Abstract
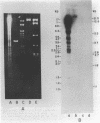
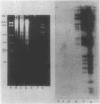
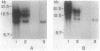
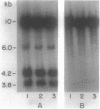
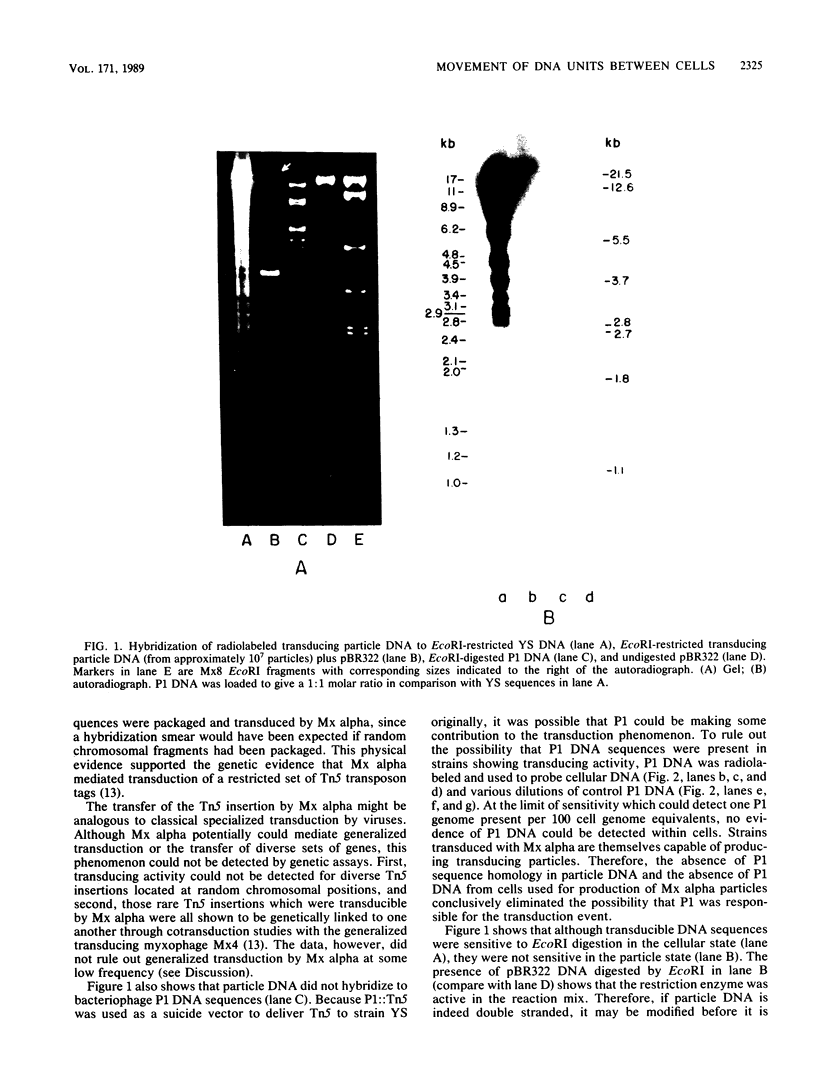
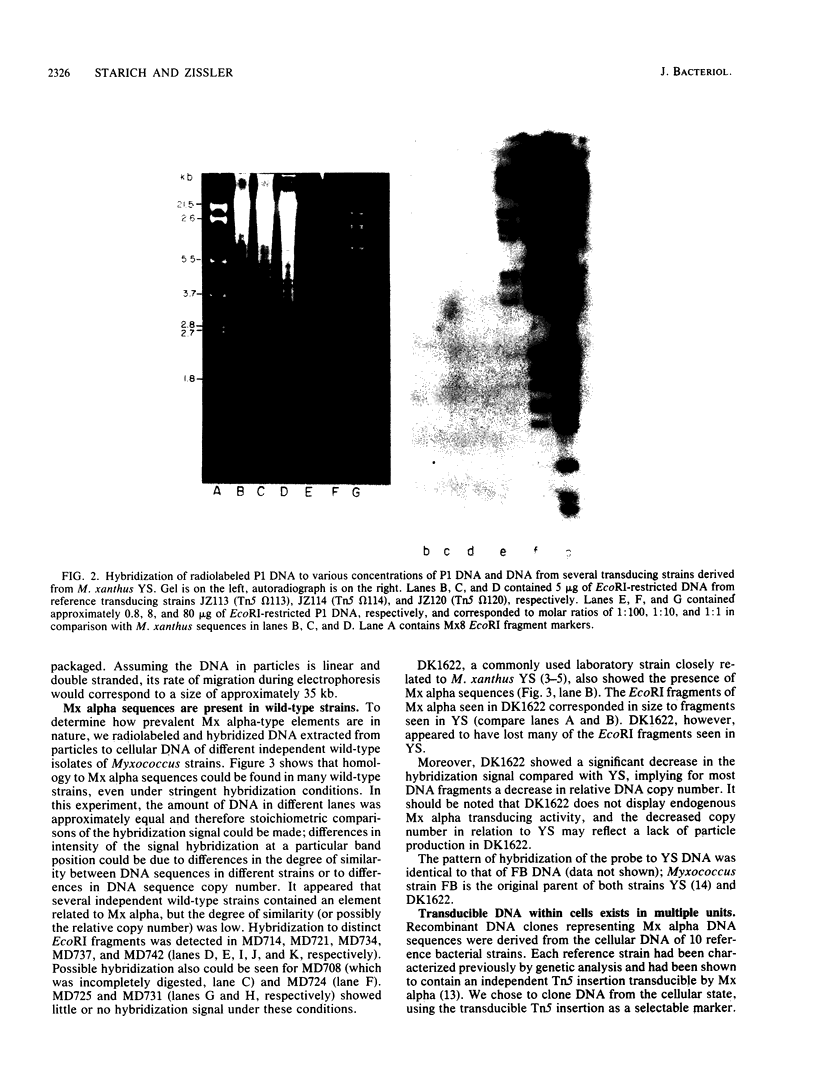
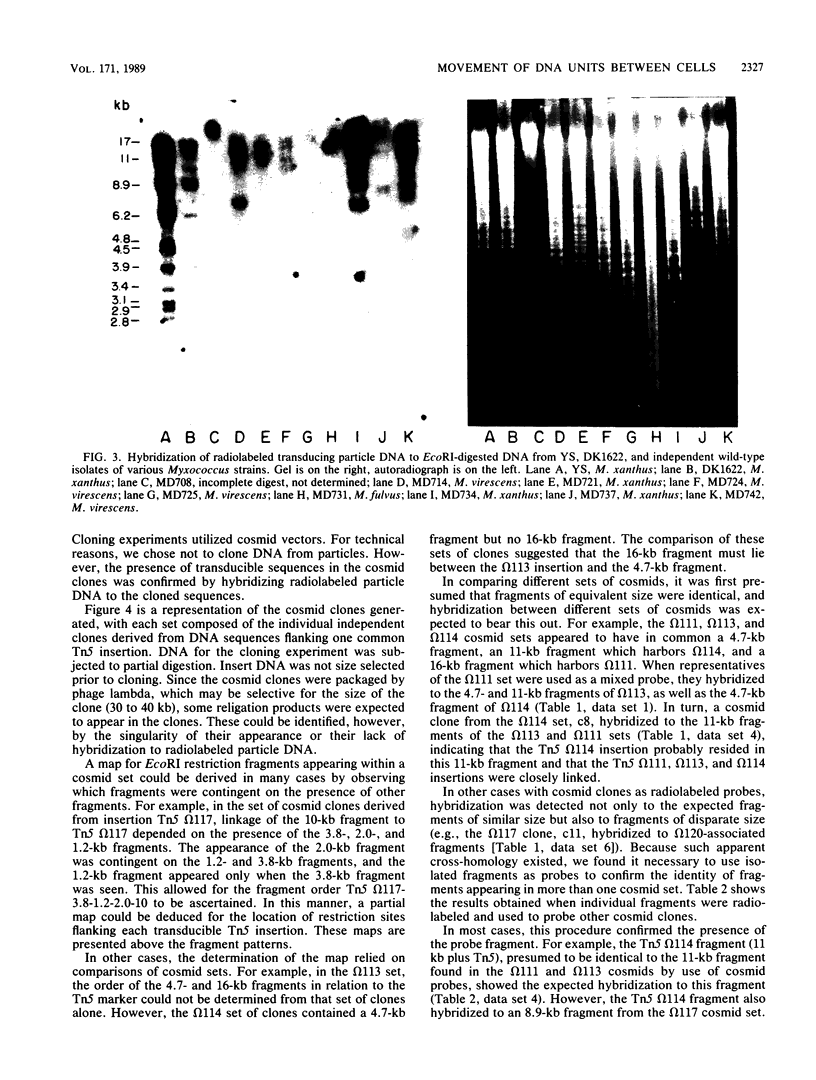
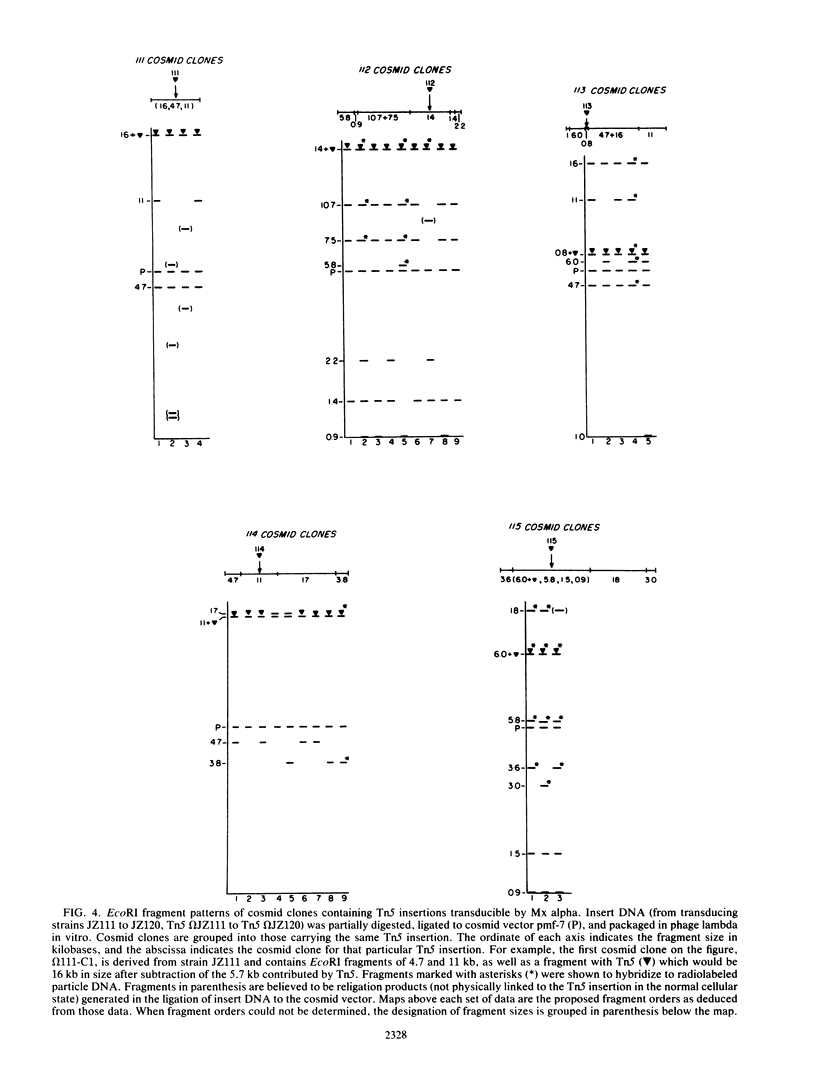
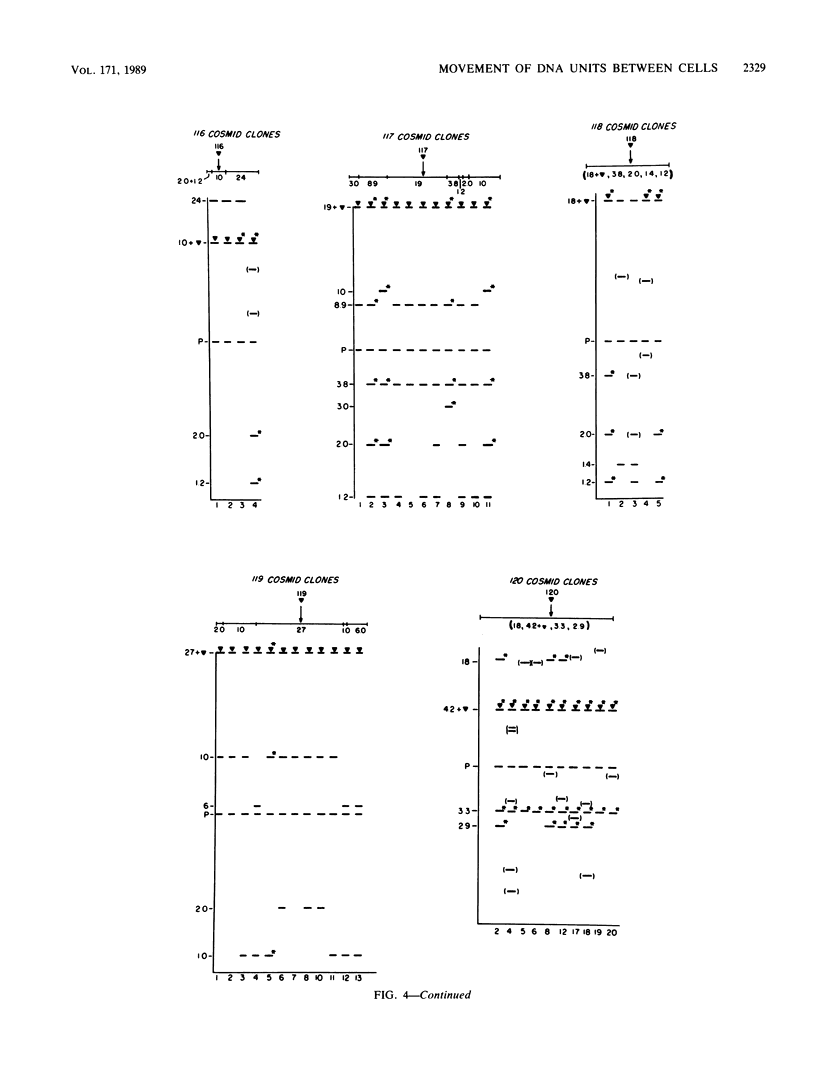
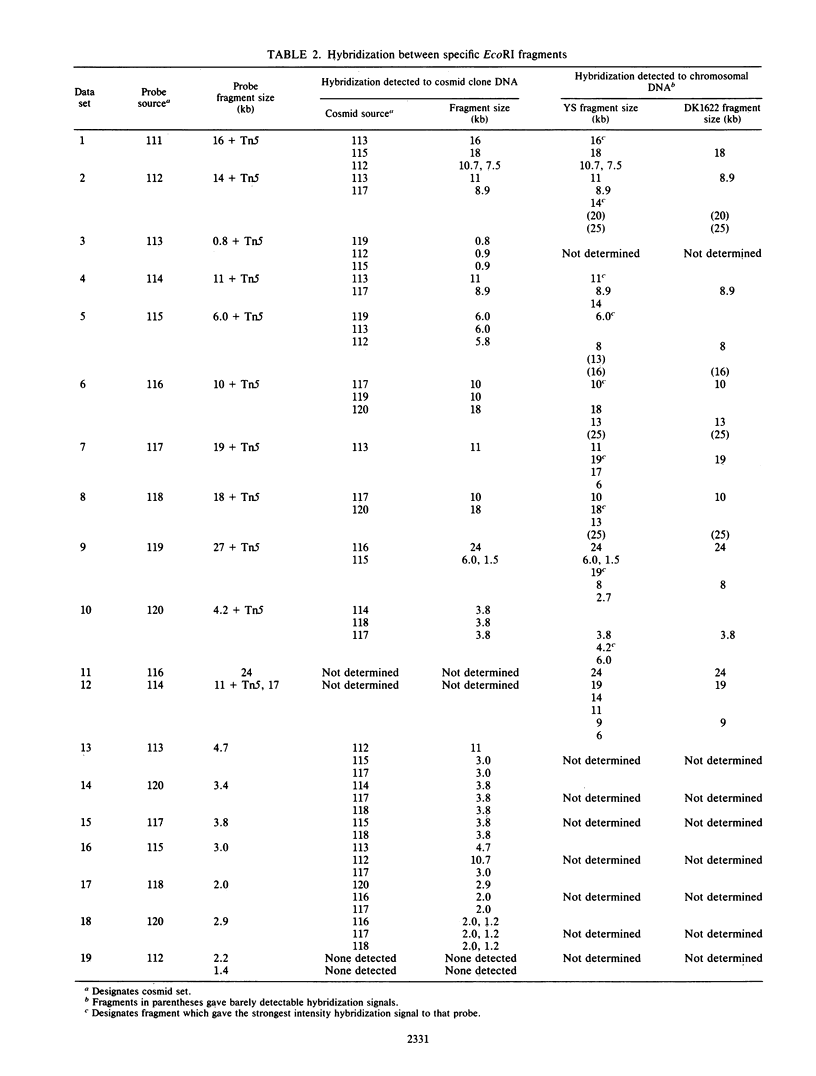
