Abstract
Although apoptosis has been extensively studied in developing neurons, the dynamic changes in this pathway after neuronal maturation remain largely unexplored. We show that as neurons mature, cytochrome c– mediated apoptosis progresses from inhibitor of apoptosis protein–dependent to –independent regulation because of a complete loss of Apaf-1 expression. However, after DNA damage, mature neurons resynthesize Apaf-1 through the cell cycle–related E2F1 pathway and restore their apoptotic potential. Surprisingly, we find that E2F1 is sufficient to induce Apaf-1 expression in developing but not mature neurons. Rather, Apaf-1 up-regulation in mature neurons requires both chromatin derepression and E2F1 transcriptional activity. This differential capacity of E2F1 to induce Apaf-1 transcription is because of the association of the Apaf-1 promoter with active chromatin in developing neurons and repressed chromatin in mature neurons. These data specifically illustrate how the apoptotic pathway in mature neurons becomes increasingly restricted by a novel mechanism involving the regulation of chromatin structure.
Introduction
Although apoptosis occurs extensively during neuronal development, it is rarely observed and presumably no longer desired in the mature brain (for review see Benn and Woolf, 2004). However, apoptosis has been observed in adult neurons in response to several pathological situations including neurodegenerative diseases and brain injury, and as a side effect of chemotherapy (Lazarus et al., 1981; Yuan and Yankner, 2000). How the apoptotic pathway is restricted in mature neurons and how these restrictions are overcome after pathological stimuli is unclear.
Multiple cellular stressors cause activation of the caspase proteases and apoptosis in neurons (Putcha and Johnson, 2004). The intrinsic pathway of apoptosis is regulated by the Bcl-2 family of proteins, which control mitochondrial release of cytochrome c. Once in the cytosol, cytochrome c binds to Apaf-1 to form the apoptosome complex. Recruitment of procaspase-9 to the apoptosome results in its activation, allowing it to cleave and activate executioner caspases, such as caspase-3, which orchestrate the destruction of the cell (Boatright and Salvesen, 2003). Using knockout mice, we have previously demonstrated that Apaf-1, caspase-9, and caspase-3 are necessary for cytochrome c–induced apoptosis in sympathetic neurons (Wright et al., 2007).
Developing sympathetic neurons (postnatal day 5 [P5]) are acutely dependent on NGF for survival and undergo Bax-mediated cytochrome c release and caspase activation when deprived of NGF (Putcha and Johnson, 2004). In contrast, mature sympathetic neurons (P28) fail to undergo apoptosis after NGF deprivation because of the inability of Bax to translocate to the mitochondria (Easton et al., 1997; Putcha et al., 2000; Orike et al., 2001). Whether other fundamental differences exist in the regulation of apoptosis between developing and mature sympathetic neurons remains unclear.
Cytochrome c–dependent apoptosis in developing sympathetic neurons relies on overcoming endogenous X-linked inhibitor of apoptosis protein (XIAP), which inhibits active caspases. Cytosolic cytochrome c alone is incapable of inducing apoptosis in developing P5 sympathetic neurons (Deshmukh and Johnson, 1998; Neame et al., 1998) unless XIAP is inactivated (Potts et al., 2003). Thus, developing sympathetic neurons engage a XIAP-mediated “safety brake” that ensures they do not undergo apoptosis unless required.
Here, we find that neurons acquire an additional, inhibitor of apoptosis protein (IAP)–independent resistance to apoptosis as they mature. Importantly, we identify chromatin modification as important mechanisms by which apoptotic resistance is regulated in maturing neurons.
Results and discussion
Mature sympathetic neurons restrict their cytochrome c–dependent apoptotic pathway by a mechanism independent of IAPs
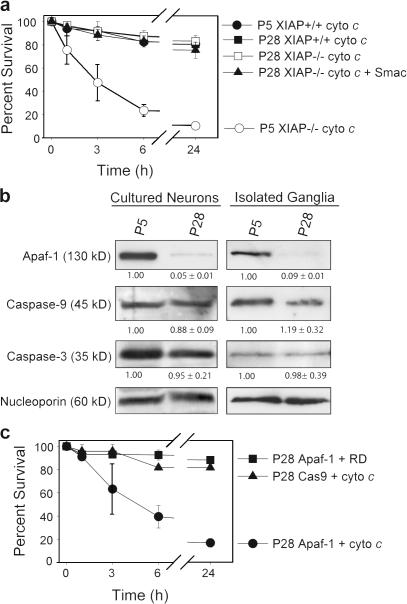
In developing P5 neurons, apoptosome-dependent caspase activation is strictly regulated by endogenous XIAP, as cytochrome c is insufficient to induce apoptosis in wild-type neurons but does so effectively in XIAP-deficient neurons (Fig. 1 a; Potts et al., 2003). Surprisingly, XIAP-deficient mature P28 neurons remained completely resistant to injection of cytochrome c (Fig. 1 a). This resistance was not caused by increased regulation by other IAPs, as coinjection of cytochrome c and second mitochondria-derived activator of caspases (Smac; an inhibitor of IAPs) was unable to induce apoptosis (Fig. 1 a). These results indicate that the apoptotic pathway in mature P28 neurons becomes further restricted by mechanisms independent of IAPs.
Figure 1.
