Abstract
Juvenile hormone esterase (JHE; EC 3.1.1.1), which is intrinsically involved in regulation of development of some insect larvae, is rapidly removed from the hemolymph by the pericardial cells. Lys-29 and Lys-524, which are implicated in the degradation of JHE, were mutated to Arg. Neither the half-life of the modified JHE in the hemolymph nor the catalytic parameters were changed significantly, but when combined, these mutations resulted in apparent failure of lysosomal targeting in the pericardial cell complex. A hypothesis for the mechanism of reduced efficiency of lysosomal targeting is presented. Infection of larvae with a recombinant baculovirus expressing the modified JHE resulted in a 50% reduction in feeding damage compared with larvae infected with the wild-type virus, thus demonstrating improved properties as a biological insecticide. These data demonstrate that alteration of specific residues of JHE that disrupted lysosomal targeting, dramatically increased the insecticidal activity of this protein.
Keywords: binding protein, insect control
One of the key regulatory molecules controlling insect development is juvenile hormone (JH; Fig. 1 Inset). This sesquiterpenoid is involved with regulation of larval development, metamorphosis, and reproduction in insects (1). The titer of JH is regulated by the rates of synthesis and degradation within the insect larva. One of the key enzymes involved in JH degradation (2) is juvenile hormone esterase (JHE; EC 3.1.1.1; Fig. 1). It has been postulated that an artificial increase in the titer of JHE and resulting decrease in the titer of JH would be detrimental to insect development and that this strategy might be exploited for the control of insect pests (3).
Figure 1.
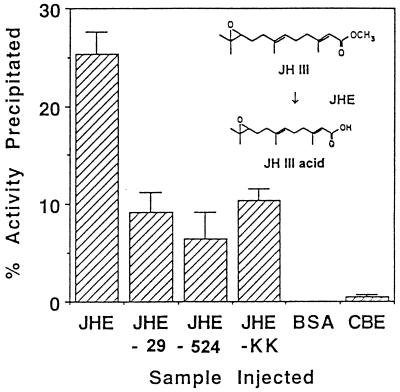
Immunoprecipitation of JHE with universal Hsp70 antiserum to quantify the extent of JHE-BP binding to modified forms of JHE. Larvae of M. sexta were injected with affinity-purified JHE or modified JHE, or the control samples BSA or CBE. Pericardial cells were removed 1 hr postinjection and homogenized in sample buffer. Aliquots of the homogenate were immunoprecipitated with the Hsp70 antiserum and Immunobead second antibody reagent. Data shown are for mean percent catalytic activity precipitated from three or four pericardial cell homogenates. (Inset) JH III is hydrolyzed to JH III acid by JHE of H. virescens with a kcat/Km that is indistinguishable from other JH homologs.
On the basis that overexpression of JHE at an inappropriate time would disrupt larval development, the cDNA sequence for JHE derived from the moth Heliothis virescens (4) was incorporated into a baculovirus expression vector for delivery of JHE to the insect (3). The insecticidal effects of the recombinant JHE produced by the baculovirus were slight and affected only first instar larvae (3). Pharmacokinetic analysis of JHE following injection into larvae suggests that JHE is cleared rapidly from the hemolymph (5). Investigations have shown that the tissue responsible for the clearance of JHE in lepidopteran larvae is the pericardial cell complex (5–7).
Previous studies have demonstrated that JHE associates with at least two proteins within the pericardial cell complex (8). The first of these binding proteins, JHE-BP, was found to cross-react with an antiserum raised to the ATPase fragment of Hsp70, but does not appear to be Hsp70 (8). Approximately 25% of the JHE activity in the pericardial cell complex was associated with JHE-BP 1 hr after injection.