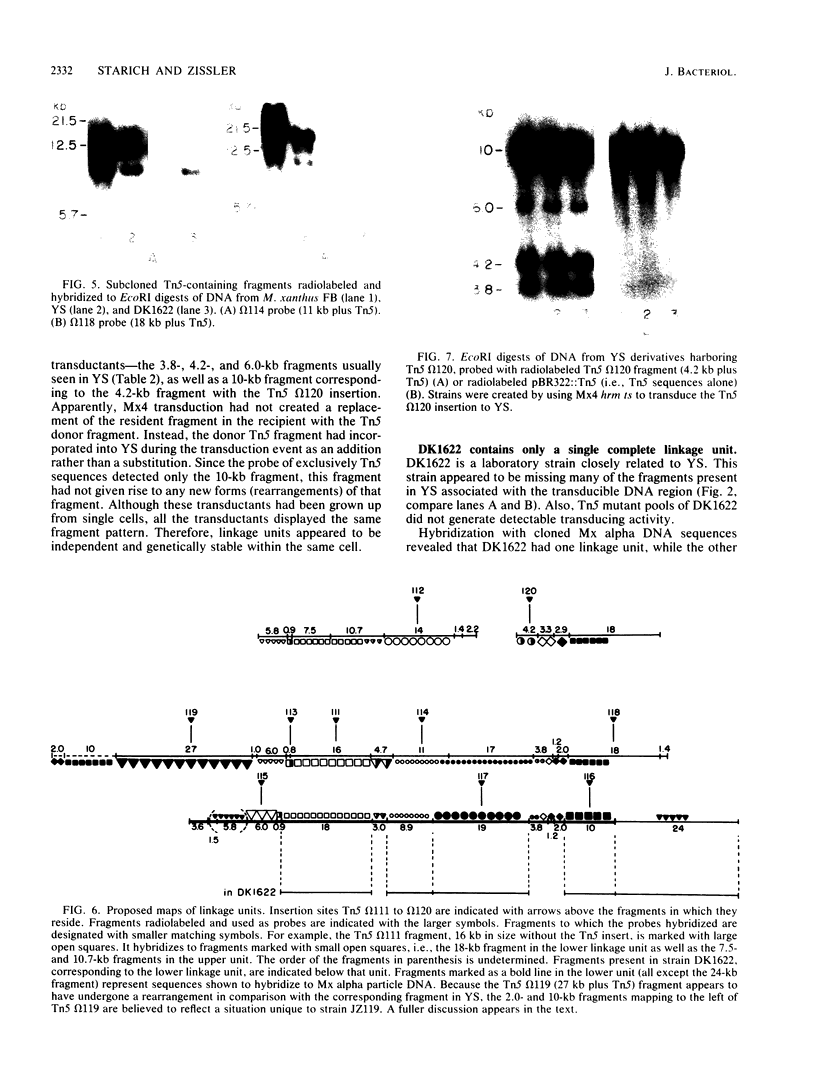
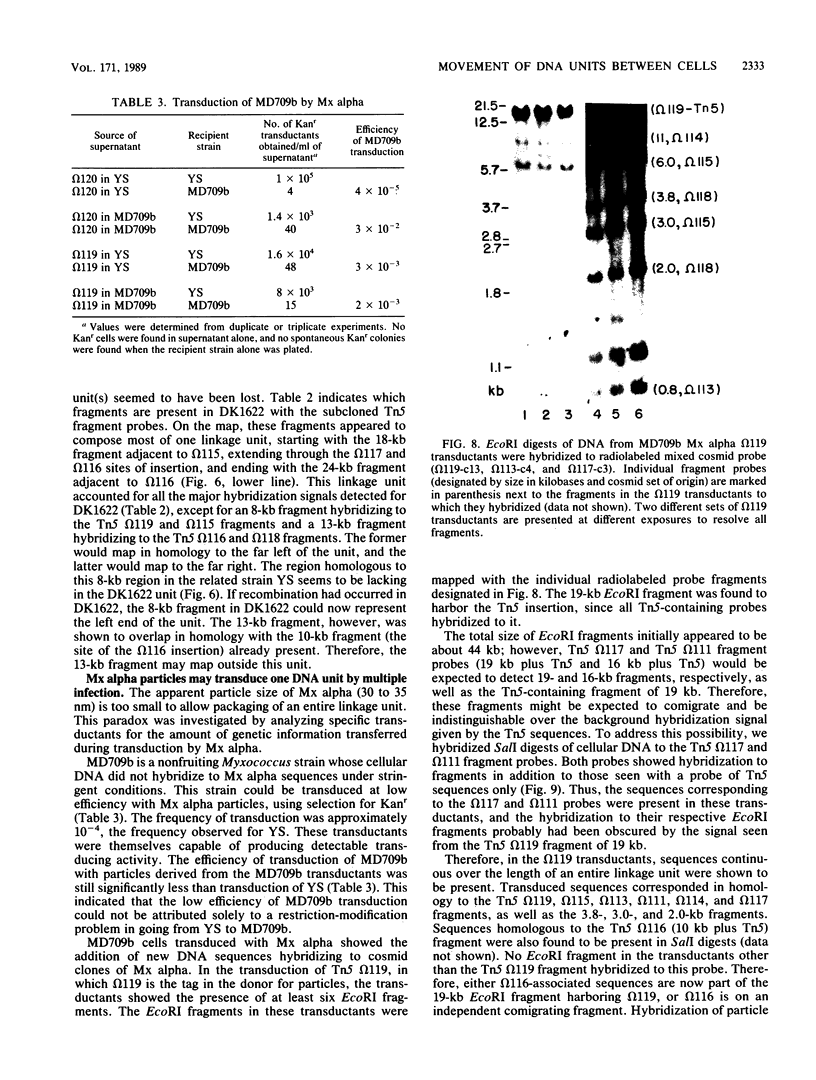
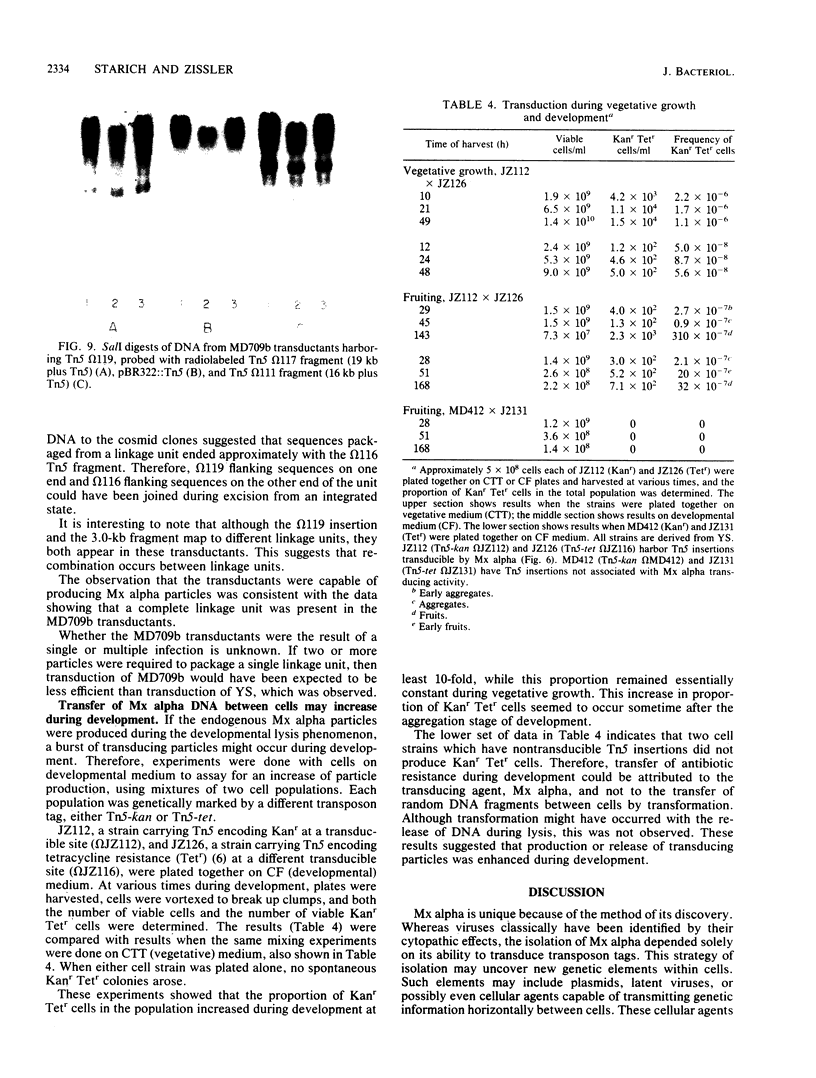
Myxococcus xanthus YS produces particles (Mx alpha particles) that transmit genetic information between cells. Mx alpha particles might be viruses, although no host able to sustain lytic growth of Mx alpha has been discovered. The particles could be detected by their ability to transduce