Mature sympathetic neurons develop an IAP- independent restriction of the apoptosome pathway because of a loss of Apaf-1 expression. (a) P5 and 28 sympathetic neurons were isolated from XIAP−/− mice or wild-type littermates (XIAP+/+) and injected with cytochrome c or cytochrome c and Smac. After injection, cell viability was assessed at the indicated time points. (b) Wild-type sympathetic neurons were maintained in culture until the P5 or 28 equivalent or superior cervical ganglia were isolated from P5 and 28 mice. Protein levels were analyzed by Western blotting. Densitometry of protein levels are represented as fold change ± SEM and normalized to nucleoporin levels. (c) Wild-type P28 sympathetic neurons were injected with constructs encoding EGFP and either Apaf-1 or procaspase-9. After 24 h, expressing cells were injected with rhodamine dextran (RD) with or without cytochrome c and survival was assessed at the indicated time points. Error bars denote ± SEM.
To determine the underlying mechanism of resistance to cytochrome c in mature neurons, we compared levels of proapoptotic proteins in developing P5 and mature P28 neurons. To obtain P5 neurons, P0 neurons were isolated and cultured for 5 d in vitro, and P6–13 neurons were maintained in culture until the P28 equivalent before experimentation. Although the levels of caspase-9 and -3 remained relatively unchanged, the levels of Apaf-1, which were already low in P5 neurons (Wright et al., 2004), decreased to nearly undetectable levels in P28 neurons (Fig. 1 b). A similar loss of Apaf-1 expression was seen in sympathetic ganglia isolated from P28 mice (Fig. 1 b), confirming the observations in cultured neurons. These results are consistent with previous papers showing a marked reduction in Apaf-1 levels in the adult cortex (Yakovlev et al., 2001) and retina (Donovan and Cotter, 2002).
We tested whether restoration of Apaf-1 would sensitize these cells to cytochrome c. P28 neurons were injected with cDNAs expressing GFP and either Apaf-1 or procaspase-9 (as a control), and, after 24 h, to allow for DNA expression, GFP-expressing cells were injected with rhodamine dextran alone or rhodamine dextran and cytochrome c. P28 neurons (both wild type and XIAP deficient) expressing Apaf-1 became sensitive to cytochrome c, indicating that restoring Apaf-1 expression alone was sufficient to restore apoptosome function in mature neurons (Figs. 1 c and S1 a, available at http://www.jcb.org/cgi/content/full/jcb.200708086/DC1).
DNA damage restores the ability of mature sympathetic neurons to undergo apoptosis through transcriptional up-regulation of Apaf-1
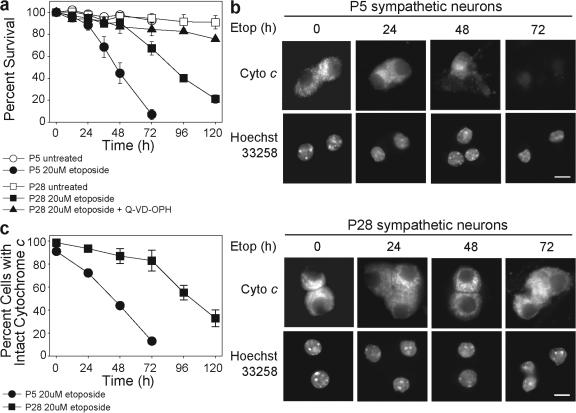
Despite this resistance to cytochrome c, mature neurons are capable of dying in response to DNA damage (Besirli et al., 2003), albeit slower than developing neurons (Fig. 2 a). Etoposide-induced apoptotic death in mature neurons was effectively blocked with broad spectrum caspase inhibitors Q-VD-OPH and zVAD-fmk (Figs. 2 and S1 b). Interestingly, the time course of cytochrome c release (Fig. 2, b and c) was coincident with the time course of death (Fig. 2 a) in both developing and mature neurons, indicating that apoptosis in mature neurons is slower because of the delayed release of cytochrome c.
Figure 2.
Mature sympathetic neurons release cytochrome c and undergo apoptosis in response to DNA damage. (a) P5 and 28 sympathetic neurons were either left untreated or treated with etoposide. Where indicated, the caspase inhibitor Q-VD-OPH was added. Cell viability, indicated by intact phase-bright cell bodies, was assessed at the indicated time points. (b) Status of cytochrome c in etoposide-treated P5 and 28 neurons was assessed by immunohistochemistry for the indicated times. Mitochondrial cytochrome c retains an intact punctate staining pattern, which disappears after release to the cytosol. Nuclei were stained with Hoechst 33258 and Q-VD-OPH was added to the cultures to maintain the nuclei. Bar, 15 μm. (c) Quantification of P5 and 28 sympathetic neurons with intact mitochondrial cytochrome c after etoposide treatment for the indicated times. Error bars denote ± SEM.