If JHE is degraded in the pericardial cells, it is likely that either the ubiquitin or the lysosomal protein degradation pathway is involved. If this assumption is correct, it should be possible to affect these processes by specifically altering amino acid residues involved in recognition of JHE by either degradation pathway. Disruption of processes in the pericardial cells that result in degradation of JHE could improve the insecticidal efficacy of the recombinant baculovirus. A number of amino acid motifs have been implicated in lysosomal and/or ubiquitin targeting of proteins (9–11). Of particular note is the presence of a lysine residue proximal to the N terminus of mature proteins for ubiquitin conjugation (11), and the KFERQ-like sequence motif, which has been implicated in lysosomal targeting (9, 10). The sequence of JHE has two lysine residues that could be associated with degradation. Lysine residue 29 is near the N terminus, and lysine residue 524 is located within a KFERQ-like putative lysosome targeting sequence. Both of these lysine residues are in hydrophilic regions of the protein sequence, indicating that they are likely to be on the surface of the protein (12). Based on these observations the lysine residues at positions 29 and 524 in JHE were altered by site-directed mutagenesis to arginine (13, 14). The lysine residue in the putative lysosomal targeting sequence was selected for mutation because lysines are subject to ubiquitin conjugation, should ubiquitin-associated degradation of JHE occur. Based on previous studies, a single amino acid alteration of a KFERQ-like lysosomal targeting motif would result in reduced efficiency of lysosomal targeting (15).
In addition to control mutations (14), three modified forms of JHE were produced; JHE-29 and JHE-524 with single Lys-Arg mutations, and JHE-KK with both mutations. Recombinant Autographa californica nuclear polyhedrosis viruses (AcNPV) were generated to direct synthesis of each of the modified JHEs. We tested whether mutation of JHE at these specific residues resulted in (i) reduced uptake of JHE by the pericardial cells, (ii) reduced association with JHE-BP within the pericardial cells, and (iii) alteration of the intracellular location within the pericardial cells. In addition to elucidating the mechanisms of regulation of a key component in the endocrine control of insect development, disruption of normal intracellular processing could result in an insecticidal effect. Recombinant baculovirus insecticides that reduce feeding damage of pest species are extremely promising as novel insect pest control agents (16).
MATERIALS AND METHODS
Construction of AcJHE-29, AcJHE-524, and AcJHE-KK.
The JHE clone 3hv16B (3), which contains the 1,734-bp coding region without the 3′ untranslated region of the JHE cDNA (4), was used for construction of the modified JHEs. This clone was removed from the pBluescript phagemid vector by BglII digestion and recloned in the reverse orientation in the same vector. This procedure enabled removal of the coding region with either BglII or EcoRI, and rescue of the appropriate strand for annealing to the oligonucleotides. Site-directed mutagenesis was carried out (13) by using the oligonucleotides described in Ward et al. (14) to mutate Lys-29 to Arg and Lys-524 to Arg, to produce the modified proteins JHE-29 and JHE-524, respectively. All mutations were confirmed by double-stranded sequencing by using a Sequenase kit (United States Biochemical). For JHE-29, the JHE cDNA sequences were removed from pBluescript by BglII digestion and ligated into the BglII cloning site of the baculovirus transfer vector pAcUW21 (17). JHE-524 was removed from pBluescript by digestion with EcoRI, and cloned into the EcoRI site immediately adjacent to the BglII cloning site in pAcUW21. The double mutant JHE-KK was generated by inserting the fragment of JHE-29 with the K29R mutation directly into the JHE-524 clone from which the equivalent region had been removed. This was performed as follows. The JHE-524 clone in the baculovirus transfer vector pAcUW21 was digested with BamHI to release a DNA fragment that included a portion of the polyhedrin gene, the p10 and pol promoters, and the N-terminal region of JHE. This digest was treated with alkaline phosphatase (Boerhinger-Mannheim) to prevent religation. The transfer vector containing the JHE-29 mutant of JHE was digested with BamHI to excise the equivalent region. This fragment was gel-purified from an agarose gel using a Qiaex gel extraction kit (Qiagen, Chatsworth, CA) and ligated into the BamHI-digested JHE-524 transfer vector. The insertion of this fragment in the correct orientation replaced the portion of the polyhedrin gene and promoters removed by BamHI digestion as well as placed the K29R mutation into the same JHE clone as the K524R mutation, thus making the double mutant JHE-KK. The presence of both mutations and the orientation of the inserted fragment were confirmed by restriction endonuclease digestion.
The recombinant baculoviruses AcJHE-29, AcJHE-524, and AcJHE-KK were produced by cotransfection of cells derived from Spodoptera frugiperda (Sf21) with plasmid DNA and linearized DNA of the virus AcRP6-SC (18). Recombinant viruses were purified by plaque assay by using standard techniques (19, 20) and by assaying plaques for JHE activity (3).
Determination of Kinetic Parameters.