a Tn5 transposon tag to recipient bacteria. DNA from purified particles hybridized to a limited number of DNA restriction fragments of strain YS, suggesting that Mx alpha particles contain only specific DNA sequences. A set of Tn5 insertions residing in the transducible region provided genetic markers for cloning cellular DNA packaged by Mx alpha. A map of this region showed that transducible DNA comprised multiple units of approximately 80 kilobases each. Individual units share DNA homology but are divergent in the location of restriction sites. Other wild-type isolates of Myxococcus species contained DNA sequences with homology to Mx alpha DNA, indicating that Mx alpha DNA is widespread in nature. Experiments on the transfer of Mx alpha DNA in strain YS suggested that DNA transfer is enhanced during the developmental cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campos J. M., Geisselsoder J., Zusman D. R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Introduction of transposon Tn5 into Myxococcus for analysis of developmental and other nonselectable mutants. Proc Natl Acad Sci U S A. 1981 Jan;78(1):425–429. doi: 10.1073/pnas.78.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Dworkin M. Separation and properties of the cytoplasmic and outer membranes of vegetative cells of Myxococcus xanthus. J Bacteriol. 1980 Feb;141(2):914–927. doi: 10.1128/jb.141.2.914-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Starich T., Cordes P., Zissler J. Transposon tagging to detect a latent virus in Myxococcus xanthus. Science. 1985 Nov 1;230(4725):541–543. doi: 10.1126/science.2996138. [DOI] [PubMed] [Google Scholar]
- Wireman J. W., Dworkin M. Developmentally induced autolysis during fruiting body formation by Myxococcus xanthus. J Bacteriol. 1977 Feb;129(2):798–802. doi: 10.1128/jb.129.2.798-802.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wireman J. W., Dworkin M. Morphogenesis and developmental interactions in myxobacteria. Science. 1975 Aug 15;189(4202):516–523. doi: 10.1126/science.806967. [DOI] [PubMed] [Google Scholar]