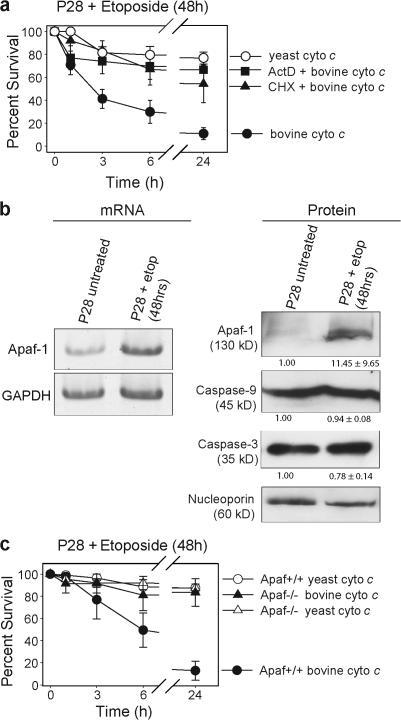
Etoposide-treated P28 neurons die shortly after the time of cytochrome c release, indicating that they are permissive for caspase activation by this point. To determine the time course by which caspase activation became permissive, mature neurons were treated with etoposide for 24 or 48 h, which are time points before endogenous cytochrome c release (Fig. 2, b and c), and injected with cytochrome c. Single cell microinjection of cytochrome c allows bypass of any restrictions upstream of the mitochondria and direct focus on assessing the ability of the apoptosome to induce apoptosis. Mature neurons treated with etoposide for 24 h remained resistant (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200708086/DC1); however, at 48 h they underwent rapid apoptosis after injection of cytochrome c (Fig. 3 a). Injecting yeast cytochrome c, which is unable to induce apoptosome formation, did not result in significant death. Notably, P28 neurons treated with etoposide in the presence of either actinomycin D to inhibit transcription or cycloheximide to inhibit protein synthesis remained resistant to cytochrome c (Fig. 3 a). This indicates that both transcription and translation are required for sensitization to cytochrome c–induced apoptosis after DNA damage in mature neurons.
Figure 3.
Transcriptional up-regulation of Apaf-1 after DNA damage sensitizes mature sympathetic neurons to cytochrome c. (a) P28 sympathetic neurons were treated with etoposide either alone or in the presence of 0.1 μg/ml actinomycin D (ActD) or 1 μg/ml cycloheximide (CHX) for 48 h and injected with either bovine or yeast cytochrome c. After injection, cell viability was assessed at the indicated time points. (b) P28 sympathetic neurons were left untreated or treated with etoposide for 48 h and mRNA levels were analyzed by RT-PCR analysis. PCR primers used to amplify Apaf-1 and GAPDH mRNA yielded bands of 170 and 196 nucleotides, respectively. Protein levels were analyzed by Western blot. Densitometry of protein levels are represented as fold change ± SEM and normalized to nucleoporin levels. (c) P28 sympathetic neurons isolated from Apaf-1−/− mice or wild-type littermates (Apaf-1+/+) were treated with etoposide for 48 h and injected with either bovine or yeast cytochrome c. After injection, cell viability was assessed at the indicated time points. Error bars denote ± SEM.
We reasoned that mature P28 neurons must restore expression of Apaf-1 after treatment with etoposide. Indeed, after 48 h of etoposide treatment, a time that precedes endogenous cytochrome c release (Fig. 1, b and c), Apaf-1 was dramatically increased at both the mRNA and protein level (Fig. 3 b). Importantly, Apaf-1–deficient P28 neurons were unable to regain sensitivity to cytochrome c after 48 h of etoposide treatment (Fig. 3 c). These results show that mature neurons are critically dependent on the induction of Apaf-1 after DNA damage to permit cytochrome c–mediated caspase activation.
Cell cycle gene expression is necessary but not sufficient for transcriptional up-regulation of Apaf-1 and sensitivity to cytochrome c in mature neurons
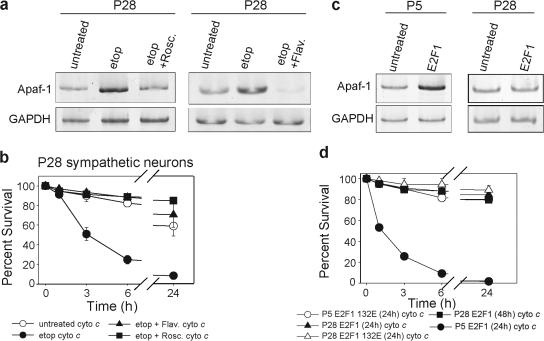
To determine how Apaf-1 is transcriptionally induced in mature neurons after etoposide treatment, we examined Apaf-1 transcription factors p53 and E2F1 (Fortin et al., 2001; Moroni et al., 2001). We narrowed our focus to the E2F1 pathway, as the majority of etoposide-treated p53-deficient mature neurons showed sensitivity to cytochrome c (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200708086/DC1). Inhibition of the E2F1 pathway by multiple mechanisms has been shown to be sufficient to delay or inhibit apoptosis in developing neurons (Park et al., 1996; Greene et al., 2004). E2F transcription factors are held inactive by hypophosphorylated retinoblastoma protein pocket proteins, which dissociate after their phosphorylation by the Cdks. We used two different Cdk inhibitors, roscovitine and flavopiridol, to block etoposide-induced E2F activation (Padmanabhan et al., 1999). The addition of either inhibitor completely blocked the etoposide-induced up-regulation of Apaf-1 and the sensitization of mature neurons to cytochrome c (Fig. 4, a and b), demonstrating that the Cdk-dependent activation of the E2F pathway is necessary for the transcription of Apaf-1 by DNA damage in mature neurons.
Figure 4.
