Abstract
There are many occasions in which humans and other animals must inhibit the production of some behavior or inhibit the processing of some internal representation. Success in inhibitory processing under normal circumstances can be revealed by the fact that certain brain pathologies render inhibitory processing ineffective. These pathologies often have been associated with damage to frontal cortex, including lateral and inferior aspects. We provide behavioral evidence of a verbal working memory task that, by hypothesis, engaged inhibitory processing, and we show (by using positron emission tomograpny) that the inhibitory processing is associated with a lateral portion of the left prefrontal cortex. The task in which subjects engaged was item-recognition: Four target letters were presented for storage followed, after a brief interval, by a probe letter that could match a target letter or not. On some trials, when the probe did not match a target letter and therefore required a “no” response, the probe had matched a target letter of the previous trial, so on these trials a “yes” response was prepotent and had to be inhibited, by hypothesis. Compared with a condition in which no prepotent response was created, this condition yielded brain activation in left inferior frontal gyrus, in the region of Brodmann’s area 45.
Humans and other animals have the ability to inhibit a wide variety of sensory input, motor output, and internal processes during the normal course of cognition. For example, people effectively inhibit distracting sensory information (1), undesired response tendencies (2), and irrelevant cognitive processes (3) as the need arises. The study of human patients who suffer from reduced inhibitory control and of animals who have had selective brain lesions or electrical stimulation all suggest that the lateral prefrontal cortex is involved critically in these inhibitory functions (4–14). Indeed, it has been proposed that inhibitory control could be a central function of some regions of prefrontal cortex (15–18). Yet, there is little direct evidence from normal humans for the putative role of lateral prefrontal structures in inhibitory control [although some evidence may derive from a study of the Stroop task (19) and of proactive interference (20)]. We provide such evidence from a verbal working memory task in which subjects had to inhibit a prepotent response to respond accurately. Under these conditions, we found brain activation [(measured by positron emission tomography (PET)] in left lateral prefrontal cortex.
METHODS
Design.
These results derive from an experiment in which PET measurements were collected from 12 normal adults (ages 19–30 years). The experiment included three conditions (two experimental and one control) in which subjects were presented a target set of letters to be retained in memory during a retention interval of 3 s. This interval was followed on a random half of the trials by a probe letter that required a positive response because it matched a member of the target set (“positive probes”) and on half the trials by a probe that required a negative response because it did not match any target (“negative probes”).
The two experimental conditions were designed to differ in a very specific way. In the High Recency condition, half of the negative probes had been members of the target set on the immediately previous trial (“recent negative probes”). We hypothesized that correct responses to these recent negative probes on the current trial would require inhibition of a prepotent “yes” response associated with their status as target set members on the prior trial. Similarly, half of the positive probes on the current trial had been members of the previous target set as well (“recent positive probes”). Constructing the High Recency condition required that there be overlap in the constituents of the target sets on successive trials; this overlap was always 50%. The Low Recency condition was designed to minimize inhibition in that negative probes were never members of the target set on either of the previous two trials, so there was no need to inhibit a positive response for any negative probe. For the two experimental conditions, there were always four letters in the target set on each trial.
Subjects also were tested in a Minimal Memory condition in which only a single letter had to be held in memory for a retention interval of only 200 ms. This condition served as a control (whose activations were subtracted from the two experimental conditions) because it included encoding, retention, and response properties of the two experimental conditions, and the memory requirement was minimal.
Procedure.
Subjects participated in the three conditions (High Recency, Low Recency, and Minimal Memory) for five PET scans each counterbalanced for order of presentation. Before this session, subjects received practice on the High and Low Recency tasks in a session that included 180 trials of each. Stimuli were consonants, and they were presented on a Macintosh computer controlled by psyscope software (21). Subjects responded with the index finger of the left hand for positive responses and with the middle finger for negative responses. For all conditions, the proportion of positive and negative probes was 50% each. In the High Recency condition, a recent negative probe occurred on half of all negative trials; on the remaining half, it was a letter that had not been a member of the target set on either of the previous two trials. Positive probes in the High Recency condition were members of the prior trial’s target set on half of all positive trials; for the remaining half, they were not members of the target set for either of the previous two trials. In the Low Recency condition, no probe (whether positive or negative) was a member of the target set on either of the previous two trials, which was also true of the Minimal Memory control condition.