To ascertain whether the catalytic activity of the enzymes had unintentionally been affected by the lysine mutations, recombinant JHE, JHE-29, JHE-524, and JHE-KK were purified from insect cell culture by DEAE chromatography as described by Ichinose et al. (5). The recombinant enzymes were produced by infection of Sf21 cells (21) in 80-cm2 flat cultures. Medium was harvested 5 days postinfection. Cell-free medium was diluted 4-fold with distilled water and the pH adjusted to 8.5 with Tris⋅HCl at a final concentration of 2.5 mM. The diluted samples were purified using DEAE-Sepharose equilibrated in 10 mM Tris⋅HCl, pH 8.5, containing 50 mM NaCl and eluted in the same buffer using a gradient of 50–200 mM NaCl. The JHE-containing fractions were determined using the radiochemical assay for JHE (22) and concentrated using Centricon-30 microconcentrators (Amicon). The samples were equilibrated in 0.05 M sodium phosphate buffer during concentration. The Km, Vmax, and kcat were determined by using both the radiochemical substrate [3H]JH III (21), and the colorimetric substrate HEXTAT [S-(methyl) thiohexylthioethanoate] in a microtiter-plate format (23), with cell culture medium and BSA as control samples, as described previously (14). Conditions that gave linear dependence on both time and protein concentration were used. All assays were performed in triplicate.
In Vitro and in Vivo Expression of Modified JHEs.
The expression of the modified JHEs was compared with that of wild-type JHE in cell culture. Cultures of Sf21 cells were seeded at 1.5 × 106 cells in 35-mm Petri dishes and allowed to settle for 2 hr. Cells were infected with AcJHE-KK, AcJHE-29, AcJHE-524, or AcJHE at 10 pfu per cell in a volume of 100 μl. After 1 hr, the inoculum was removed and 2 ml ExCell 401 (JRH Biosciences, Lenexa, KS) with 100 units of penicillin and 100 μg streptomycin per ml, and 3% fetal calf serum (Intergen) was added. Because it previously had been demonstrated that 96–98% of active enzyme was exported in all cases, aliquots of medium only were removed every 4 to 8 hr and stored at −20°C prior to assay for enzyme activity using the colorimetric substrate HEXTAT (23) as described previously (24).
For analysis of expression of JHE, JHE-29, JHE-524, and JHE-KK in vivo, third instar larvae of Heliothis virescens were injected with 2 μl of virus (5 × 107 pfu/ml). Control larvae were injected with cell culture medium. Injected larvae were bled at 2 and 3 days postinfection with three or four larvae bled at each time point. Hemolymph samples were stored with a crystal of phenyl thiourea at −20°C prior to assay with the radiochemical assay for JHE activity.
Pharmacokinetic Analysis of JHE-KK.
Larvae of Manduca sexta were anesthetized with CO2 on the second day of the second instar (33–34 mg), and injected with DEAE-purified enzyme using a finely drawn microcapillary tube (5). At this stage, the hemolymph volume is 16–18 μl (5). Each larva was injected with 1 μl of purified enzyme with activity of 1–1.5 nmol JH III hydrolyzed/min per ml for JHE, JHE-29, JHE-524, or JHE-KK. The protein concentration of the injection sample was adjusted to 5 μg/ml with BSA. Hemolymph samples were taken from six larvae at 20 min, and 1, 2, 3, and 4 hr postinjection and diluted 1/25 in sodium phosphate buffer (pH 7.4), I = 0.2, 5% sucrose, 0.01% phenylthiourea, and 0.02% sodium azide. Samples were stored at −20°C before assay for JHE activity was performed using the radiochemical assay. The half-life of each enzyme was calculated using exponential regression analysis, and the rate constants for clearance were determined.
Immunoprecipitation of the JHE/JHE-BP Complex.
