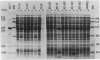
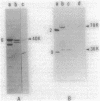
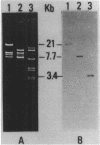
Abstract
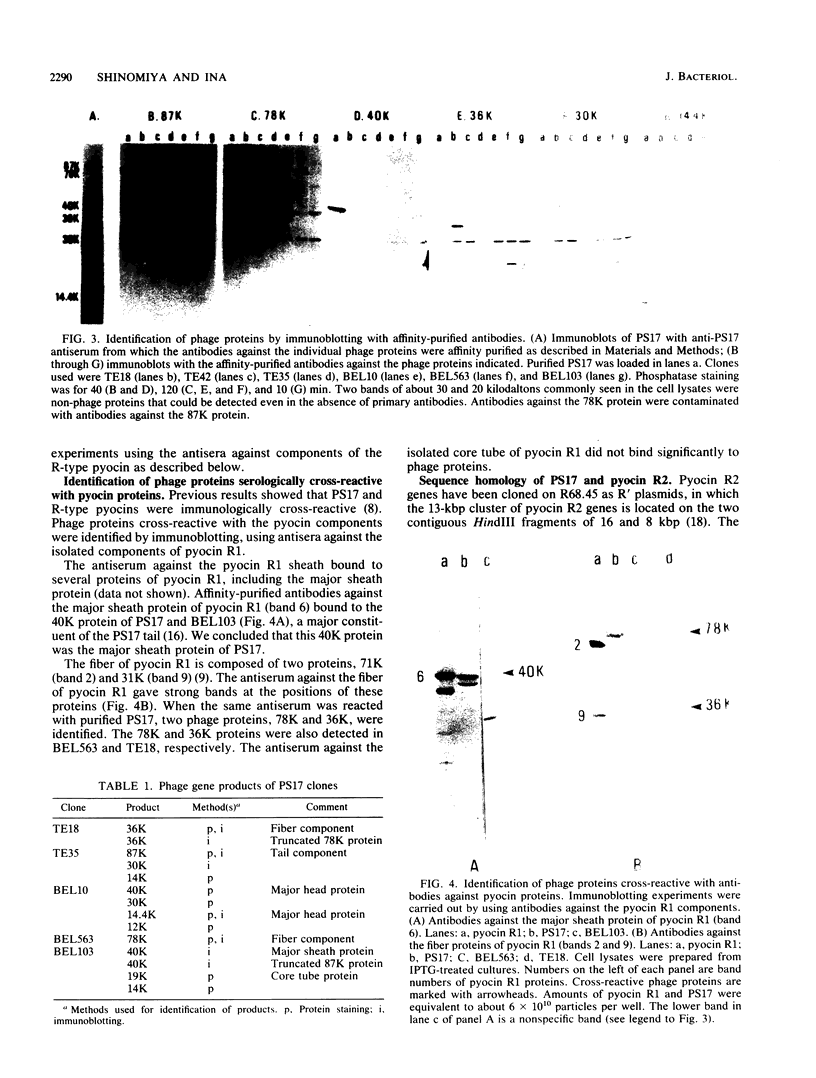
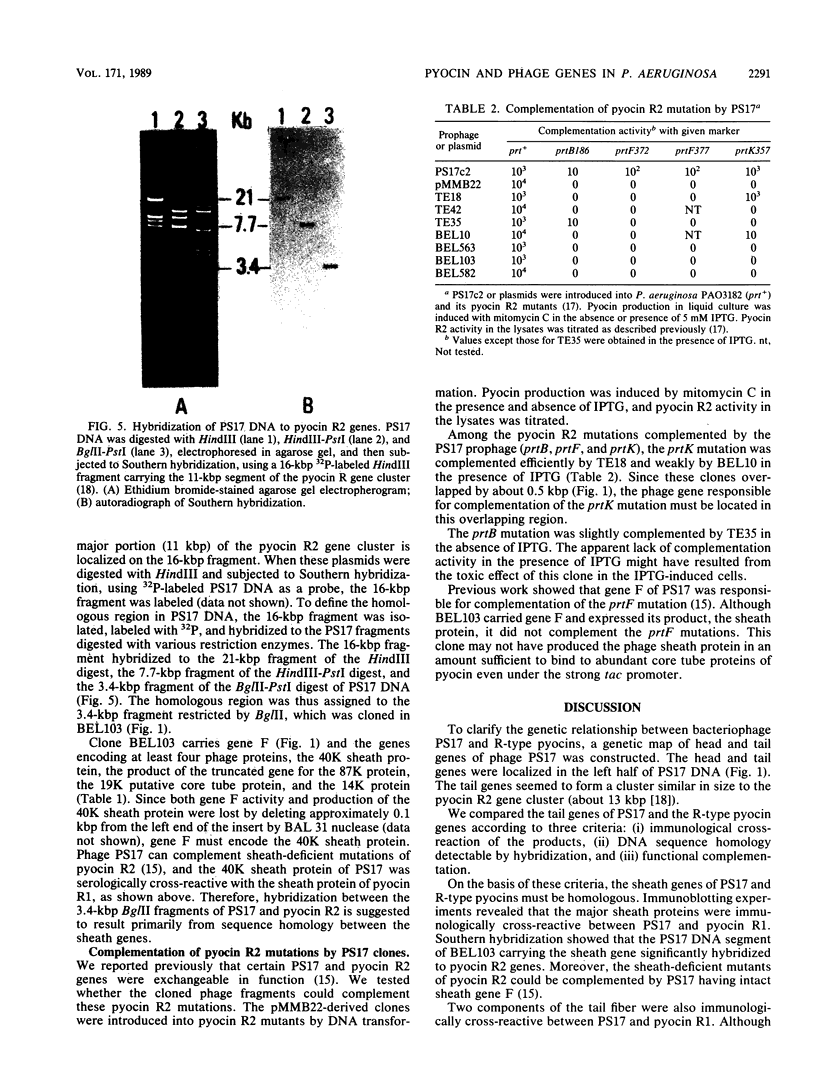
PS17 is a bacteriophage of Pseudomonas aeruginosa that is serologically cross-reactive with phage tail-like bacteriocins called R-type pyocins. In addition to having immunological cross-reactivity, certain genes are functionally complementable between PS17 and R-type pyocins. To compare the genetic structures of PS17 and R-type pyocins, a physical map of PS17 genes was constructed by cloning phage DNA fragments on RSF1010-derived vector plasmids. The head and tail gene clusters were tandemly arrayed and together occupied about half of the 41-kilobase-pair PS17 chromosome. With use of these phage clones, the following results were obtained with respect to the genetic relationship between PS17 and R-type pyocins: (i) serological cross-reaction between PS17 and pyocin occurred for the major sheath protein and two components of the fiber, (ii) a certain pyocin mutation was complemented by cloned phage fragments, and (iii) the phage DNA fragment carrying sheath and core tube genes was shown to hybridize to the DNA fragment carrying the pyocin R2 genes.