Image Acquisition.
A Siemens ECAT EXACT-47 PET scanner acquired data in three-dimensional mode (septa retracted), with measured attenuation but no correction for scattered events. Three-dimensional reconstruction yielded 47 contiguous slices that were 3.375 mm (center-to-center) apart with in-plane resolution of 10 mm at full-width half-maximum. Subjects were positioned in the scanner, and head position was recorded and verified before each scan. A bolus of 10 mCi of [15O] water was delivered i.v. over a 10-s interval as the subject began the sequence of trials. Acquisition began when the true coincidence rate exceeded half the random coincidence rate. Eight minutes separated each scan to allow the residual radiation to return to an acceptable level.
Image Analysis.
A complete description of the image analysis protocol is given elsewhere (22). In brief, it consisted of the following steps: Intra-subject registration corrected motion between scans for each subject (23). Each subject’s image sets then were transformed to a stereotactic system (24). A subtraction image set then was created for each subject between the averaged images for each contrast of interest. The subtraction image sets then were averaged across subjects. SDs for the voxels were averaged within the brain to create a pooled estimate of variance, and a t statistical value was calculated for each voxel by using a pooled variance estimate and correcting for multiple nonindependent comparisons (25, 26). Significant CBF changes were localized by using stereotactic coordinates and are displayed in Fig. 1 on a standard nonlinearly warped MRI for visual interpretation.
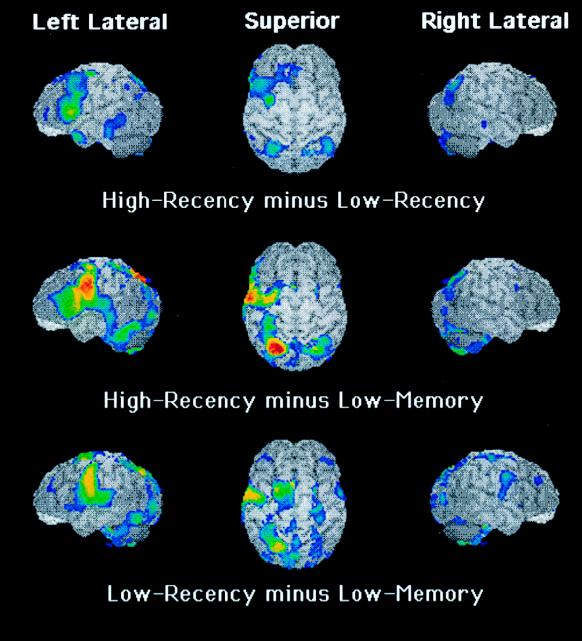
Figure 1.
Images reflecting the activations in three subtractions. Each row presents left lateral, superior, and right lateral views of the brain, with t statistics of activations at or within 15 mm of the surface superimposed in color on a gray MRI of a single subject (not one from this study). Red represents the largest ± value, followed by yellow, green, and blue. Shown are all activations that passed a criterion of P < 0.05 uncorrected for multiple comparisons (although statistical analysis corrected for multiple comparisons in revealing the sites of activations that are listed in Table 2). The top row depicts the sites of activation subtracting the Low from the High Recency condition; the second row depicts the sites from the subtraction of the Low Memory from the High Recency condition; and the bottom row shows the subtraction of the Low Memory from the Low Recency condition.