To test whether mutation of JHE at Lys-29 and/or Lys-524 would decrease the extent of binding to JHE-BP, immunoprecipitation experiments were conducted from pericardial cell homogenates. Fourth instar larvae of M. sexta were injected with 25 to 50 μl of buffer containing affinity-purified JHE or modified JHE (25) (1.5 to 10 μM [3H]JH III hydrolyzed/min per ml), or the control sample BSA (0.5 mg/ml). Pericardial cells were removed 1 hr postinjection and placed in 20 μl sample buffer (20 mM Tris⋅HCl, pH 6.8/150 mM NaCl/1 mM EDTA/1 mM phenylmethylsulfonyl fluoride) on ice. Pericardial cells were homogenized, and 5–15 μl of the homogenate (in a total volume of 15 μl) was immunoprecipitated with 1 μl of universal Hsp70 antiserum and Immunobead second antibody reagent (Bio-Rad; 200 μl). The universal Hsp70 antiserum was raised to the peptide sequence TVPAYFNDSQRQATDA, which is highly conserved in the ATPase fragment of the Hsp70 family from multiple taxa. Crude recombinant rabbit carboxylesterase (CBE; 20 μl; 47 mmol α-naphthyl acetate hydrolyzed/min per ml) from insect cell culture was also immunoprecipitated as a control enzyme using the same procedure. The amount of catalytic activity in (i) the original homogenate, (ii) the precipitated pellet, and (iii) the supernatant was determined by radiochemical assay for JHE and BSA injections, or by using α-naphthyl acetate for CBE precipitations. Determination of the amount of catalytic activity in the homogenate prior to immunoprecipitation allowed detection of any loss of activity during the immunoprecipitation process.
Electron Microscope Analysis of Wild-Type and Modified JHE.
For analysis of the intracellular location of wild-type and modified JHE, larvae were injected with 106 pfu on the second and third days of the fourth instar with the wild-type AcNPV, or with the recombinant viruses AcJHE, AcJHE-29, AcJHE-524, or AcJHE-KK. Infected larvae were dissected 6 days postinitial infection. Tissues were fixed by using aldehyde fixation without osmium tetroxide fixation to enhance antiserum binding, and were embedded in LR White resin (London Resin, Basingstoke, U.K.). Sections were immunogold-labeled by using JHE antiserum or preimmune serum as described previously (7). The JHE antiserum that was raised to JHE derived from H. virescens does not recognize the JHE of M. sexta under the conditions employed as illustrated previously (8). This is due in part to the low titers of JHE present in M. sexta at early fourth instar. At least four insects (16 sections of the pericardial cell complex per insect) were examined by electron microscope for each treatment. The degree of immunolabeling of the lysosomes was quantified by using a 0.078-μm2 quadrat placed at random over lysosome images (n = 13 for each virus) from different sections of different insects. Mean and standard deviation values were calculated for immunogold labeling per μm2.
Bioassay of Recombinant Baculoviruses.
For analysis of feeding damage caused to lettuce by uninfected first instar larvae of H. virescens, or larvae infected with wild-type virus or recombinant viruses, 30 neonate larvae were infected per virus with 2,000 polyhedra/μl by droplet feeding (26). Larvae were transferred individually to inverted Petri dishes containing damp filter paper and a piece of iceburg lettuce. Dishes were sealed with parafilm and maintained at 26°C. Lettuce leaves were changed every 48 hr and the area scanned before and after feeding using a CI-202 area meter (CID, Inc., Vancouver, WA). Data were analyzed by one-way ANOVA followed by the Fisher PLSD means comparison test (27).
RESULTS
Determination of Kinetic Parameters.
The kinetic parameters Km, Vmax, and kcat were determined for JHE, JHE-29, JHE-524, and JHE-KK using [3H]JH III (22) or HEXTAT (23) as substrates. For both substrates, the kinetic constants for JHE-KK were similar to those of the recombinant wild-type JHE, and JHE-29 and JHE-524 with single mutations (Table 1). When enzymes assayed at the same time were compared in a pair-wise fashion, no major differences were found. Slight differences between the wild-type and mutant proteins are attributed to variations among preparations of the recombinant protein. A 2-fold variation in kinetic parameters was seen among multiple preparations of the same protein.
Table 1.
Kinetic parameters for wild-type and modified JHE
| Substrate | Enzyme | Km, μM | Vmax, μmol/min per mg | kcat, min−1 |
|---|---|---|---|---|
| [3H]JH III | JHE | 0.03 ± 0.01 | 1.57 ± 0.06 | 104 |
| JHE-29 | 0.05 ± 0.01 | 1.89 ± 0.05 | 125 | |
| JHE-524 | 0.06 ± 0.01 | 3.00 ± 0.02 | 198 | |
| JHE-KK | 0.02 ± 0.001 | 2.01 ± 0.02 | 133 | |
| HEXTAT* | JHE | 46 ± 6 | 55 ± 3 | 3,649 |
| JHE-29 | 49 ± 7 | 67 ± 4 | 4,466 | |
| JHE-524 | 77 ± 19 | 118 ± 14 | 7,800 | |
| JHE-KK | 59 ± 11 | 44 ± 3 | 2,904 |
Mean ± SD values are given for six replicates on a single recombinant protein preparation.