Activation of the cell cycle machinery is necessary but not sufficient for transcriptional up-regulation of Apaf-1 in mature neurons. (a) P28 sympathetic neurons were untreated, treated with etoposide alone, or treated with etoposide and a CDK inhibitor, either 50 μM roscovitine (Rosc) or 1 μM flavopiridol (Flav), for 48 h. mRNA levels were analyzed by RT-PCR. PCR primers used to amplify Apaf-1 and GAPDH mRNA yielded bands of 170 and 196 nucleotides, respectively. (b) P28 sympathetic neurons were either left untreated or treated with etoposide alone or etoposide and a CDK inhibitor, either 50 μm roscovitine or 1 μm flavopiridol, for 48 h. Neurons were injected with cytochrome c and cell viability was assessed at the indicated time points. (c) P5 and 28 sympathetic neurons were infected with E2F1-expressing adenovirus. 36 h after infection, mRNA levels were analyzed by RT-PCR. PCR primers used to amplify Apaf-1 and GAPDH mRNA yielded bands of 170 and 196 nucleotides, respectively. (d) P5 and 28 sympathetic neurons overexpressing either E2F1 or DNA-binding mutant E2F1 132E were injected with cytochrome c and their survival was assessed at the indicated time points. Error bars denote ± SEM.
Surprisingly, although expression of E2F1 alone was sufficient to induce Apaf-1 transcription in P5 neurons, it was unable to do so in P28 neurons (Fig. 4 c). Consistent with this observation, P5 neurons expressing E2F1 developed sensitivity to cytochrome c, whereas P28 neurons expressing E2F1 remained resistant regardless of the time allowed after E2F1 expression (Fig. 4 d). Importantly, the ability of E2F1 to induce sensitivity to cytochrome c in P5 neurons was specific for its transcriptional activity, as a DNA-binding mutant of E2F1 (E2F1 132E; Johnson et al., 1993) failed to do so (Fig. 4 d).
Together, these results show that E2F1 has a differential ability to induce Apaf-1 expression and therefore affect sensitivity to cytochrome c in the P5 developing versus P28 mature neurons. The observation that the activation of the E2F pathway is necessary but not sufficient for Apaf-1 up-regulation in mature neurons suggests that additional molecular events are required.
Apaf-1 undergoes increased chromatin-mediated repression in mature neurons
Transactivation of target genes by transcription factors is critically dependent on the accessibility of chromatin, which is regulated in part by histone modifications. We speculated that changes in chromatin accessibility may underlie the differential ability of E2F1 to induce Apaf-1 transcription in developing but not mature neurons.
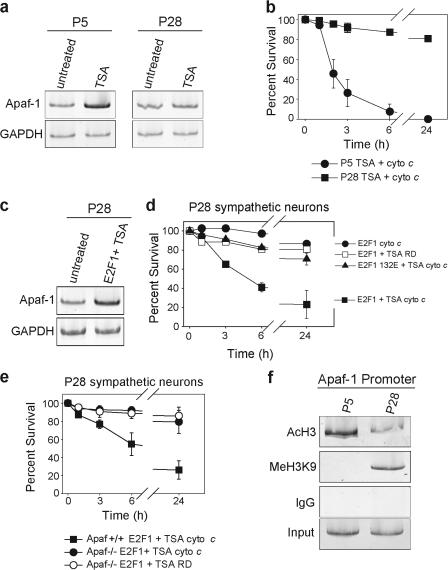
To test this prediction, we examined whether chromatin derepression with the addition of the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) was sufficient to induce sensitivity to cytochrome c via the up-regulation of Apaf-1 in developing and mature neurons. Treatment with TSA induced a significant increase in Apaf-1 expression in developing P5 neurons but was unable to do so in mature P28 neurons (Fig. 5 a). Consistent with these results, P5 neurons treated with TSA underwent rapid apoptosis after the injection of cytochrome c, whereas P28 neurons remained resistant (Fig. 5 b).
Figure 5.
Mature sympathetic neurons require simultaneous chromatin derepression and transcriptional activation for Apaf-1 up-regulation. (a) P5 and 28 sympathetic neurons were treated with 250 nM TSA for 24 h and mRNA levels were analyzed by RT-PCR. PCR primers used to amplify Apaf-1 and GAPDH mRNA yielded bands of 170 and 196 nucleotides, respectively. (b) P5 and 28 sympathetic neurons were treated with 250 nM TSA for 24 h and injected with cytochrome c. After injection, cell viability was assessed at the indicated time points. (c) P28 sympathetic neurons were infected with E2F1 adenovirus. 12 h later, 250 nM TSA was added. 36 h after the initial E2F1 adenovirus infection, mRNA levels were examined by RT-PCR. PCR primers used to amplify Apaf-1 and GAPDH mRNA yielded bands of 170 and 196 nucleotides, respectively. (d) P28 sympathetic neurons expressing either E2F1 or E2F1 132E were treated with or without 250 nM TSA. After 24 h, neurons were injected with cytochrome c or rhodamine dextran (RD), and cell viability was assessed at the indicated time points. (e) P28 sympathetic neurons from Apaf-1−/− mice or wild-type littermates (Apaf-1+/+) were infected with E2F1 adenovirus and treated with 250 nM TSA. After 24 h, neurons were injected with cytochrome c or rhodamine dextran, and cell viability was assessed at the indicated time points. (f) Chromatin was extracted from superior cervical ganglia isolated from P5 or 28 mice. Chromatin was immunoprecipitated with the indicated antibodies or IgG control. Immunoprecipitated chromatin was used in a PCR reaction with primers designed to amplify a 570-nucleotide region of the Apaf-1 promoter. Error bars denote ± SEM.