In contrast to the Low Recency condition, the High Recency condition was designed to create a relationship between successive trials that would recruit inhibitory processes. We anticipated that this recruitment would be evident in both brain activation and behavioral data. The behavioral prediction was confirmed in two comparisons of the data in Table 1. Recent negative probes in the High Recency condition had responses that were slower and less accurate overall than (i) nonrecent negative probes in this condition and (ii) nonrecent negative probes in the Low Recency condition [(i), Response Time: PET session, P = 0.009; practice session, P = 0.009; Accuracy: PET session, P = 0.32; practice session, P = 0.005; (ii, Response Time: PET session, P = 0.03; practice session, P = 0.02; Accuracy: PET session, P = 0.45; practice session, P = 0.01)]. These data invite the interpretation that a memory trace of items from at least one previous trial lingers during a current trial, leading to higher latency and lower accuracy in making a decision about a negative probe item for the current trial (27, 28). By contrast, the responses for recent positive probes in the High Recency condition were not reliably different in latency or accuracy from responses to nonrecent positive probes in this condition or from responses to nonrecent positive probes in the Low Recency condition (all Ps > 0.10).
Table 1.
Response times and accuracies for each type of trial in the three conditions
| Type of probe | Condition
|
|||||
|---|---|---|---|---|---|---|
| PET session
|
Practice session
|
|||||
| High Recency | Low Recency | Minimal Memory | High Recency | Low Recency | ||
| Response times, ms | Recent negative | 772 ± 33 | 757 ± 37 | |||
| Nonrecent negative | 739 ± 33 | 741 ± 30 | 596 ± 26 | 704 ± 34 | 695 ± 33 | |
| Recent positive | 688 ± 33 | 682 ± 46 | ||||
| Nonrecent positive | 695 ± 26 | 683 ± 34 | 529 ± 23 | 672 ± 42 | 655 ± 37 | |
| Accuracies | Recent negative | 0.99 ± 0.004 | 0.91 ± 0.017 | |||
| Nonrecent negative | 0.98 ± 0.010 | 0.99 ± 0.006 | 0.98 ± 0.019 | 0.97 ± 0.010 | 0.96 ± 0.010 | |
| Recent positive | 0.99 ± 0.005 | 0.92 ± 0.021 | ||||
| Nonrecent positive | 0.99 ± 0.005 | 0.99 ± 0.004 | 0.98 ± 0.012 | 0.92 ± 0.026 | 0.93 ± 0.013 | |
Each cell contains a mean and associated SEM calculated across subjects. Note that there are data for the type of probe only in cells that are appropriate to that condition.
The pattern of differences for negative probes is what we would expect from the experimental demand to inhibit a competing positive response in the High Recency condition. What neural mechanisms underlie this inhibitory process? This question is answered by the activation data from the PET imaging shown at the top of Fig. 1 (and in Table 2), which reflect a direct subtraction of the Low from the High Recency condition. The left lateral view reveals the one site of activation that was statistically reliable in this comparison between conditions, centered in the region of Brodmann’s area 45 of lateral prefrontal cortex. As shown in the middle and bottom rows of Fig. 1, this site appears prominently in the subtraction of the Minimal Memory control condition from the High Recency (2.55% activation) but not the Low Recency (−0.69% activation) condition (corrected for multiple comparisons, P = 0.025 for the former and p = .17 for the latter; these results were determined by a region-of-interest analysis in which the regions-of-interest were identified by activated regions from the subtraction of the control from the average of the High plus the Low Recency conditions; this site does not appear as a peak voxel in Table 2 because its activation is contiguous to other activated sites). Because this site appears both to differentiate the High from the Low conditions and to be activated in the High but not in the Low conditions, we interpret the activation of this lateral prefrontal site to reflect an inhibitory process required in the High but not in the Low Recency condition. [(This site is somewhat similar to one documented in a study of proactive interference, a study that also may have recruited inhibitory processes (20).]
Table 2.