HEXTAT is S-(methyl) thiohexylthioethanoate, a colorimetric substrate.
In Vitro and in Vivo Expression of Recombinant JHEs.
The in vitro and in vivo expression of the recombinant JHEs was determined to ensure that the mutations made did not affect the level of expression from the recombinant baculoviruses, compared with expression of wild-type JHE. The in vitro expression of JHE, JHE-29, JHE-524, and JHE-KK by the recombinant baculoviruses was compared by monitoring JHE activity in cell culture medium in Petri dishes, every 4 hr up to 96 hr postinfection, as described previously (24). Expression of the modified JHEs in cell culture was not significantly different from expression of wild-type JHE, with maximum activities of 30 ± 5 nmol JH III hydrolyzed/min per ml medium by 72 hr postinfection (data not shown). Activities were within 20% of each other at each time point. Analysis of in vivo expression of the recombinant enzymes following injection of non-occluded virus into third instar larvae of H. virescens showed that the expression of the modified JHEs was not significantly different from expression of the recombinant wild-type JHE by 3 days postinfection (data not shown). The activity detected by 3 days postinfection was approximately 3 μmol JH III hydrolyzed/min per ml hemolymph following injection with 105 pfu recombinant virus. Activity levels in the hemolymph of larvae injected with medium were not above background levels for the radiochemical assay.
Pharmacokinetic Analysis.
The catalytic activity of injected JHE-29, JHE-524, and JHE-KK was cleared rapidly from the hemolymph of larvae of M. sexta. Clearance followed first order kinetics. Activity detected in control larvae was less than 1 nmol JH III hydrolyzed/min per ml. The rate constant of clearance for JHE-29, JHE-524, and JHE-KK was similar to that of wild-type JHE with a half-life of approximately 40 min (Table 2).
Table 2.
Pharmacokinetic parameters* for clearance of JHE following injection into the hemolymph of Manduca sexta
| Enzyme | Rate constant of clearance,† hr−1 | Mean t½‡ ±SD, min |
|---|---|---|
| JHE | 1.28 | 37.3 ± 6.6 |
| 0.98 | ||
| 0.90 | ||
| JHE-29 | 0.93 | 45.5 ± 1.2 |
| 0.89 | ||
| 1.15 | ||
| JHE-524 | 1.34 | 34.2 ± 4.8 |
| 0.89 | ||
| 0.72 | ||
| JHE-KK | 1.51 | 46.2 ± 16 |
| 1.05 | ||
| 0.97 |
JHE was injected into hemolymph of larval M. sexta. Clearance of catalytic activity from the hemolymph was monitored up to 4 h postinjection by determination of the levels of JHE activity using JH III in a standard assay.
Plasma concentration P at any time t (hr) is given by P = P0e−kt, where P0 is the initial plasma concentration and k is the rate constant of clearance.
Data are presented for three experiments for each JHE type. Six larvae were bled to determine hemolymph activity at each of five time points for each experiment.
Immunoprecipitation of JHE.
Approximately 25% of the recombinant wild-type JHE activity in the homogenate of pericardial cells was associated with JHE-BP and was immunoprecipitated with the Hsp70 antiserum, compared with 5–10% for JHE-29, JHE-524, and JHE-KK (Fig. 1). The percentage of activity precipitated following injection of JHE-29, JHE-524, or JHE-KK was not significantly different. Data shown (Fig. 1) are for mean percent catalytic activity precipitated for precipitations from three or four pericardial cell homogenates. Following immunoprecipitation from pericardial cells from larvae injected with BSA, the JHE activity detected was not above background levels. Five-tenths percent of the total CBE activity (935 μmol α-naphthyl acetate hydrolyzed/min per 20 μl) was precipitated with the Hsp70 antiserum. Analysis of catalytic activity in the pericardial cell homogenate prior to immunoprecipitation indicated that JHE activity recovered following immunoprecipitation was greater than 80% of the initial activity in the homogenate. Neither antiserum nor immunobeads affected the catalytic activity of JHE.
