our previous work. Because water-soluble estradiol is metabolized within 24 hrs (Pitha, Harman & Michel, 1986; Taylor, Weiss & Pitha, 1989b), memory was tested in the absence of estradiol in the circulation. As such, the post-training injections were not meant to mimic hormone replacement in humans, but to pinpoint effects of estradiol on memory consolidation. Because the chronic and acute pre-training non-water soluble estradiol treatments used in most previous studies can affect non-mnemonic aspects of task performance (e.g., arousal, motivation, or sensorimotor function), administering estradiol post-training differs from pre-training treatments in that post-training treatment allows specific effects on memory consolidation to be observed in the absence of these non-mnemonic confounds. However, it should be noted that the neural mechanisms underlying post-training and pre-training may differ. Because post-training treatments with water-soluble estradiol influence memory rapidly (Packard, 1998), they may act primarily via non-genomic mechanisms whereas pre-training treatments likely act primarily via genomic mechanisms (McEwen, 2001). With regard to enrichment, unlike our previous study in which mice were exposed to enriched environments for only 3 hrs/day (Gresack & Frick, 2004), mice in this experiment were exposed to enrichment for 24 hrs/day in their home cages because this treatment produces much greater improvements in the spatial water maze among aged male mice than exposure for 3 hrs/day (Bennett, McRae, Levy & Frick, 2006).
Of particular interest were interactions between estradiol and enrichment in middle-aged and aged females. If enrichment enhances neural function to the extent that no further improvement by estradiol is possible, then estradiol treatment should improve memory in standard-housed, but not enriched, females. We hypothesized this to be the case in young and middle-aged females based on previous studies (Gresack & Frick, 2004; Matthews et al., 1999). However, if effects of aging on the brain in aged females leave considerable room for improvement after enrichment or provide multiple routes for improvement, then the combination of both treatments may improve memory in aged females more than either alone. Also, if the age at the start of treatment affects the response to estradiol such that this hormone is most effective soon after hormone loss (i.e., at middle-age), then we hypothesized that estradiol treatment would improve memory in middle-aged standard-housed females more than in aged standard-housed females.
2. Methods
2.1. Subjects
Female C57BL/6 mice were obtained from Taconic (Germantown, NY). All mice arrived at 3 weeks of age (n = 302) and were randomly assigned to the standard or enriched housing conditions. They were handled for 5 min/day at least five times after arrival to habituate them to being picked up by the experimenters. The mice were housed in a room with a 12:12 light/dark cycle (lights on at 07:00), with all testing performed during the light phase. Ad libitum access to food and water was provided.
Upon arrival, mice were randomly assigned to groups to be behaviorally tested at one of three ages: young (5 months, n = 46), middle-aged (17 months, n = 128), or aged (22 months, n = 128). Environmental enrichment was continued up to and through behavioral testing, such that the young group was enriched for approximately 5 months, the middle-aged group approximately 17 months, and the aged group approximately 22 months. All mice of one age were tested at the same time; that is, all young mice were tested approximately one year before middle-aged mice, who were all tested approximately 5 months before aged mice. Approximately two weeks prior to behavioral testing, mice were ovariectomized to remove the primary endogenous source of estrogen and progesterone. After ovariectomy, mice were randomly assigned to vehicle and estradiol groups and treated as described below. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University, and conformed to the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Housing conditions
As described above, mice were assigned to the standard or enriched housing conditions upon arrival in the lab. Standard-housed mice were housed up to 5 per shoebox cage and had no exposure to enriching objects. Enriched mice were housed as described previously (Bennett et al., 2006) in groups of up to 11 in large transparent plastic bins (Ancare, Bellmore, NY; 66 cm long × 46 cm wide × 38 cm high) fitted with a transparent snap-on lid. The lid was modified for ventilation by cutting 2 rectangular holes (30 cm long × 18.5 cm wide) 6 cm apart into the lid and fitting them with wire feeding racks covered by ventilation lids from standard shoebox cages. Water was provided by drilling a small hole (10 mm × 25 mm) 7 cm above the base of the bins on one end and inserting the spout from an exteriorly mounted stainless steel water bottle holder (Ancare, Bellmore, NY). Food pellets were placed in a stainless steel food bowl on the floor of the bins. The bins were placed on a stainless steel cage rack in the same colony room as the standard shoebox cages. Bins were changed twice per week (a clean bin, bedding, and objects were provided at each change) and contained a large assortment of enrichment objects which always included 2 running wheels, 2 rodent dwellings, a large plastic tube configuration with vertical climbing aspects (watchtowers, spiral loops, bridges, etc.), and 4 or 5 other objects. New objects were presented in new configurations each time the bins were changed.
2.3. Ovariectomy
Surgeries were conducted as described previously (Fernandez & Frick, 2004; Gresack & Frick, 2004). Briefly, mice were anesthetized using 2% isoflourane gas in 100% oxygen. Two incisions were made on the dorsal surface of the body, just above the pelvic bones. The ovary, oviduct, and tip of the uterine horn were isolated, clamped off, and removed. The rest of the uterine horn was returned to the abdominal cavity, the muscle wall was sutured, and the skin closed with wound clips. Mice were housed singly for one week, during which time analgesia was provided by 300 mg/kg children's acetaminophen in the drinking water for one week post-surgery. During recovery, enriched mice were provided with 1−2 small toys in their cages. After recovery, mice were re-housed with their original cage-mates and enrichment treatment resumed as before.
2.4. Hormone treatment
After ovariectomy, mice were randomly assigned to receive intraperitoneal (i.p.) injections of vehicle or 17β-estradiol (E2; 0.2 mg/kg). Cyclodextrin-encapsulated 17β-estradiol (Sigma Chemical Co., St. Louis, MO) was dissolved in physiological saline in a volume of 4 ml/kg. The vehicle, 2-hydroxypropyl-β-cyclodextrin, contained the same amount of cyclodextrin as the cyclodextrin-encapsulated E2 and was dissolved in an equivalent volume of saline. Cyclodextrin is a solubility-enhancing carrier for the estradiol which allows this hormone to be metabolized within 24 hrs (Pitha & Pitha, 1985; Taylor, Weiss & Pitha, 1989a). As such, this formulation allowed for estradiol injections to be given each day of testing without gradual buildup of the hormone in circulation.
Injections were not given prior to behavioral testing, and were only administered after the completion of testing each day. The combination of this post-training administration and the rapid metabolism of cyclodextrin-encapsulated E2 allowed for memory to be tested each day in the absence of exogenous estradiol in circulation, thereby eliminating hormone effects on factors that can affect performance independent of memory such as motivation, attention, or motor ability. We have shown that immediate post-training i.p. injection of the 0.2 mg/kg dose of cyclodextrin-encapsulated E2 improves spatial memory in the water maze in young (Gresack & Frick, 2006b) and aged (Harburger, Bennett & Frick, 2007) female mice, and both object recognition and spatial working memory in young female mice (Gresack & Frick, 2004). We have previously reported that this dose results in estradiol level increases in the physiological range (approximately 80 pg/ml in aged females relative to vehicle control; Gresack & Frick, 2006a). Previous studies have shown that estradiol injection 2 hrs post-training has no beneficial effect on spatial (Packard & Teather, 1997) or object memory (Luine, Jacome & MacLusky, 2003) consolidation in young females, suggesting that estradiol acts within the first two hours after injection to modulate memory consolidation.
2.5. Behavioral testing
2.5.1. Experimental design
Approximately two weeks after ovariectomy, mice were tested in the object recognition task using a 24-hr delay. Five days of spatial Morris water maze testing began approximately one week after object recognition, followed two days later by three days of cued Morris water maze testing. Object recognition testing using a 48-hr delay began approximately one week after cued testing. Hormone injections were given each day at the completion of testing as described below.