Because neither E2F1 overexpression nor derepression with the addition of TSA alone was able to induce Apaf-1 up-regulation in mature neurons (Figs. 4 c and 5 a), we hypothesized that this region of chromatin may be highly repressed in mature neurons. Indeed, expression of E2F1 in the presence of TSA induced a marked transcriptional increase in Apaf-1 (Fig. 5 c) and rendered mature P28 neurons sensitive to cytochrome c (Fig. 5 d). This sensitivity of P28 neurons was dependent on the up-regulation of Apaf-1, as neurons isolated from Apaf-1–deficient mice failed to undergo cytochrome c–induced apoptosis after TSA and E2F1 treatment (Fig. 5 e). These data support the model that neuronal maturation is accompanied by increased repression of Apaf-1 at the level of chromatin structure.
We tested this model directly with a chromatin immunoprecipitation (ChIP) assay using antibodies to acetylated histone 3 (AcH3), which is indicative of active chromatin, and histone 3 trimethylated on lysine 9 (MeH3K9), which is indicative of chromatin in a repressed state (Fischle et al., 2003). The AcH3 antibody selectively precipitated significantly more Apaf-1 promoter from developing P5 ganglia than mature P28 ganglia, indicating that Apaf-1 is primarily associated with active chromatin during development (Fig. 5 f). In contrast, the MeH3K9 antibody precipitated the Apaf-1 promoter only from mature P28 ganglia, indicating that after neuronal maturation, this region of chromatin is actively repressed (Fig. 5 f). Together, these results show that Apaf-1 undergoes a dynamic change from an active chromatin state in developing neurons to a repressed chromatin state in mature neurons to effectively restrict the apoptotic potential as neurons mature.
Although developing neurons undergo substantial apoptosis, restrictions in the apoptotic pathway are physiologically important for the long-term survival of mature neurons. Previous studies have shown that mature neurons develop apoptotic restriction at the premitochondrial point of Bax function (Easton et al., 1997; Vekrellis et al., 1997; Vogelbaum et al., 1998). Bax and Apaf-1 represent logical points of control, as they represent two critical points in the apoptotic pathway with no functional redundancy in neurons. The engagement of these specific restraints on the apoptotic pathway with neuronal maturation provides a mechanistic basis for the observed greater incidence of apoptotic death in neonatal versus adult models of brain injury (Blomgren et al., 2003). However, in response to some apoptotic insults such as DNA damage and traumatic brain injury (Yakovlev et al., 2001), mature neurons are able to overcome these restraints and reengage the apoptotic pathway, thus minimizing detrimental effects such as inflammation associated with nonapoptotic forms of death.
Although several studies have demonstrated the intriguing requirement for cell cycle activity in neuronal apoptosis (Greene et al., 2004; Becker and Bonni, 2005), its exact function has been less clear. Our hypothesis is that because the expression of certain proapoptotic genes is regulated by cell cycle–related transcriptional factors, exit from cell cycle after differentiation results in decreased transcription of proapoptotic genes. Therefore, neurons undergoing apoptosis must first reactivate cell cycle machinery and synthesize proapoptotic proteins. Neurons can use the E2F1 pathway to reach their apoptotic threshold by inducing the expression of Bim (Biswas et al., 2005) and Bad (Konishi and Bonni, 2003) upstream of cytochrome c release and Apaf-1 downstream of cytochrome c.
The ability of the E2F pathway to induce expression of proapoptotic target genes during neuronal apoptosis can occur through either direct transactivation or derepression (Greene et al., 2004; Becker and Bonni, 2005). Disruption of a repressive complex containing E2F4 and the retinoblastoma protein pocket protein p130 is sufficient for the up-regulation of proapoptotic E2F target genes in embryonic cortical neurons (Liu et al., 2005). Likewise, derepression with an HDAC inhibitor alone is sufficient to activate E2F1 target genes in early postnatal cerebellar granule neurons (Boutillier et al., 2003) and developing but not mature retina (Wallace et al., 2006). Here, we report that either overexpression of E2F1 or HDAC inhibition was indeed sufficient to induce Apaf-1 transcription and sensitivity to cytochrome c in developing but not mature sympathetic neurons (Figs. 4 and 5).
In contrast, we found that both gene derepression and E2F1 transactivation were required for Apaf-1 expression in mature neurons (Fig. 5, c and d), indicating that neuronal maturation is accompanied by the progressive repression of Apaf-1. This was confirmed by demonstrating that the Apaf-1 promoter was associated with active chromatin (AcH3) in developing neurons and repressed chromatin (MeH3K9) in mature neurons (Fig. 5 f). These results identify the regulation of Apaf-1 transcription at the level of chromatin structure as a key component in restricting the apoptotic pathway during neuronal maturation.
Although changes in the chromatin state of proapoptotic genes adds a level of control for restricting apoptosis in mature neurons, defects in this mechanism could contribute to neurodegeneration by maintaining neuronal sensitivity to apoptosis during adulthood (Mattson and Magnus, 2006). The potential use of HDAC inhibitors for treatment of various cancers has recently gained prominence (Bolden et al., 2006). Our results caution that these compounds may have unintended consequences, sensitizing these otherwise resistant neurons to pathological stimuli.