Electron Microscopic Analysis.
There was no evidence of virus infection of the pericardial cell complex 6 days postinfection of fourth instar larvae under the conditions described. Following infection with AcJHE, JHE was concentrated in the lysosomes of the pericardial cells with a mean of 177 ± 48 gold particles/μm2 (n = 13: Fig. 2A). The intracellular localization of JHE-29 and JHE-524 was the same as for wild-type JHE. In contrast, JHE-KK had a mean density of only 33 ± 16 gold particles/μm2 in the lysosomes (n = 13: Fig. 2B). These values were determined for micrographs with comparable amounts of JHE antigen. Background labeling of lysosomes that was detected following infection with wild-type virus was 8 ± 9 gold particles/μm2 (n = 13) and by using preimmune serum was 11 ± 8 gold particles/μm2 (n = 13).
Figure 2.
Distribution of JHE (A) and JHE-KK (B) within the pericardial cells. JHE is concentrated in the lysosomes at a fivefold greater density than JHE-KK (177 ± 48 compared with 33 ± 16 gold particles/μm2, respectively: P < 0.05). There were no significant differences detected between the intracellular distribution of JHE-29, JHE-524, and JHE. At least 64 sections from a total of four or more insects were examined for each treatment.
Bioassay of Recombinant Baculoviruses.
A 50% reduction in feeding damage was seen on lettuce for insects infected with AcJHE-KK relative to damage caused by insects infected the wild-type virus [probable least-squares difference (PLSD) = 0.588; P < 0.05: Fig. 3]. Feeding damage caused by larvae infected with AcJHE-29 or AcJHE-524 was not significantly different from that caused by larvae infected with the wild-type virus. Under these conditions the recombinant virus expressing an insect-selective scorpion toxin, AcAaIT (28, 29), gave a 73% reduction in feeding damage compared with the wild-type virus (PLSD = 0.582; P < 0.05), which is not significantly different from the damage caused by larvae infected with AcJHE-KK (PLSD = 0.588; P > 0.05).
Figure 3.
Feeding damage caused to lettuce by uninfected first instar larvae of H. virescens, or larvae infected with wild-type virus or AcJHE-KK. Thirty neonate larvae were infected per virus. A 50% reduction in feeding damage was seen for insects infected with AcJHE-KK relative to damage caused by insects infected the wild-type virus (PLSD = 0.588; P < 0.05).
DISCUSSION
We have tested the hypothesis that site-directed mutagenesis of JHE could be used to disrupt the intracellular processing and/or degradation of JHE by the pericardial cells of M. sexta. Initially, kinetic parameters were determined for the modified JHEs to confirm that the catalytic properties of JHE were not dramatically altered by mutation at Lys-29 and/or Lys-524. Examination of in vivo and in vitro expression of catalytically active enzyme indicated that mutation of JHE did not significantly affect baculovirus expression when compared with expression of wild-type JHE. Pharmacokinetic analysis was then used to determine whether mutagenesis of JHE had altered the rate of removal of the enzyme from the hemolymph by the pericardial cells. The rate of removal of the modified JHEs from the hemolymph was not significantly different from the rate of removal of wild-type JHE. This observation suggests that Lys-29 and Lys-524 do not play a critical role in the uptake of JHE from the hemolymph by the pericardial cells.
To test whether mutation of JHE resulted in decreased association of JHE with JHE-BP in the pericardial cells, immunoprecipitation experiments were carried out. Mutation of Lys-29 and/or Lys-524 resulted in a decreased proportion of the active JHE being associated with JHE-BP: 5–10% of the active, modified JHEs in the pericardial cells was associated with JHE-BP, compared with 25% for wild-type JHE. The function of JHE-BP in the pericardial cells is unknown. Previous studies suggest that JHE is removed from the hemolymph by receptor-mediated endocytosis rather than by the ubiquitin-based degradation pathway (5, 8). In this case JHE would bind to a cell-surface receptor, be internalized in clathrin coated pits, dissociate from the receptor in the acid environment of the early endosome, and be transported in an endosomal carrier vesicle (ECV) to the late endosome, where degradation would begin (30). The data suggest that JHE-BP is not the JHE membrane receptor, since reduced association of JHE-BP with JHE for all three of the mutants (Fig. 1) does not correlate with a reduction in the rate of removal of JHE from the hemolymph (Table 2), as would be expected if JHE-BP were the JHE receptor. It has been stated that ligand-binding proteins are likely to be involved in endosomal sorting of proteins destined for ECV transport to the late endosome (30). Whether or not JHE-BP plays a role in this sorting process remains to be determined.