2.5.2. Object recognition
The object recognition task, a test of non-spatial hippocampal-dependent memory, was conducted in a wooden open field box (58 × 58 × 46 cm high) painted white and located in a quiet room under dim lighting. A video camera was mounted on the ceiling above the box and connected to a video recorder, monitor, and computer in an adjoining room. Throughout testing, the door to the testing room was closed and mice were observed on the monitor.
Testing was conducted as described previously (Frick & Gresack, 2003; Gresack & Frick, 2004). This task takes advantage of the natural affinity of mice for novelty. Briefly, three phases (habituation, sample, and choice) were conducted on separate test days. During habituation, each mouse was placed in the empty open field box and allowed to explore for 5 min. During this time, locomotor activity was assessed by recording the number of crossings of a 5 × 3 grid laid over the field on the computer monitor. No injections were administered after habituation. The next day, all mice completed the sample phase. Following a 1-min re-habituation to the box, two identical objects were placed in the northwest and northeast corners of the box (approximately 5 cm from the walls) and the mice explored the objects until they accumulated 30 sec of exploration. Each mouse was then removed from the box, immediately injected with vehicle or E2, and returned to its home cage. Mice were tested in the choice phase 24 or 48 hrs after injection as described in the Experimental Design section above. Young female mice typically remember the familiar object in the choice phase after 24 hrs, but not after 48 hrs (Gresack & Frick, 2004). Thus, the 24-hr delay allowed for impairing effects of E2 and enrichment on object recognition to be observed, whereas the 48-hr delay allowed for the observation of enhancing effects of these treatments on object recognition. During the choice phase, one familiar object (identical to that which was used in the sample phase) and one novel object were placed in the same corners of the box occupied during the sample phase. The location of the novel object was counterbalanced across mice in each group. Mice remained in the box until they accumulated 30 sec of object exploration. If a mouse did not accumulate 30 sec of exploration within 20 min during either the sample or choice phases, then testing was discontinued and the mouse was excluded from the data analyses. During all phases of testing, the box and objects were cleaned with 70% ethanol between mice.
Time spent exploring each object was recorded using a video tracking system and a custom-written computer program. Object exploration was scored only when the mouse's nose or front paws touched the object. Intact memory for the familiar object was demonstrated if the mouse exhibited a “preference” for the novel object in the choice phase. A preference was indicated if the mouse spent significantly more time than chance (15 sec) with the novel object.
2.5.3. Morris water maze
A white circular tank (97 cm in diameter) was filled with water (24 ± 2 °C) and surrounded by a variety of extramaze cues including a countertop and sink, colorful 2 and 3 dimensional objects attached to the walls, and abstract black and white designs (approximately 30 × 30 cm) located around the tank approximately 40 cm from the edge of the tank. The tank was located in a rectangular room measuring approximately 242 cm wide and 340 cm long. It was divided into four quadrants, and four start positions (north, south, east, and west) were located at the intersections of the quadrants. Data were recorded using an HVS 2020 automated tracking system (HVS Image, Hampton, England). Prior to water maze testing, all mice were habituated to the water using a four-trial shaping procedure in which a smaller ring (55 cm) was inserted inside of the larger 97 cm ring to decrease the total swimming area. This procedure habituated the mice to the water and taught them to escape from the water by climbing onto a platform. Each mouse was first placed on a visible red lucite platform (10 × 10 cm) for 10 sec, and then placed at three progressively further distances from the platform where it was allowed 30 sec to escape onto the platform. No data were collected during this procedure and no compounds were administered after the procedure.
Spatial water maze
Spatial water maze testing began two days after shaping. This task, designed to test spatial reference memory, was conducted as described previously (Bennett et al., 2006; Frick et al., 2002). A transparent lucite platform (10 × 10 cm) was submerged just underneath the surface of the water in the southeast quadrant of the tank, where it remained for the duration of the five spatial test sessions. The sequence of the four start positions in the tank (north, west, south, and east) varied each trial for each mouse. Six trials/mouse were conducted for five consecutive days (1 session/day). During the first five trials (platform trials), the platform was raised and available to the mouse for escape. Each mouse was given 120 sec to locate the platform, after which time the experimenter placed the mouse on the platform and allowed it to remain there for 10−15 sec. Each mouse was dried with a cloth towel upon removal from the platform and placed in its home cage for an intertrial interval of approximately 20 min. Swim time (sec), swim distance (m) and swim speed (m/sec) were recorded during these trials. Lower numbers indicate better performance for swim time and swim distance, as well as slower swim speeds.
The sixth trial was a variable-interval probe trial (Markowska, Long, Johnson & Olton, 1993) in which the platform was collapsed, remaining submerged and unavailable for escape for 20, 30, or 40 sec. At the end of this time, the platform was raised and made available for escape for the duration of the 60 sec total trial time. While the platform was lowered and unavailable for escape, quadrant time (the percent of time spent in each quadrant of the tank) and platform crossings were recorded. For platform crossings, the number of times the mouse crossed the submerged platform location/10 sec was used as the dependent variable in order to account for the varying duration of the intervals. Thus, the numbers of crossings were divided by 2, 3, or 4 for the 20, 30, and 40 sec intervals, respectively. For both quadrant time and platform crossings, higher numbers indicate better performance.
Immediately following the probe trial, each mouse was removed from the platform, dried with a cloth towel, and given an i.p. injection of vehicle or E2. They were then returned to their home cages where they remained until testing the next day.
Cued water maze
Cued water maze testing began two days after the conclusion of spatial testing. The cued task was utilized primarily to control for non-mnemonic factors (e.g., swimming ability, vision, motivation) that may influence task performance. This task was conducted as described previously (Bennett et al., 2006; Frick et al., 2002). Briefly, the platform was made visible by raising it just above the surface of the water, covering it with red tape, and attaching the circular top of a plastic container (8 cm in diameter, 0.5 cm wide) to the platform oriented perpendicular to the surface of the platform and water. Three test sessions were conducted on consecutive days, with each session consisting of 6 trials in which the start position (north, south, east, and west) and quadrant containing the platform varied for each trial. Mice were given 120 sec to locate the escape platform and the inter-trial interval was approximately 20 min. Swim time, swim distance, and swim speed were recorded.
Immediately following the sixth trial of each day, each mouse was removed from the platform, dried with a cloth towel, and given an i.p. injection of vehicle or E2. They were then returned to their home cages where they remained until testing the next day.
2.6. Uterine weights
After the completion of 48-hr object recognition testing, mice were injected with vehicle or E2, and 1 hr later were briefly euthanized with CO2 and cervically dislocated. Uteri were removed and weighed (g) as a bioassay of the physiological effectiveness of estradiol treatment. Although 1 hr is a rapid time frame in which to observe changes in uterine weights, this time point was selected because it was within the 2 hr window in which post-training estradiol affects spatial memory (Packard & Teather, 1997). We have previously shown that the 0.2 mg/kg dose of E2 significantly increases uterine weights in aged female mice within 2 hrs after injection (Harburger et al., 2007). In the present study, however, effects on uterine weights could have been the results of either the acute injection prior to tissue collection or of multiple injections given throughout the experiment.
2.7. Data analysis
Because each age group was tested separately, analyses were conducted within each age rather than between ages. Thus, for each age, four groups are included in each analysis: Standard-Vehicle, Standard-E2, Enriched-Vehicle, and Enriched-E2. Analyses for each task were conducted as described below.