Finally, other proapoptotic genes that are targets of the E2F1 pathway during neuronal apoptosis could also be repressed by a similar mechanism in mature neurons. Indeed, dynamic changes in chromatin-mediated repression of proapoptotic genes with maturation may represent a general mechanism designed to ensure the long-term survival of not only neurons but other postmitotic cells as well.
Materials and methods
Reagents
All reagents were obtained from Sigma-Aldrich or Thermo Fisher Scientific unless stated otherwise. Collagenase and trypsin were obtained from Worthington Biochemical Corporation. Q-VD-OPH was obtained from MP Biomedicals. Flavopiridol was a gift of D. Park (University of Ottawa, Ottawa, Canada). Plasmid DNAs expressing E2F1 wild type and mutant 132E were a gift of A. Bonni (Harvard University, Cambridge, MA). Our method of generating Smac protein has been described previously (Potts et al., 2003). p53-deficient mice were purchased from the Jackson Laboratory. XIAP-deficient mice were a gift of C. Thompson (University of Pennsylvania, Philadelphia, PA). Apaf-1–deficient mice were generated by J. Herz (The University of Texas Southwestern Medical Center, Dallas, TX) and provided by S. Ackerman (The Jackson Laboratory, Bar Harbor, MA).
Cell culture
Primary sympathetic neurons were isolated from superior cervical ganglia as described previously (Potts et al., 2003), with some modifications. For mature neurons, superior cervical ganglia were dissected from P6–13 mice and treated with 1 mg/ml collagenase followed by 1 mg/ml trypsin for 1 h each at 37°C. P0 neurons were maintained in culture for 5 d (for P5 neurons) and P6–13 neurons were maintained in culture until the P28 equivalent before experimentation.
Cell treatments
Where indicated, cells were treated with 20 μM etoposide. The broad spectrum caspase inhibitor Q-VD-OPH was used at a concentration of 25 μM. Cell survival was assessed at multiple time points by morphology, with cells that had intact, phase-bright cell bodies scored as alive. This method of assessing neuronal survival correlates well with other cell survival assays such as trypan blue exclusion and staining with calcien AM (Potts et al., 2003). E2F1 and GFP adenoviruses were provided by J. Cook (University of North Carolina at Chapel Hill, Chapel Hill, NC). Virus particles were purified on a cesium chloride gradient and tittered in 293T cells. P5 sympathetic neurons were infected at an MOI of 100–250 and P28 sympathetic neurons were infected at an MOI of 1,000. Data shown are mean ± SEM of three independent experiments with a minimum of 100 cells counted for each condition.
Microinjections
Microinjections were performed as described previously (Wright et al., 2007). DNA microinjections contained 50 ng/μl EGFP (Clontech Laboratories, Inc.) and 200 ng/μl of indicated plasmid. Cytochrome c injections were 10 mg/ml and Smac injections were 1 mg/ml. Data shown are mean ± SEM of at least three independent experiments and each experiment represents a minimum of 50 cells for each condition.
Western blot
Western blots were performed as described previously (Potts et al., 2003). Primary antibodies were as follows: anti-Apaf-1 (13F11; Qbiogene); anti–procaspase-3 (9665; Cell Signaling Technology); anti–caspase-9 (M0543; MBL International); and anti-nucleoporin (BD Biosciences). HRP-conjugated secondary antibodies were purchased from Thermo Fisher Scientific. Proteins were detected using ECL-Plus and scanned with an imager (Typhoon; both from GE Healthcare). Data shown are representative of three independent experiments. Densitometry was preformed using ImageJ software and normalized to nucleoporin levels. The densitometry is represented as fold change ± SEM.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Wright et al., 2007). Data shown are representative of three independent experiments. Status of cytochrome c with etoposide treated was preformed in the presence of Q-VD-OPH to block caspase activation, which allows us to block cell death and therefore access the status of cytochrome c (released or intact).
Semiquantitative RT-PCR
Our method for semiquantitative RT-PCR analysis has been described previously (Potts et al., 2003). Data shown are representative of three independent experiments. Primer sets used for PCR amplification of Apaf-1 and GAPDH were as follows: Apaf-1 forward (5′-GAGGCACAATGGATGCAAAGG-3′) and reverse (5′-GGCTGCTCGTTGATATTGAGTGG-3′) and GAPDH forward (5′-CCATGGAGAAGGCTGGGG-3′) and reverse (5′-CAAAGTTGTCATGGATGACC-3′).