For sections with a comparable amount of immunogold labeling, fivefold less JHE-KK was seen in the lysosomes compared with JHE, JHE-29, and JHE-524 (Fig. 2). This suggests that the intracellular processing of JHE-KK has been affected by mutation of both Lys-29 and Lys-524. We hypothesize that these two mutations have disrupted the endosomal sorting process. This would account for the observations that uptake from the hemolymph is not affected by the mutations made, and that the efficiency of JHE-KK transport to the lysosome is significantly reduced compared with that for wild-type JHE.
Assessment of feeding damage caused by larvae infected with the recombinant baculoviruses expressing wild-type or modified JHEs indicates that JHE-KK has insecticidal activity. Larvae infected with the virus AcJHE-KK caused 50% less damage to lettuce compared with larvae that were infected with the wild-type virus. The viruses AcJHE-29 and AcJHE-524 did not show enhanced insecticidal activity relative to the wild-type virus or virus expressing wild-type JHE. The insecticidal activity of JHE-KK is correlated with reduced efficiency of lysosomal targeting of JHE-KK in the pericardial cells. If JHE-KK accumulates in the cytoplasm of the pericardial cells, it could reduce the titer of JH in these cells. Because JH has been implicated in regulation of gene expression (31), a localized anti-JH effect might affect protein synthesis by the pericardial cells (32).
The reduced association of the JHE mutants with JHE-BP does not correlate with decreased efficiency of lysosomal targeting or insecticidal activity, which were seen only for JHE-KK. This fact suggests that decreased association of JHE-KK with JHE-BP may be unrelated to, or provides only a partial explanation for, the reduced efficiency of lysosomal transport and the insecticidal effect. The precise mechanism of the insecticidal activity of JHE-KK, the details of the receptor-mediated endocytosis pathway in insects, and the role, if any, of the putative lysosome targeting sequence in this process remain to be established.
Numerous studies implicate JHE as an important enzyme in the degradation and thus regulation of JH. This study provides evidence that some insects also have a sophisticated mechanism for the regulation of JHE. From an applied perspective, we have demonstrated that an insecticidal agent can be generated by minor modification of an insect protein that does not normally exhibit toxicity. Disruption of lysosomal targeting of other insect proteins, particularly those involved in regulation of larval development or maintenance of homeostasis, represents a new approach for generation of novel insecticidal agents.
Acknowledgments
We thank K. Hoover, Dr. A. Parker, Dr. T. Shiotsuki, Dr. T. Huang, A. Brockhouse, and Dr. Dusan Cmarko for technical assistance with various aspects of this project. We thank Dr. F. Matsumura of University of California, Davis, for provision of the universal Hsp70 antiserum. This is paper no. J-17163 of the Iowa Agriculture and Home Economics Experiment Station (Ames, Iowa), project no. 3301, which was supported by Hatch Act and State of Iowa funds. This work was supported by grants from the National Science Foundation (DCB-91-19332), the U.S. Department of Agriculture (91-37302-6186), the U.S. Department of Agriculture Forest Service (23-696), University of California Systemwide Biotechnology Program, and the North Atlantic Treaty Organization (CRG 951318). B.D.H. was supported by a McMaster’s Fellowship from the Commonwealth Scientific and Industrial Research Organization and a Fellowship from the National Science Foundation.