For the object recognition task, a preference for one object over another in the choice phase was assessed using one-sample t-tests to determine whether the time spent with the novel object differed significantly from the chance value of 15 sec (Frick & Gresack, 2003; Gresack & Frick, 2004). This type of t-test was used because the times spent with each object are not independent; the total time exploring must equal 30 sec, so time spent with one object reduces time spent with the other. T-tests were conducted for each group and each delay separately. For grid crossings, a two-way analysis of variance (ANOVA) was conducted with Environment and Estrogen treatment as the independent variables (SuperANOVA, Abacus Concepts, Berkeley, CA). Spatial and cued water maze measures were averaged within a group for each session and analyzed using two-way repeated-measures ANOVAs with Environment and Estrogen treatment as the between-subject variables and Session as the within-subjects variable. One-way ANOVAs without repeated measures were performed on uterine weights with Environment and Estrogen as the independent variables.
3. Results
3.1. Subjects
Of the mice that arrived at 3 weeks of age, the following numbers survived up until behavioral testing: young (n = 41), middle-aged (n = 79), and aged (n = 53). Those that did not survive died of natural causes or were euthanized due to conditions such as ulcerative dermatitis and tumors. The mice ultimately included in the data analyses were in good health throughout enrichment, surgery, and behavioral testing. No young mice were excluded from any of the data analyses. Among middle-aged mice, one Standard-Vehicle mouse was excluded from all object recognition analyses because she took longer than 20 min to accumulate 30 sec of exploration at both delays. Other middle-aged mice were excluded from the analysis at one of the two delays for the same reason as follows: 2, 3, and 4 mice were excluded from the analyses of the 24-hr delay in the Standard-Vehicle, Standard-E2, and Enriched-Vehicle groups, respectively, whereas one mouse was excluded from each of the Standard-Vehicle, Standard-E2, and Enriched-E2 groups in the analyses of the 48-hr delay. Two middle-aged females died prior to uterine dissections. Among aged females, one mouse was excluded from each of the Enriched-Vehicle and Enriched-E2 groups in the analysis of the 24-hr delay for taking longer than 20 min to accumulate 30 sec of exploration time. Five aged females died prior to uterine dissections.
3.2. Uterine weights
Estradiol significantly increased uterine weights in females of all ages (Young: F(1,37) = 48.57, p < 0.0001; Middle-aged: F(1,73) = 116.63, p < 0.0001; Aged: F(1,44) = 66.45, p < 0.0001). For young females, mean ± SEM weights were 0.026 ± 0.004 g for vehicle-treated females and 0.064 ± 0.003 g for E2-treated females. For middle-aged females, mean ± SEM weights were 0.031 ± 0.003 g in vehicle-treated females and 0.087 ± 0.004 g in E2-treated females. For aged females, mean ± SEM weights were 0.032 ± 0.004 g in vehicle-treated females and 0.095 ± 0.006 g in E2-treated females. The main effects of Environment and the Estrogen × Environment interactions were not significant for any age for uterine weight.
3.3. Object recognition
3.3.1. Young females
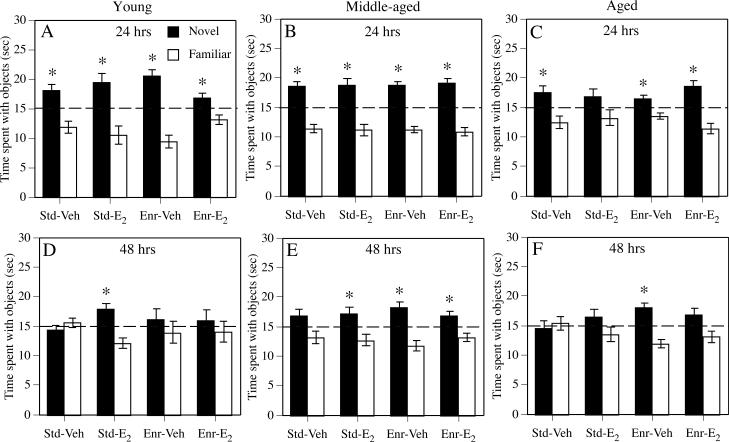
Locomotor activity in the open field did not differ among the groups, as indicated by non-significant main effects of Estrogen treatment and Environment, and a non-significant Estrogen × Environment interaction for the grid crossings measure (Table 1). During the choice phase at the 24-hr delay (Fig. 1A), all groups spent significantly more time with the novel object than chance (Standard-Vehicle: t(9) = 3.05, p < 0.02; Standard-E2: t(8) = 2.92, p < 0.02; Enriched-Vehicle: t(10) = 5.07, p < 0.0001; Enriched-E2: t(10) = 2.33, p < 0.05), suggesting that all groups remembered the familiar object after 24 hrs well enough to show a preference for the novel object. The fact that all groups including the Standard-Vehicle group exhibited a preference for the novel object at the 24-hr delay indicates no effect of either treatment at this delay in young females. During the choice phase for the 48-hr delay (Fig. 1D), only the Standard-E2 group showed a preference for the novel object (t(8) = 3.13, p < 0.02). The fact that the Standard-E2 group, but not the Standard-Vehicle group, preferred the novel object indicates a beneficial effect of estradiol in standard-housed females. The lack of a significant preference by either enriched group suggests that enrichment alone did not facilitate object recognition relative to chance in young females and that enrichment reduced the beneficial effect of estradiol on object recognition at the 48-hr delay.
Table 1.
Grid crossings during object recognition habituation
| Group | Young | Age Middle-aged | Aged |
|---|---|---|---|
| Standard-Vehicle | 177.5 ± 15.1 | 165.0 ± 16.1 | 159.0 ± 17.4 |
| Standard-E2 | 155.4 ± 13.4 | 166.2 ± 9.8 | 159.9 ± 14.1 |
| Enriched-Vehicle | 184.5 ± 8.3 | 142.1 ± 7.2 | 173.0 ± 11.9 |
| Enriched-E2 | 164.1 ± 10.8 | 155.4 ± 8.7 | 178.3 ± 9.4 |
Values are the mean ± the standard error of the mean (SEM).
Fig. 1.
Time spent with the objects during the choice phase of the object recognition task conducted 24 hrs (A-C) or 48 hrs (D-F) after the sample phase. Chance performance is indicated by the dotted line at 15 sec. *p < 0.05 relative to chance. Each bar represents the mean ± standard error of the mean (SEM) of each group for a single phase of testing. Std-Veh = Standard-Vehicle, Std-E2 = Standard-E2, Enr-Veh = Enriched-Vehicle; Enr-E2 = Enriched-E2.
3.3.2. Middle-aged females
Grid crossings during habituation were not affected by either treatment, as indicated by non-significant main effects of Estrogen treatment and Environment, and a non-significant Estrogen × Environment interaction (Table 1). All groups showed a significant preference for the novel object at the 24-hr delay (Fig. 1B; Standard-Vehicle: t(12) = 5.0, p < 0.0001; Standard-E2: t(12) = 4.16, p < 0.001; Enriched-Vehicle: t(18) = 7.08, p < 0.0001; Enriched-E2: t(23) = 5.44, p < 0.0001), suggesting that neither estradiol nor enrichment influenced performance at this delay. In contrast, all groups but the Standard-Vehicle group spent significantly more time than chance with the novel object at the 48-hr delay (Fig. 1E; Standard-E2: t(14) = 2.33, p < 0.04; Enriched-Vehicle: t(22) = 3.64, p < 0.001; Enriched-E2: t(21) = 2.49, p < 0.03), suggesting that both estradiol and enrichment enhanced object recognition.