Activations and deactivations in three sets of subtractions among the three conditions of the experiment.
| Subtraction | Stereotaxic coordinates | Activation magnitude, % | z-score | Anatomical region |
|---|---|---|---|---|
| High recency minus low recency | (−48, 21, 9) | 3.07 | 4.30 | Left area 45 of Lateral Prefrontal Cortex |
| High recency minus low memory control | (−24, −62, 40) | 5.21 | 7.49 | Left area 7/40 of Posterior Parietal Cortex |
| (−46, −4, 32) | 4.62 | 6.65 | Left area 6 of Premotor Cortex | |
| (−37, 8, 25) | 4.33 | 6.23 | Left area 6/44 of Premotor/Broca’s region | |
| (−26, −6, 43) | 3.92 | 5.63 | Left area 4/6 of Motor/Premotor Cortex | |
| (26, −60, 34) | 3.46 | 4.98 | Right area 7 of Superior Parietal Cortex | |
| (10, −64, −20) | 3.22 | 4.63 | Right midline cerebellum | |
| (24, −55, −25) | 3.13 | 4.51 | Right lateral cerebellar hemisphere | |
| (−3, 41, 0) | −4.60 | −6.62 | Left area 32 of anterior cingulate cortex | |
| (17, 41, 38) | −3.02 | −4.34 | Right area 8 of medial frontal cortex | |
| Low recency minus low memory control | (−17, 1, 45) | 3.64 | 5.43 | Left area 32/24 of anterior cingulate |
| (−48, −6, 32) | 3.36 | 5.01 | Left area 6 of premotor cortex | |
| (−21, −64, 38) | 3.15 | 4.71 | Left area 7/40 of posterior parietal cortex | |
| (−60, −6, 22) | 3.10 | 4.62 | Left area 4/6 of motor/premotor cortex | |
| (12, −60, −14) | 2.99 | 4.47 | Right midline cerebellum | |
| (−17, 30, 50) | −3.70 | −5.51 | Left area 8 of medial frontal cortex | |
| (8, 32, 50) | −3.24 | −4.83 | Right area 8 of medial frontal cortex | |
| (1, 37, −11) | −3.20 | −4.78 | Right area 11 of medial frontal cortex | |
| (−39, 14, 47) | −3.19 | −4.76 | Left area 6/8 of lateral frontal cortex | |
| (−1, 39, 40) | −3.14 | −4.68 | Left area 8 of medial frontal cortex |
Activations are shown in normal font, and deactivations (i.e., negative values from the subtractions) are shown in italics. Shown are the stereotaxic coordinates of the peak voxels that passed a criterion of P < 0.05 after correction for multiple comparisons (26).
A region-of-interest analysis also revealed several areas of activation common to the High and Low Recency conditions. These appear in left hemisphere parietal and premotor and cortex in right cerebellum. These sites are typical of ones reported in studies of verbal working memory (22). Although these regions appear to be more activated in the High Recency condition, there are, in fact, no reliable differences in activation between the High and Low Recency conditions in these regions; the only reliable difference between these conditions, as indicated above, is in the left lateral prefrontal cortex.
To the extent that activation may be greater overall in the High Recency condition, this may reflect greater recruitment of working memory processes. For example, previous investigators have argued that one effect of recent negative probes in a task is to make the discrimination between positive and negative responses more difficult (26, 27); this point may be reflected in the greater activation in the High condition in areas that are activated in both conditions. Nevertheless, our data do not sustain an argument that the only difference between the High and Low Recency conditions is that the former requires more overall effort, undifferentiated by the type of process that is recruited. Two pieces of evidence contradict this explanation. First, the High Recency condition has a very selective effect on performance that is restricted to the recent negative probes only (responses to nonrecent negative probes do not differ in response time or accuracy between High and Low Recency conditions; all Ps > 0.10). Second, all other things being equal, a hypothesis based on overall effort predicts a similar pattern of brain activations when comparing the Low Recency condition to the Minimal Memory condition as when comparing the High to the Low Recency condition. This is because the Low Recency condition is more difficult than the control (see Table 1). However, as noted above, the difference between the High and Low Recency conditions involves activation in left lateral prefrontal cortex, activation that is not evident in a comparison of the Low Recency to the Minimal Memory control. These results cannot be explained by the hypothesis that the High Recency condition is simply more difficult overall.