ABBREVIATIONS
- JHE
juvenile hormone esterase
- JHE-BP
JHE binding protein
- CBE
carboxylesterase
- PLSD
probable least-squares difference
References
- 1.Riddiford L M. Adv Insect Physiol. 1994;24:213–274. [Google Scholar]
- 2.Hammock B D. In: Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Kerkut G A, Gilbert L I, editors. New York: Pergamon; 1985. pp. 431–472. [Google Scholar]
- 3.Hammock B D, Bonning B C, Possee R D, Hanzlik T N, Maeda S. Nature (London) 1990;344:458–461. [Google Scholar]
- 4.Hanzlik T N, Abdel-Aal Y A I, Harshman L G, Hammock B D. J Biol Chem. 1989;264:12419–12425. [PubMed] [Google Scholar]
- 5.Ichinose R, Kamita S G, Maeda S, Hammock B D. Pestic Biochem Physiol. 1992;42:13–23. [Google Scholar]
- 6.Ichinose R, Nakamura A, Yamoto T, Booth T F, Maeda S, Hammock B D. Insect Biochem Mol Biol. 1992;22:893–904. [Google Scholar]
- 7.Booth T F, Bonning B C, Hammock B D. Tissue Cell. 1992;24:267–282. doi: 10.1016/0040-8166(92)90100-l. [DOI] [PubMed] [Google Scholar]
- 8.Bonning B C, Booth T F, Hammock B D. Arch Insect Biochem Physiol. 1997;34:275–286. doi: 10.1002/(SICI)1520-6327(1997)34:3<275::AID-ARCH3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Chiang H L, Terlecky S R, Plant C P, Dice J F. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 10.Dice J F. Trends Biochem Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 11.Bachmair A, Varshavsky A. Cell. 1989;56:1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 12.Chou P Y, Fasman G D. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- 13.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward V K, Bonning B C, Huang T, Shiotsuki T, Griffith V N, Hammock B D. Int J Biol Chem. 1992;24:1933–1941. doi: 10.1016/0020-711x(92)90289-d. [DOI] [PubMed] [Google Scholar]
- 15.Terlecky S R, Chiang H L, Olson T S, Dice J F. J Biol Chem. 1992;267:23490–23495. [Google Scholar]
- 16.Miller L K. J Invert Pathol. 1995;65:211–216. doi: 10.1006/jipa.1995.1032. [DOI] [PubMed] [Google Scholar]
- 17.Bishop D H L. Sem Virol. 1992;3:253–264. [Google Scholar]
- 18.Kitts P, Ayres M D, Possee R D. Nucleic Acids Res. 1990;18:5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King L A, Possee R D. The Baculovirus Expression System. London: Chapman & Hall; 1992. [Google Scholar]
- 20.O’Reilly D R, Miller L K, Luckow V A. Baculovirus Expression Vectors: A Laboratory Manual. New York: Freeman; 1992. [Google Scholar]
- 21.Vaughn J L, Goodwin R H, Tompkins G J, McCawley P. In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 22.Hammock B D, Sparks T C. Anal Biochem. 1977;82:573–579. doi: 10.1016/0003-2697(77)90196-8. [DOI] [PubMed] [Google Scholar]
- 23.McCutchen B F, Uematsu T, Székács A, Huang T L, Shiotsuki T, Lucas A, Hammock B D. Arch Biochem Biophys. 1993;307:231–241. doi: 10.1006/abbi.1993.1584. [DOI] [PubMed] [Google Scholar]
- 24.Bonning B C, Hammock B D. J Virol Methods. 1995;51:103–114. doi: 10.1016/0166-0934(94)00095-x. [DOI] [PubMed] [Google Scholar]
- 25.Shiotsuki T, Huang T L, Uematsu T, Bonning B C, Ward V K, Hammock B D. Protein Expression Purif. 1994;5:296–306. doi: 10.1006/prep.1994.1045. [DOI] [PubMed] [Google Scholar]
- 26.Hughes P R, van Beek N A M, Wood H A. J Invert Pathol. 1986;48:187–92. [Google Scholar]
- 27.Steel R G D, Torrie J H. Principles and Procedures in Statistics: A Biometrical Approach. New York: McGraw–Hill; 1980. [Google Scholar]
- 28.Stewart L M D, Hirst M, Ferber M L, Merryweather A T, Cayley P J, Possee R D. Nature (London) 1991;352:85–88. doi: 10.1038/352085a0. [DOI] [PubMed] [Google Scholar]
- 29.McCutchen B F, Choudary P V, Crenshaw R, Maddox D, Kamita S G, Palekar N, Volrath S, Fowler E, Hammock B D, Maeda S. Bio/Technology. 1991;9:848–852. doi: 10.1038/nbt0991-848. [DOI] [PubMed] [Google Scholar]
- 30.Mellman I. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 31.Jones G. Annu Rev Entomol. 1995;40:147–169. doi: 10.1146/annurev.en.40.010195.001051. [DOI] [PubMed] [Google Scholar]
- 32.Fife H G, Palli S R, Locke M. Insect Biochem. 1987;17:829–840. [Google Scholar]