3.3.3. Aged females
Grid crossings during habituation did not differ among the groups, as indicated by non-significant main effects of Estrogen treatment and Environment, and a non-significant Estrogen × Environment interaction (Table 1). During the choice phase at the 24-hr delay (Fig. 1C), all groups but the Standard-E2 group showed a significant preference for the novel object (Standard-Vehicle: t(10) = 2.52, p < 0.03; Enriched-Vehicle: t(13) = 2.59, p < 0.03; Enriched-E2: t(14) = 4.0, p < 0.001), suggesting that estradiol treatment was detrimental for 24-hr object recognition in standard, but not enriched, females. At the 48-hr delay (Fig. 1F), only the Enriched-Vehicle group showed a preference for the novel object (t(14) = 4.71, p < 0.0001), suggesting that only enrichment alone enhanced 48-hr object recognition relative to chance. The fact that the Standard-E2 group did not show a significant preference for the novel object indicates no effect of estradiol on 48-hr object recognition, whereas the fact that the Enriched-E2 group showed no preference indicates that estradiol reduced the beneficial effects of enrichment on 48-hr object recognition.
3.4. Spatial water maze
3.4.1. Young females
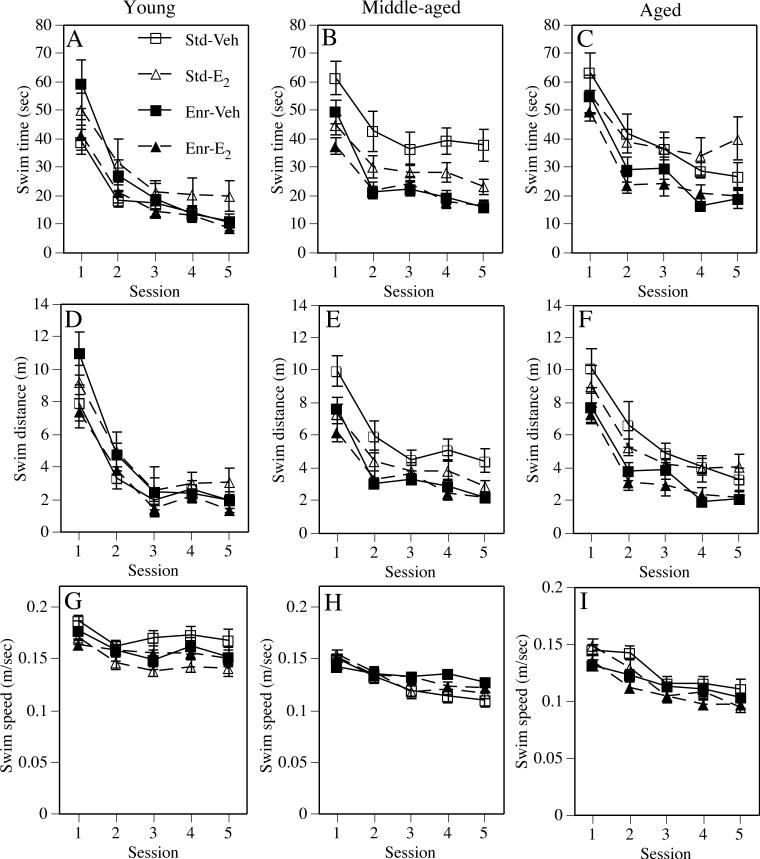
Of the three platform trial measures (swim time, swim distance, and swim speed), the main effect of Estrogen treatment was significant only for swim speed (F(4,148) = 49.55, p < 0.0001; Fig. 2G), such that swim speeds were slower in estradiol-treated females relative to vehicle controls. The main effect of Environment was not significant for any of these measures. In contrast, the Estrogen × Environment interaction was significant for all three measures (Fs(1,37) = 4.51−10.35, ps < 0.05). As evident in Fig. 2, estradiol increased swim time (Fig. 2A) and swim distance (Fig. 2D) in standard-housed females, but reduced time and distance in enriched females. Estradiol also decreased swim speed in standard, but not enriched, females. Significant Session effects in all three measures (Fs(4,148) = 8.25−49.55, ps < 0.0001) indicated that the groups, as a whole, learned to find the platform. The Session × Estrogen, Session × Environment, and Session × Estrogen × Environment interactions were not significant for any measure.
Fig. 2.
Performance during the spatial platform trials as assessed by swim time (A-C), swim distance (D-F), and swim speed (G-H). Data from young females are presented in the left column, from middle-aged females are presented in the center column, and from aged females are presented in the right column. Each data point represents the mean ± SEM of each group for each session. Lines for the Vehicle-treated groups are solid and those for the Estradiol-treated groups are dashed. See text for discussion of estradiol and enrichment effects for each measure at each age. Std-Veh = Standard-Vehicle, Std-E2 = Standard-E2, Enr-Veh = Enriched-Vehicle; Enr-E2 = Enriched-E2.
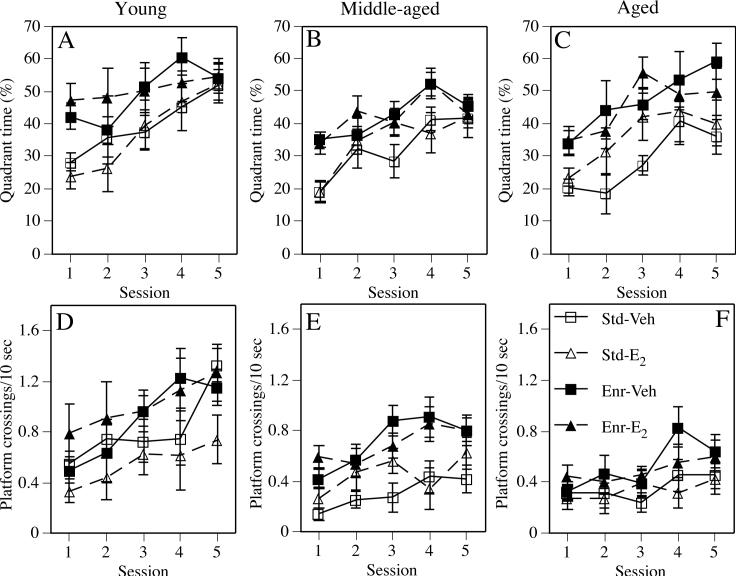
Enrichment significantly improved quadrant time (Fig. 3A; F(1,37) = 7.45, p < 0.01) and platform crossings (Fig. 3D; F(1,37) = 6.61, p < 0.02) during the probe trials. Estradiol, alone or in combination with enrichment, did not affect either measure, as suggested by non-significant main effects of Estrogen treatment and Estrogen × Environment interactions. Significant Session effects for both quadrant time and platform crossings (Fs(4,148) = 8.07 and 5.93, respectively, ps < 0.0002) indicated that the groups learned the location of the hidden platform. No interactions with Session were significant for either measure.
Fig. 3.
Performance during the spatial probe trials as assessed by quadrant time (A-C) and platform crossings (D-F). Data from young females are presented in the left column, from middle-aged females are presented in the center column, and from aged females are presented in the right column. Each data point represents the mean ± SEM of each group for each session. Lines for the Vehicle-treated groups are solid and those for the Estradiol-treated groups are dashed. See text for discussion of estradiol and enrichment effects for each measure at each age. Std-Veh = Standard-Vehicle, Std-E2 = Standard-E2, Enr-Veh = Enriched-Vehicle; Enr-E2 = Enriched-E2.