Full text
PDF

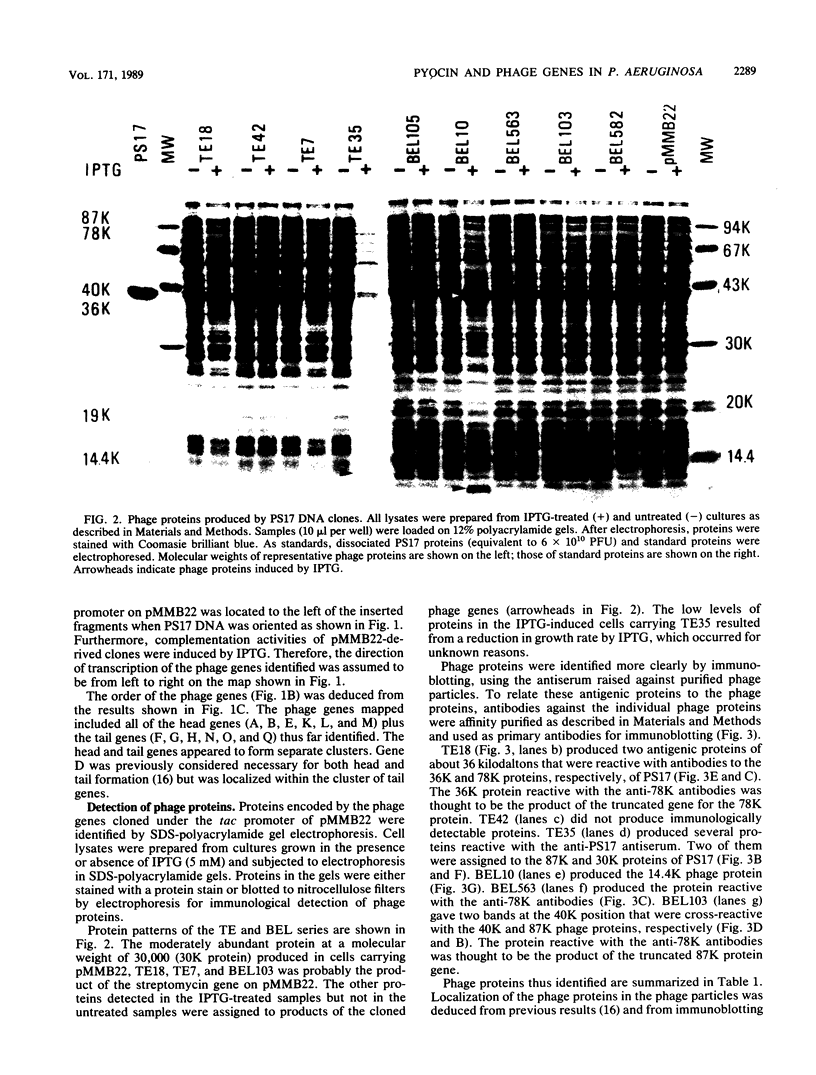

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Ishii S. Isolation, homogeneity, and properties of core particle from pyocin R1. J Biochem. 1979 Feb;85(2):403–411. doi: 10.1093/oxfordjournals.jbchem.a132347. [DOI] [PubMed] [Google Scholar]
- Kageyama M., Shinomiya T., Aihara Y., Kobayashi M. Characterization of a bacteriophage related to R-type pyocins. J Virol. 1979 Dec;32(3):951–957. doi: 10.1128/jvi.32.3.951-957.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki T., Ogura Y., Ishii S. Isolation and characterization of pyocin R1 fibers. J Biochem. 1982 Mar;91(3):825–835. doi: 10.1093/oxfordjournals.jbchem.a133770. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohsumi M., Shinomiya T., Kageyama M. Comparative study on R-type pyocins of Pseudomonas aeruginosa. J Biochem. 1980 Apr;87(4):1119–1125. [PubMed] [Google Scholar]
- Reanney D. C., Ackermann H. W. Comparative biology and evolution of bacteriophages. Adv Virus Res. 1982;27:205–280. doi: 10.1016/s0065-3527(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Shinomiya T. Phenotypic mixing of pyocin R2 and bacteriophage PS17 in Pseudomonas aeruginosa PAO. J Virol. 1984 Feb;49(2):310–314. doi: 10.1128/jvi.49.2.310-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S. Bactericidal activity of the tail of Pseudomonas aeruginosa bacteriophage PS17. J Virol. 1979 Dec;32(3):958–967. doi: 10.1128/jvi.32.3.958-967.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S., Kageyama M. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. I. Localization of the pyocin R2 gene cluster between the trpCD and trpE genes. Mol Gen Genet. 1983;189(3):375–381. doi: 10.1007/BF00325898. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S., Kikuchi A., Kageyama M. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. II. Physical characterization of pyocin R2 genes using R-prime plasmids constructed from R68.45. Mol Gen Genet. 1983;189(3):382–389. doi: 10.1007/BF00325899. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui C. Structure of pyocin R. I. Isolation of sheath from pyocin R by alkali treatment and its properties. J Biochem. 1971 Jan;69(1):101–110. doi: 10.1093/oxfordjournals.jbchem.a129437. [DOI] [PubMed] [Google Scholar]