Rather, we conclude that the High Recency condition recruits inhibitory processes to reject unwanted response tendencies associated with probes that recently had been members of the target set. These inhibitory processes appear to be mediated by left lateral prefrontal structures in our task. This finding is consistent with various studies of humans and other animals that have documented the involvement of frontal structures in inhibition of motor and other cognitive processes (4–14). Together, these data recommend further examination of the various types of inhibitory processes that are mediated by frontal cortex and the role that these inhibitory processes play in a range of cognitive tasks.
Acknowledgments
This research was supported in part by a grant from the Office of Naval Research and in part by a grant from the National Institute on Aging, both to the University of Michigan.
ABBREVIATION
- PET
positron emission tomography
References
- 1.Stroop J R. J Exp Psych. 1935;18:643–662. [Google Scholar]
- 2.Roberts R J, Hager L D, Heron C. J Exp Psych: General. 1994;123:374–393. [Google Scholar]
- 3.Wickens D D. Psychol Rev. 1970;22:1–15. [Google Scholar]
- 4.Chao L L, Knight R T. NeuroReport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Woods D L, Knight R T. Neurology. 1986;36:212–216. doi: 10.1212/wnl.36.2.212. [DOI] [PubMed] [Google Scholar]
- 6.Perret E. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 7.Shimamura A P. In: The Cognitive Neurosciences. Gazzaniga M, editor. Cambridge, MA: MIT Press; 1995. pp. 803–813. [Google Scholar]
- 8.Milner B, Petrides M, Smith M L. Hum Neurobiol. 1985;4:137–142. [PubMed] [Google Scholar]
- 9.Brutkowski S, Mishkin M, Rosvold H E. In: Central and Peripheral Mechanisms of Motor Function. Gutmann E, Hnik P, editors. Prague, Czech Republic: Czechoslovak Academy of Sciences; 1963. pp. 133–141. [Google Scholar]
- 10.Drewe E A. Cortex. 1975;11:8–16. doi: 10.1016/s0010-9452(75)80015-3. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki K, Gemba H, Tsujimoto T. Brain Res. 1989;495:100–107. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- 12.Bartus R T, Levere T E. Brain Res. 1977;119:233–248. doi: 10.1016/0006-8993(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 13.Malmo R. J Neurophysiol. 1942;5:295–308. [Google Scholar]
- 14.Watanabe M. Brain Res. 1986;382:15–27. doi: 10.1016/0006-8993(86)90105-8. [DOI] [PubMed] [Google Scholar]
- 15.Fuster J M. The Prefrontal Cortex. 3rd Ed. New York: Lippincott-Raven; 1997. [Google Scholar]
- 16.Diamond A. In: The Development and Neural Bases of Higher Cognitive Functions. Diamond A, editor. New York: New York Academy of Sciences Press; 1990. pp. 267–317. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J D, Servan-Schreiber D. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Hasher L, Zacks R T. In: The Psychology of Learning and Motivation. Bower G H, editor. San Diego: Academic; 1988. pp. 193–224. [Google Scholar]
- 19.Taylor S F, Kornblum S, Lauber E J, Minoshima S, Koeppe R A. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- 20.Dolan R J, Fletcher P C. Nature (London) 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J D, MacWhinney B, Flatt M R, Provost J. Behav Res Meth Instru Comput. 1993;25:257–271. [Google Scholar]
- 22.Jonides J, Schumacher E H, Smith E E, Lauber E J, Awh E, Minoshima S, Koeppe R A. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 23.Minoshima S, Koeppe R A, Fessler J A, Kuhl D E. In: Quantification of Brain Function-Tracer Kinetics and Image Analysis in Brain PET. Uemura K, Lassen N A, Jones T, Kanno I, editors. Excepta Medica, Tokyo: International Congress Series 1030; 1993. pp. 409–418. [Google Scholar]
- 24.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 25.Friston K J, Frith C D, Liddle P F, Frackowiak R S J. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 26.Worsley K J, Evans A C, Marrett S, Neelin P. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 27.Monsell S. Cognitive Psych. 1978;10:465–501. [Google Scholar]
- 28.McElree B, Dosher B A. J Exp Psych General. 1989;118:346–373. [Google Scholar]