3.4.2. Middle-aged females
Estrogen treatment significantly reduced swim time (Fig. 2B; F(1,74) = 8.88, p < 0.004) and swim distance (Fig. 2E; F(1,74) = 6.96, p < 0.01) during the platform trials, as did Enrichment (swim time: F(1,74) = 25.78, p < 0.0001; swim distance: F(1,74) = 20.45, p < 0.0001). The Estrogen × Environment interaction was significant for swim time (F(1,74) = 4.6, p < 0.04), and nearly significant for swim distance (F(1,74) = 3.35, p = 0.07). As illustrated in Fig. 2, these interactions were driven by the fact that estradiol reduced swim time (Fig. 2B) and distance (Fig. 2E) in standard-housed, but not enriched, females. The Standard-Vehicle group performed poorly in both measures, leaving room for both estradiol and enrichment to improve performance. However, the combination of estrogen and enrichment did not improve performance more than enrichment alone, although this treatment did improve performance more than estradiol alone. These effects on swim time and swim distance occurred in the absence of effects on swim speed (Fig. 2H), as the main effects of Estrogen treatment and Environment, as well as the Estrogen × Environment interaction, were not significant for this measure. Significant main effects of Session for all three measures (Fs(4,296) = 39.98−60.62, ps < 0.0001) indicated learning by all groups. The only Session-related interaction that was significant for any of the three measures was a Session × Environment interaction for swim speed, which reflects that fact that standard-housed females swam more slowly than enriched females during later sessions (Fig. 2H).
Enrichment significantly increased both quadrant time (Fig. 3B; F(1,74) = 14.47, p < 0.0003) and platform crossings (Fig. 3E; F(1,74) = 25.65, p < 0.0001) during the probe trials, whereas Estrogen treatment did not affect either measure. The Estrogen × Environment interactions were also not significant for either measure. Significant Session effects for quadrant time and platform crossings (Fs(4,296) = 10.89 and 5.61, respectively, ps < 0.0002) indicated that all groups learned to find the hidden platform. No Session-related interactions were significant for either measure.
3.4.3. Aged females
The main effects of Environment, but not Estrogen treatment, were significant for swim time (Fig. 2C; F(1,49) = 21.71, p < 0.0001), swim distance (Fig. 2F; F(1,49) = 26.7, p < 0.0001), and swim speed (Fig. 2I; F(1,49) = 4.62, p < 0.04) during the platform trials. Enrichment decreased swim time, distance, and speed relative to Standard housing. Estrogen × Treatment interactions were not significant for any measure. Performance in all groups improved throughout testing, as indicated by significant Session effects in all measures (Fs(4,196) = 31.88−54.53, ps < 0.0001). The Session × Environment interaction was significant for swim speed (F(4,196) = 2.46, ps < 0.05), reflecting the fact that enriched females swam slower in some sessions than standard-housed females. No other Session-related interactions were significant for any platform trial measure.
As in the platform trials, enrichment significantly improved both quadrant time (Fig. 3C; F(1,49) = 20.21, p < 0.0001) and platform crossings (Fig. 3F; F(1,49) = 8.81, p < 0.005) during the probe trials, whereas Estrogen treatment did not affect either measure. Again, the Estrogen × Environment interactions were not significant for either measure. Significant Session effects for quadrant time and platform crossings (Fs(4,196) = 7.46 and 2.85, respectively, ps < 0.03) indicated that performance improved in all groups throughout testing. No interactions with Session were significant for either measure.
3.5. Cued water maze
3.5.1. Young females
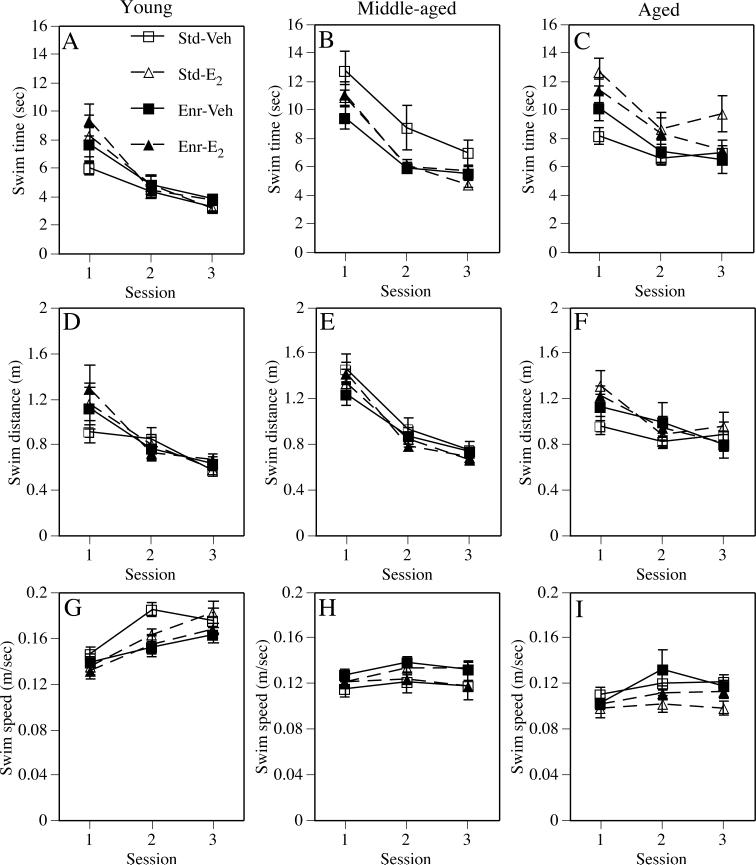
There were no significant main effects of Estrogen treatment on any cued measure, although a Session × Estrogen interaction for swim time (Fig. 4A; F(2,74) = 3.26, p < 0.05) likely reflected the fact that vehicle-treated females found the platform slower than estradiol-treated females during the first test session (Fig. 4A). The main effect of Environment was significant only for swim speed (Fig. 4G; F(1,37) = 5.94, p < 0.02), such that enriched females swam slower than standard-housed females. Estrogen × Environment interactions were not significant for any cued measure. The main effect of Session was significant for all measures (Fs(2,74) = 24.91−49.45, ps < 0.0001), suggesting learning among all groups. With the exception of the significant Session × Estrogen interaction for swim time, no interactions with Session were significant for any measure.
Fig. 4.
Performance during the cued trials as assessed by swim time (A-C), swim distance (D-F), and swim speed (G-H). Data from young females are presented in the left column, from middle-aged females are presented in the center column, and from aged females are presented in the right column. Each data point represents the mean ± SEM of each group for each session. Lines for the Vehicle-treated groups are solid and those for the Estradiol-treated groups are dashed. See text for discussion of estradiol and enrichment effects for each measure at each age. Std-Veh = Standard-Vehicle, Std-E2 = Standard-E2, Enr-Veh = Enriched-Vehicle; Enr-E2 = Enriched-E2.
3.5.2. Middle-aged females
The main effects of Estrogen and Environment were not significant for any of the cued measures (Fig. 4B, E, H). The Estrogen × Environment interaction was significant for swim time (Fig. 4B; F(1,74) = 5.49, p < 0.03), indicating that estradiol reduced swim time in standard-housed, but not enriched, females. The Estrogen × Environment interaction was also significant for swim speed (Fig. 4H; F(1,74) = 5.25, p < 0.03), reflecting the fact that estradiol increased speeds in standard-housed females and decreased speeds in enriched females. Performance improved in all groups throughout training, as indicated by significant Session effects for all measures (Fs(2,148) = 3.79−93.58, ps < 0.0001), but non-significant Session-related interactions for all measures indicated that this improvement proceeded at a similar rate among the groups.
3.5.3. Aged females
The main effects of Environment and the Estrogen × Enrichment interactions were not significant for any cued task measure (Fig. 4C, F, I). The main effect of Estrogen treatment was significant for swim time (Fig. 4C; F(1,49) = 6.99, p < 0.02), such that estradiol-treated females exhibited slower swim times than vehicle-treated females. Estradiol, however, did not affect swim distance (Fig. 4F) or swim speed (Fig. 4I). Performance improved throughout testing as indicated by significant Session effects in all three measures (Fs(2,98) = 4.97−31.13, respectively, ps < 0.01), and the lack of significant interactions between Session and the two treatment suggested a similar rate of improvement among the groups.