ChIP assay
ChIP was performed using the Chromatin Immunoprecipitation Assay kit (Millipore) according to the manufacturer's instructions. In brief, superior cervical ganglia were isolated from P5 or 28 mice and washed in ice-cold PBS. Ganglia were cross-linked by incubating with 0.1% formaldehyde in PBS for 30 min at room temperature. Cross-linking was halted with the addition of glycine to 0.125 M for 10 min. Ganglia were washed three times in ice-cold PBS and resuspended in 300 μl of SDS lysis buffer and tumbled for 30 min at 4°C. Ganglia were then sonicated for 13 pulses of 5 s each at 35% amplitude with a 1-min rest period between each pulse. Sonicated chromatin was diluted to a final volume of 1,600 μl with ChIP dilution buffer and precleared by incubation with 35 μl of protein A–agarose/salmon sperm DNA for 1 h at 4°C. After preclearing, 100 μl of the sample was set aside for input control and the remaining chromatin was divided equally between conditions. Samples were incubated with 5 μg of the indicated antibodies for 16 h at 4°C. Antibody–histone complexes were recovered by incubating with 35 μl of protein A–agarose/salmon sperm DNA for 5 h at 4°C. Beads were centrifuged, washed, and eluted according to the manufacturer's instructions. Cross-linking was reversed with the addition of NaCl to a final concentration of 200 mM for 4 h at 65°C and protein complexes were degraded with the addition of 2 μg proteinase K for 1 h at 45°C. DNA was recovered with phenol-chloroform extraction followed by ethanol precipitation at −20°C overnight and resuspended in 50 μl TE buffer. 5 μl DNA was used for each PCR reaction. A no-antibody condition was run as a negative control and a fraction of the preimmunoprecipitated chromatin was used to determine input. Data shown are representative of three independent experiments.
Primers used to amplify the region identified as the minimal core promoter for Apaf-1 (available from GenBank/EMBL/DDBJ under accession no. AJ970309) were as follows: forward (5′-GTAGGCACACAGCTCTAAATAGGAG-3′) and reverse (5′-CAAGATCTTCAGGGATCCAGAC-3′).
Image acquisition and processing
All images were acquired by a digital black and white charge-coupled device camera (ORCA-ER; Hamamatsu) mounted on an inverted fluorescence microscope (DMIRE 2; Leica). The secondary antibody used for immunohistochemistry was Cy3. Cy3 was detected by a filter set (TRITC DiL HQ) and the Hoechst 33258 was detected by a Hoechst filter set (both from Chroma Technology Corp.). The objective used was HC PL APO CS 63× 1.32.0.60 oil (Leica). Samples were mounted in glycerol containing mounting media and imaged by oil immersion. All images were taken at room temperature. The image acquisition software was Metamorph 5.0 (MDS Analytical Technologies). Images were scaled down and cropped in Photoshop (Adobe) to prepare the final figures.
Online supplemental material
Fig. S1 shows the assessment of neuronal death of P28 XIAP-deficient neurons expressing exogenous Apaf-1 after cytochrome c microinjection. This figure also shows the time course of neuronal survival of P28 neurons after treatment with etoposide in the presence of the general caspase inhibitor zVAD-fmk. Fig. S2 shows the assessment of neuronal survival of P28 neurons treated with etoposide for 24 h followed by cytochrome c microinjection. Fig. S3 shows the assessment of neuronal death of P28 p53-deficient neurons treated with etoposide for 48 h followed by cytochrome c microinjection. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200708086/DC1.
Acknowledgments
We thank Allyson Vaughn and Yolanda Huang for critical review of this manuscript. We also thank Drs. Xiangdong Lu and Brian Strahl for helpful discussions.
This work was supported by National Institutes of Health grants NS42197 and NS049745 to M. Deshmukh and K.M. Wright, respectively.
K.M. Wright and M.I. Smith contributed equally to this paper.
Abbreviations used in this paper: AcH3, acetylated histone 3; ChIP, chromatin immunoprecipitation; HDAC, histone deacetylase; IAP, inhibitor of apoptosis protein; MeH3K9, histone 3 trimethylated on lysine 9; P, postnatal day; Smac, second mitochondria-derived activator of caspases; TSA, trichostatin A; XIAP, X-linked IAP.
References
- Becker, E.B., and A. Bonni. 2005. Beyond proliferation—cell cycle control of neuronal survival and differentiation in the developing mammalian brain. Semin. Cell Dev. Biol. 16:439–448. [DOI] [PubMed] [Google Scholar]
- Benn, S.C., and C.J. Woolf. 2004. Adult neuron survival strategies–slamming on the brakes. Nat. Rev. Neurosci. 5:686–700. [DOI] [PubMed] [Google Scholar]
- Besirli, C.G., T.L. Deckwerth, R.J. Crowder, R.S. Freeman, and E.M. Johnson Jr. 2003. Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ. 10:1045–1058. [DOI] [PubMed] [Google Scholar]
- Biswas, S.C., D.X. Liu, and L.A. Greene. 2005. Bim is a direct target of a neuronal E2F-dependent apoptotic pathway. J. Neurosci. 25:8349–8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren, K., C. Zhu, U. Hallin, and H. Hagberg. 2003. Mitochondria and ischemic reperfusion damage in the adult and in the developing brain. Biochem. Biophys. Res. Commun. 304:551–559. [DOI] [PubMed] [Google Scholar]
- Boatright, K.M., and G.S. Salvesen. 2003. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15:725–731. [DOI] [PubMed] [Google Scholar]
- Bolden, J.E., M.J. Peart, and R.W. Johnstone. 2006. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5:769–784. [DOI] [PubMed] [Google Scholar]