4. Discussion
Young females
Enrichment alone had no effect on object recognition in young females, but improved spatial water maze performance during the probe trials without affecting spatial platform trial or cued task performance. These data suggest that enrichment alone can improve challenging measures of spatial memory, but cannot enhance object recognition in young females. Using the same object recognition protocol, we have previously reported that 6 months of enrichment for 3 hrs/day also did not significantly affect object recognition in young ovariectomized C57BL/6 mice (Gresack & Frick, 2004). However, other studies in young mice have reported beneficial effects of enrichment on object recognition (Duffy et al., 2001; Rampon et al., 2000; Tang, Wang, Feng, Kyin & Tsien, 2001). This discrepancy between data from our lab and others may result from numerous factors including the use of different object recognition protocols (ours ensures consistent duration of exposure to the objects across mice whereas others fix total trial time and, thus, allow for differences in motivation or neophobia to produce differential exposure to the objects) and the fact that our lab used ovariectomized mice. A possible effect of ovariectomy on the results is suggested by a previous report in young female rats showing that impaired spatial Morris water maze performance in intact females relative to ovariectomized females was abolished by environmental enrichment, whereas enrichment did not alter performance in ovariectomized females (Daniel et al., 1999).
In contrast to object recognition, the data from the spatial water maze are in general agreement with those from previous reports using short-term enrichment (60 days or less). Two previous studies using young gonadally intact female C57BL/6 mice found that approximately 40 or 60 days of enrichment moderately, but significantly, improved spatial swim time (Williams, Luo, Ward, Redd, Gibson, Kuczaj & McCoy, 2001) and swim distance (Kempermann et al., 1997) in the Morris water maze. Other studies in which young gonadally intact female rats were enriched for 2−3 months reported similar improvements in spatial swim time and swim distance (Daniel et al., 1999; Nilsson, Perfilieva, Johansson, Orwar & Eriksson, 1999). Although enrichment alone in the present study did not affect spatial swim time and swim distance, the fact that enrichment significantly improved performance in both spatial probe trial measures does suggest a beneficial effect of enrichment on spatial reference memory. In fact, it could be argued that this effect is of greater magnitude than those of previous studies due to the increased difficulty of the probe trial measures relative to swim time and distance. However, because none of the previous studies used probe trials, it is difficult to directly compare these data to the present study. Nevertheless, the present data, combined with previous reports, suggest that enrichment benefits spatial reference memory in the water maze in young females regardless of hormone status.
In the object recognition task, post-training 0.2 mg/kg 17β-estradiol enhanced 48-hr object recognition in standard-housed females, which is consistent with our previous reports in young female mice (Gresack & Frick, 2004, 2006b) and with improved object recognition after post-training estradiol injection in young female rats (Luine et al., 2003; Walf, Rhodes & Frye, 2006). It is also consistent with a report in young female mice in which pre-training estradiol treatment improved object recognition (Vaucher et al., 2002). Interestingly, the Enriched-E2 group did not exhibit a preference for the novel object at the 48-hr delay, suggesting that enrichment reduces the estradiol-induced enhancement of object recognition seen in standard-housed females. Enrichment also tended to reduce the beneficial effects estradiol on object recognition at the 24-hr delay, as the Enriched-E2 group spent less time with the novel object than the Standard-E2 group. These findings are consistent with our previous work in young female mice tested at the 48-hr delay (Gresack & Frick, 2004).
The lack of a significant main effect of Estrogen treatment on all spatial water maze measures would initially seem to suggest no effect of estradiol on spatial reference memory. However, the significant Estrogen × Enrichment interaction for swim time and nearly significant interaction for swim distance suggest that estradiol differentially affected spatial learning in standard-housed and enriched females. For example, estradiol increased spatial swim time and distance (thereby impairing performance) in young standard-housed females, but decreased spatial swim time and distance (thereby improving performance) in young enriched females. Although the estradiol-induced increase in swim time in standard-housed females was accompanied by a decrease in swim speed, perhaps suggesting that impaired performance in the Standard-E2 group was simply due to decreased swim speeds, the fact that no corresponding changes in performance of the cued task were observed in this group argues against this possibility. Interestingly, in our previous work, post-training estradiol improved spatial working memory in the radial arm maze among young standard-housed female mice but tended to impair spatial working memory among young enriched female mice (Gresack & Frick, 2004). Together with the present findings, these data may suggest that the combination of post-training estrogen treatment and enrichment is beneficial for spatial reference memory but detrimental for spatial working memory.
The fact that post-training estradiol impaired performance in the spatial water maze task among standard-housed females contradicts previous reports from our lab and others that a single immediate post-training estradiol injection can significantly improve spatial reference memory in 2-day Morris water maze tasks among young female mice (Gresack & Frick, 2006b) and rats (Packard & Teather, 1997; Rhodes & Frye, 2006). However, in these studies, estradiol-induced improvements were observed only in the first trial of the second day of testing, a data point that was obscured in the present study by averaging all five platform trials from a single session for analysis. Perhaps the beneficial effects of post-training estradiol treatment are limited to early acquisition, which would explain why the present study found no beneficial effect of estradiol over the course of five days of testing. This possibility is supported by other post-training data from our lab which show that the beneficial effects of post-training estradiol on spatial working memory in a water-escape motivated radial arm maze task are limited to the first half of testing (Gresack & Frick, 2004).
In total, the object recognition and water maze data from the present study suggest a complicated view of estradiol-enrichment interactions in young females that depends on the task and/or type of memory tested (Table 2). Whereas post-training estradiol in standard-housed females was detrimental for performance in the spatial water maze and beneficial for 48-hr object recognition, it was beneficial for the spatial water maze and somewhat detrimental for 48-hr object recognition in combination with enrichment. Further testing utilizing other spatial and object tasks should be used to determine if these effects are specific to the tasks used in this experiment or to the types of memory they test.
Table 2.
Summary of estradiol and enrichment effects on memory in each age group
| Task | Age | Treatment Estradiol alone | Enrichment alone | Interaction |
|---|---|---|---|---|
| Object recognition | Young | + at 48 hrs | --- | Enrichment reduces beneficial effect of E2 |
| Middle-aged | + at 48 hrs | + at 48 hrs | Combination as good as either alone | |
| Aged | − at 24 hrs | + at 48 hrs | E2 reduces beneficial effect of enrichment | |
| Spatial water maze | Young | --- | + probe | In platform, E2 impairs Std, but improves Enr |
| Middle-aged | + platform | + platform, probe | In platform, E2 improves Std, but not Enr | |
| Aged | --- | + platform, probe | --- | |
| Cued water maze | Young | --- | --- | --- |
| Middle-aged | --- | --- | For swim time, E2 improves Std, not Enr | |
| Aged | + swim time | --- | --- |
+ = enhanced − = impaired; --- = no effect
platform = the platform trial measures swim time and swim distance; probe = the probe trial measures quadrant time and platform crossings
Middle-aged females
Among middle-aged females, enrichment significantly improved all mnemonic spatial water maze measures without any effect on swim speed during the spatial task or on any cued task measure, thus suggesting that enrichment in middle-aged females robustly and specifically improved spatial memory. These data are consistent with previous reports of enrichment-induced improvements in spatial water maze performance among gonadally intact middle-aged male and female mice (Frick et al., 2003; Kempermann et al., 1998) and rats (Ickes, Pham, Sanders, Albeck, Mohammed & Granholm, 2000; Pham, Ickes, Albeck, Söderström, Granholm & Mohammed, 1999). Enrichment alone also enhanced object recognition at the 48-hr delay, suggesting a beneficial effect of enrichment on long-term object memory. Overall, the fact that enrichment improved both spatial and object memory among middle-aged females suggests that this treatment may be a reliable method of reducing age-related memory decline in middle-aged females, independent of hormone status.
Significant main effects of Estrogen treatment for spatial swim time and swim distance would seem to suggest that post-training estradiol generally improves spatial water maze performance in middle-aged females. However, the Estrogen × Environment interactions for both measures indicate that estradiol improved memory in the spatial task only in standard-housed females. Previous studies in which estradiol alone was administered to middle-aged female rats via silastic capsules prior to spatial water maze (Markham et al., 2002) or spatial radial arm maze (Daniel et al., 2006) testing also demonstrate an improvement after treatment. However, other studies administering estradiol orally or by systemic injection found that estradiol had either no effect or impaired spatial working memory in the radial arm maze in middle-aged mice (Fernandez & Frick, 2004) and rats (Ziegler & Gallagher, 2005). Thus, the effects of estradiol on spatial memory in middle-aged females may be dependent on numerous factors including type of memory tested, duration of hormone loss prior to treatment, route and timing of administration, dose, and duration of administration. In contrast, the congruence between the present finding that estradiol alone significantly enhanced object recognition at the 48-hr delay and previous work showing estradiol-induced improvements in object recognition using oral administration (Fernandez & Frick, 2004), suggests that object memory may be more consistently enhanced by estradiol in middle-aged females than spatial memory.
The fact that both enrichment and post-training estradiol independently improved spatial and object memory in middle-aged females (Table 2) might indicate that either treatment can be used in this age group to improve cognitive function. This conclusion would be appropriate for object memory, given that both treatments, alone or in combination, enhanced object recognition at the 48-hr delay relative to chance. However, with respect to spatial reference memory, the magnitude of the improvements produced by the treatments differed considerably. For example, although estradiol reduced spatial swim time and swim distance in standard-housed females, enrichment provided the greatest reduction in both measures irrespective of hormone status and also improved both probe trial measures, suggesting a greater benefit of enrichment than of estradiol. In fact, the magnitude of the enrichment-induced improvement in swim time and distance may have left very little room for further reductions by estradiol. This possibility is consistent with suggestions from the clinical literature that estrogen treatment is more beneficial in less educated women than in those with more education (Matthews et al., 1999). Therefore, despite the fact that both treatments can independently improve spatial water maze performance, enrichment may be a more effective method than post-training estradiol treatment of improving spatial memory in the water maze among middle-aged females. However, this conclusion is premature given the variable effectiveness of different estradiol administration protocols in middle-aged rodents. Further testing of estradiol treatments of different types, doses, durations, and timing in enriched females will be necessary to fully assess the relative effectiveness of enrichment and estradiol in middle-aged females.
Aged females
Regardless of estradiol treatment, environmental enrichment robustly and consistently improved spatial reference memory in aged females (Table 2). Enriched aged females significantly outperformed standard-housed females on all mnemonic measures from the spatial platform and probe trials, and, interestingly, did so despite the fact that enrichment decreased swim speeds during performance of the spatial task. Enrichment had no effect on any measure of performance from the cued task, demonstrating that the beneficial effect of enrichment on memory in the water maze was specific to the spatial task. These findings are consistent with our previous reports of enrichment-induced improvements in spatial water maze performance in aged female (Frick & Fernandez, 2003) and male (Bennett et al., 2006) mice. In addition to the general effects of enrichment on spatial task performance, enrichment also significantly enhanced object recognition at the 48-hr delay in vehicle-treated aged females. Previous studies in aged rodents have also demonstrated enrichment-induced improvements in other non-spatial memory tasks such as incidental learning (Warren, Zerweck & Anthony, 1982) and temporal discrimination (Soffié et al., 1999).
In contrast to enrichment, post-training estradiol had no beneficial effect, either alone or in combination with enrichment, on spatial water maze performance or 48-hr object recognition. Interestingly, estradiol alone impaired 24-hr object recognition, as indicated by the fact that Standard-Vehicle, but not Standard-E2, females showed a preference for the novel object after the 24-hr delay. Further, at the 48-hr delay, estradiol reduced the beneficial effect of enrichment on object recognition, as suggested by the fact that Enriched-Vehicle, but not Enriched-E2, females exhibited a preference for the novel object at this delay. Together, the data from the two tasks indicate that 0.2 mg/kg estradiol, injected immediately after training each day, had no effect on spatial reference memory, but had a detrimental effect on object memory in aged females. Interestingly, a single injection of this same dose of estradiol administered immediately after training in a two-day spatial water maze task significantly enhances memory for the platform location in aged female mice (Harburger et al., 2007). However, aged vehicle-treated females re-learn the platform location after the first trial on the second day of testing (Harburger et al., 2007), so the beneficial effect of estradiol in the spatial water maze may be limited to the first trial of each day. If so, then this effect would be obscured by the way testing is conducted in the five-day spatial task. Alternatively, the stress of repeated injections over the course of training may have interfered with estrogen's ability to affect memory in aged females. The aged brain is particularly vulnerable to the detrimental effects of stress (Sapolsky, Armanini, Packan & Tombaugh, 1987), and estrogen has been shown to increase corticosterone levels (Carey, Deterd, de Koning, Helmerhorst & de Kloet, 1995). Therefore, estrogen in aged females may have further increased corticosterone levels that were already elevated as a result of repeated daily injections, thereby producing hippocampal function that was either impaired or resistant to the beneficial effects of estradiol.
The inability of post-training estradiol to improve memory among aged females in this study stands in contrast to numerous reports in this age group in which chronic estradiol treatment improved various types of memory including spatial memory in the water maze (Frick et al., 2002; Frye, Rhodes & Dudek, 2005; Markham et al., 2002), radial arm maze (Heikkinen et al., 2002), and T-maze (Gibbs, 2000), object memory (Vaucher et al., 2002), and spontaneous alternation (Miller, Hyder, Assayag, Panarella, Tousignant & Franklin, 1999). However, all of these studies used either a more slowly metabolized form of estrogen (estradiol benzoate) or a much longer treatment duration (ranging from 3 weeks to 12 months), combined with the presence of estradiol in the circulation during testing. Thus, it may not be surprising that a rapidly metabolized form of estradiol given post-training had no effect on memory in aged females. Unlike middle-aged females, aged females may need for estradiol to be in the circulation or may need to be primed by estradiol 48 or 72 hours prior to testing (Sandstrom & Williams, 2001) in order to reap the more long-term mnemonic benefits of this hormone. Further, estradiol in aged females may need to bind to classical genomic estrogen receptors, rather than the fast-acting mechanisms involved in post-training administration, in order to effectively improve memory. As such, the present data indicate that repeated post-training treatments of water-soluble estradiol, such as those used here, are not an effective way in which to reduce mnemonic decline in aged females.
Potential neural mechanisms
The spatial water maze and object recognition tasks were selected for use in this study because they provide multiple measurements of hippocampal function. The spatial water maze task has long been known to require intact hippocampal function (Morris et al., 1982), and despite considerable debate surrounding the neural basis of object recognition (Mumby, 2001), the version of the object recognition task used here (which fixes the total exploration time rather than the total trial time) has also been shown to depend on the hippocampus (Baker & Kim, 2002; Clark et al., 2000). Although both tasks require the hippocampus, they each tap into different types of memory encoded by the hippocampus, thereby allowing an examination of how estrogen and enrichment affect multiple aspects of hippocampal function. Unfortunately, the design of this study, utilizing multiple tasks and estradiol treatment times, did not permit correlations between treatment-induced changes in memory with alterations in hippocampal function.