- Boutillier, A.L., E. Trinh, and J.P. Loeffler. 2003. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J. Neurochem. 84:814–828. [DOI] [PubMed] [Google Scholar]
- Deshmukh, M., and E.M. Johnson Jr. 1998. Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 21:695–705. [DOI] [PubMed] [Google Scholar]
- Donovan, M., and T.G. Cotter. 2002. Caspase-independent photoreceptor apoptosis in vivo and differential expression of apoptotic protease activating factor-1 and caspase-3 during retinal development. Cell Death Differ. 9:1220–1231. [DOI] [PubMed] [Google Scholar]
- Easton, R.M., T.L. Deckwerth, P.A. Sh, and E.M. Johnson Jr. 1997. Analysis of the mechanism of loss of trophic factor dependence associated with neuronal maturation: A phenotype indistinguishable from BAX deletion. J. Neurosci. 17:9656–9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle, W., Y. Wang, and C.D. Allis. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15:172–183. [DOI] [PubMed] [Google Scholar]
- Fortin, A., S.P. Cregan, J.G. MacLaurin, N. Kushwaha, E.S. Hickman, C.S. Thompson, A. Hakim, P.R. Albert, F. Cecconi, K. Helin, et al. 2001. APAF1 is a key transcriptional target for p53 in the regulation of neuronal cell death. J. Cell Biol. 155:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, L.A., S.C. Biswas, and D.X. Liu. 2004. Cell cycle molecules and vertebrate neuron death: E2F at the hub. Cell Death Differ. 11:49–60. [DOI] [PubMed] [Google Scholar]
- Johnson, D.G., J.K. Schwarz, W.D. Cress, and J.R. Nevins. 1993. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 365:349–352. [DOI] [PubMed] [Google Scholar]
- Konishi, Y., and A. Bonni. 2003. The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons. J. Neurosci. 23:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus, H.M., R.H. Herzig, G.P. Herzig, G.L. Phillips, U. Roessmann, and D.J. Fishman. 1981. Central nervous system toxicity of high-dose systemic cytosine arabinoside. Cancer. 48:2577–2582. [DOI] [PubMed] [Google Scholar]
- Liu, D.X., N. Nath, S.P. Chellappan, and L.A. Greene. 2005. Regulation of neuron survival and death by p130 and associated chromatin modifiers. Genes Dev. 19:719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M.P., and T. Magnus. 2006. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 7:278–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni, M.C., E.S. Hickman, E. Lazzerini Denchi, G. Caprara, E. Colli, F. Cecconi, H. Muller, and K. Helin. 2001. Apaf-1 is a transcriptional target for E2F and p53. Nat. Cell Biol. 3:552–558. [DOI] [PubMed] [Google Scholar]
- Neame, S.J., L.L. Rubin, and K.L. Philpott. 1998. Blocking cytochrome c activity within intact neurons inhibits apoptosis. J. Cell Biol. 142:1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orike, N., G. Middleton, E. Borthwick, V. Buchman, T. Cowen, and A.M. Davies. 2001. Role of PI 3–kinase, Akt, and Bcl-2–related proteins in sustaining the survival of neurotrophic factor–independent adult sympathetic neurons. J. Cell Biol. 154:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan, J., D.S. Park, L.A. Greene, and M.L. Shelanski. 1999. Role of cell cycle regulatory proteins in cerebellar granule neuron apoptosis. J. Neurosci. 19:8747–8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D.S., S.E. Farinelli, and L.A. Greene. 1996. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons. J. Biol. Chem. 271:8161–8169. [DOI] [PubMed] [Google Scholar]
- Potts, P.R., S. Singh, M. Knezek, C.B. Thompson, and M. Deshmukh. 2003. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J. Cell Biol. 163:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha, G.V., and E.M. Johnson Jr. 2004. Men are but worms: neuronal cell death in C elegans and vertebrates. Cell Death Differ. 11:38–48. [DOI] [PubMed] [Google Scholar]
- Putcha, G.V., M. Deshmukh, and E.M. Johnson Jr. 2000. Inhibition of apoptotic signaling cascades causes loss of trophic factor dependence during neuronal maturation. J. Cell Biol. 149:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis, K., M.J. McCarthy, A. Watson, J. Whitfield, L.L. Rubin, and J. Ham. 1997. Bax promotes neuronal cell death and is downregulated during the development of the nervous system. Development. 124:1239–1249. [DOI] [PubMed] [Google Scholar]
- Vogelbaum, M.A., J.X. Tong, and K.M. Rich. 1998. Developmental regulation of apoptosis in dorsal root ganglion neurons. J. Neurosci. 18:8928–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, D.M., M. Donovan, and T.G. Cotter. 2006. Histone deacetylase activity regulates apaf-1 and caspase 3 expression in the developing mouse retina. Invest. Ophthalmol. Vis. Sci. 47:2765–2772. [DOI] [PubMed] [Google Scholar]
- Wright, K.M., M.W. Linhoff, P.R. Potts, and M. Deshmukh. 2004. Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J. Cell Biol. 167:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, K.M., A.E. Vaughn, and M. Deshmukh. 2007. Apoptosome dependent caspase-3 activation pathway is non-redundant and necessary for apoptosis in sympathetic neurons. Cell Death Differ. 14:625–633. [DOI] [PubMed] [Google Scholar]
- Yakovlev, A.G., K. Ota, G. Wang, V. Movsesyan, W.-L. Bao, K. Yoshihara, and A.I. Faden. 2001. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J. Neurosci. 21:7439–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J., and B.A. Yankner. 2000. Apoptosis in the nervous system. Nature. 407:802–809. [DOI] [PubMed] [Google Scholar]