Nevertheless, there is ample evidence to suggest that estrogen- and enrichment-induced alterations in hippocampal structure and/or function underlie the behavioral changes observed in the present experiment. Estrogen and enrichment influence similar aspects of hippocampal structure and function in young and aging female rodents, including spine density (Frick, Fernandez, Bennett, Prange-Kiel, MacLusky & Leranth, 2004; Miranda, Williams & Einstein, 1999; Rampon et al., 2000; Woolley & McEwen, 1992, 1993), neurogenesis (Kempermann et al., 1997, 1998; Tanapat, Hastings, Reeves & Gould, 1999), synaptophysin levels (Bennett et al., 2006; Fernandez & Frick, 2004; Frick & Fernandez, 2003; Frick et al., 2002; Lambert, Fernandez & Frick, 2005), long-term potentiation (Duffy et al., 2001; Foy et al., 1999; Warren et al., 1995), and growth factor levels (Fernandez & Frick, 2004; Ickes et al., 2000; Pham et al., 1999). At this point, it is unclear which, if any, of these alterations might be related to the mnemonic changes seen in this study. In our previous study examining estrogen-enrichment interactions in young mice, we reported that 0.2 mg/kg estradiol alone decreased hippocampal BDNF levels, whereas enrichment alone increased hippocampal synaptophysin levels (Gresack & Frick, 2004). In enriched females, estradiol attenuated the enrichment-induced increases in synaptophysin levels, suggesting that these two treatments can interact to affect certain measures of hippocampal function. However, much more work will need to be done to more clearly elucidate the neural mechanisms underlying the interactions between these two treatments.
Conclusions
The present study is the first to examine whether life-long enrichment influences the mnemonic response to post-training estradiol treatment in young, middle-aged, and aged females. The results clearly indicate that environmental enrichment can significantly affect the ability of post-training water-soluble estradiol to improve memory, and that the nature of the interaction between the two treatments differs considerably at various points in the lifespan (Table 2). In the object recognition task, post-training estradiol alone enhanced retention at the 48-hr delay in young and middle-aged females, but did not enhance retention at this delay in aged females. Enrichment alone had no effect on retention in young females, but facilitated retention at the 48-hr delay in middle-aged and aged females. Interactions between the two treatments were dramatically different at the three ages. In the spatial water maze, enrichment alone improved performance at all ages, whereas estradiol alone had little overall effect. Again, interactions between the two treatments varied considerably among the ages as evidenced by performance during the spatial platform trials.
The fact that post-training estradiol enhanced both spatial and object memory in middle-aged, but not aged, female mice seems to support the hypothesis that hormone treatment is most effective within a critical period after age-related hormone loss (Maki, 2006; Sherwin, 2006; Zandi, Carlson, Plassman, Welsh-Bohmer, Mayer, Steffens & Brietner, 2002). Further, the finding that post-training estradiol in middle-aged females improved spatial reference memory in standard-housed, but not enriched, females is consistent with clinical data suggesting that estrogen treatment may be most effective in women with the least education (Matthews et al., 1999). Together, the present data suggest that post-training estradiol treatment is most beneficial for cognition in females who have experienced recent loss of ovarian hormones and less environmental (e.g., intellectual) stimulation. Although further study is needed to determine if these findings generalize to other species and types of memory, they provide evidence suggesting that environmental factors should be considered when assessing the effects of estrogen on cognitive functioning in aging females.
Acknowledgements
The authors would like to thank Jennifer C. Bennett for assistance with ovariectomy surgeries, and Carly Keidel, Wen Fan, and Caitlin Purcell for assistance with behavioral testing, injections, and enrichment bin changes. This project was supported by an Investigator Initiated Research Grant (IIRG-03-6051) from the Alzheimer's Association and by NIH R01 AG022525 to K.M.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiology of Learning and Memory. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's disease: Recent insights and remaining challenges. Learning and Memory. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. Journal of Endocrinology. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovareictomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiology and Behavior. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. Journal of Neuroscience. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones and Behavior. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learning and Memory. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology of Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17ß-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. Journal of Neurophysiology. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiology of Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety, and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiology of Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. European Journal of Neuroscience. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behavioral Neuroscience. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learning and Memory. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Research. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones and Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Research. 2006a;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacology Biochemistry and Behavior. 2006b;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiology of Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puoliväli J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Hormones and Behavior. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Ickes B, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Experimental Neurology. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Keating NL, Cleary PD, Rossi AS, Zaslavsky AM, Ayanian JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Annals of Internal Medicine. 1999;130:545–553. doi: 10.7326/0003-4819-130-7-199904060-00002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. Journal of Neuroscience. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiology of Learning and Memory. 2005;83:206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JRM, Dartigues J-F, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Rates and risk factors for dementia and Alzheimer's disease. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: Is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. American Journal of Psychiatry. 2001;158:227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Hormones and Behavior. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: Relevance to alterations in the estrous cycle. Journal of Neuroscience. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Long JM, Johnson CT, Olton DS. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behavioral Neuroscience. 1993;107:627–632. doi: 10.1037//0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. Journal of Neuroscience. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. Journal of the American Geriatrics Society. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? American Journal of Epidemiology. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Estrogens effects on the brain: Multiple sites and molecular mechanisms. Journal of Applied Physiology. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KBJ. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. Journal of Neuroscience. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioural Brain Research. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kobayashi S, Ohashi Y, Ando S. Age-changes of brain synapses and synaptic plasticity in response to an enriched environment. Journal of Neuroscience Research. 1999;56:307–315. doi: 10.1002/(SICI)1097-4547(19990501)56:3<307::AID-JNR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. Journal of Neurobiology. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- O'Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Hormones and Behavior. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiology of Learning and Memory. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Söderström S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Pitha J, Harman SM, Michel ME. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J Pharm Sci. 1986;75:165–167. doi: 10.1002/jps.2600750213. [DOI] [PubMed] [Google Scholar]
- Pitha J, Pitha J. Amorphous water soluble derivatives of cyclodextrins: Nontoxic dissolution enhancing excipients. Journal of Pharmacological Sciences. 1985;74:987. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang Y-P, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neuroscience. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiology of Learning and Memory. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissanen A, Puoliväli J, van Groen T, Riekkinen PJ. In mice tonic estrogen replacement therapy improves non-spatial and spatial memory in a water maze task. NeuroReport. 1999;10:1369–1372. doi: 10.1097/00001756-199904260-00039. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115:384–393. [PubMed] [Google Scholar]
- Sapolsky R, Armanini M, Packan D, Tombaugh G. Stress and glucocorticoids in aging. Endocrinology and Metabolism Clinics of North America. 1987;16:965–980. [PubMed] [Google Scholar]
- Sherwin BB. Can estrogen keep you smart? Evidence from clinical studies. Journal of Psychiatry and Neuroscience. 1999;24:315–321. [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. Journal of the American Medical Association. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women's Health Initiative Memory Study: A randomized controlled trial. Journal of the American Medical Association. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Soffié M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behavioural Brain Research. 1999;101:37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-P, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: Behavioral and physiological effects of episode-like pulses in rats. Pharmaceutical Research. 1989a;6:641–646. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: behavioral and physiological effects of episode-like pulses in rats. Pharm Res. 1989b;6:641–646. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KBJ. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiology of Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM, Zerweck C, Anthony A. Effects of environmental enrichment on old mice. Developmental Psychobiology. 1982;15:13–18. doi: 10.1002/dev.420150104. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Research. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: Effects on spatial memory and hippocampal CREB immunoreactivity. Physiology and Behavior. 2001;73:649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Winocur G. Environmental influences on cognitive decline in aged rats. Neurobiology of Aging. 1998;19:598–597. doi: 10.1016/s0197-4580(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Brietner JCS. Hormone replacement therapy and incidence of Alzheimer's disease in older women: The Cache County Study. Journal of the American Medical Association. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: assessment of estrogen replacement after ovariectomy. Brain Research. 2005;1052:163–173. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]