Abstract
Although cell surface metalloendopeptidases degrade neuropeptides in the extracellular fluid to terminate signaling, the function of peptidases in endosomes is unclear. We report that isoforms of endothelin-converting enzyme-1 (ECE-1a–d) are present in early endosomes, where they degrade neuropeptides and regulate post-endocytic sorting of receptors. Calcitonin gene-related peptide (CGRP) co-internalizes with calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), β-arrestin2, and ECE-1 to early endosomes, where ECE-1 degrades CGRP. CGRP degradation promotes CLR/RAMP1 recycling and β-arrestin2 redistribution to the cytosol. ECE-1 inhibition or knockdown traps CLR/RAMP1 and β-arrestin2 in endosomes and inhibits CLR/RAMP1 recycling and resensitization, whereas ECE-1 overexpression has the opposite effect. ECE-1 does not regulate either the resensitization of receptors for peptides that are not ECE-1 substrates (e.g., angiotensin II), or the recycling of the bradykinin B2 receptor, which transiently interacts with β-arrestins. We propose a mechanism by which endosomal ECE-1 degrades neuropeptides in endosomes to disrupt the peptide/receptor/β-arrestin complex, freeing internalized receptors from β-arrestins and promoting recycling and resensitization.
Introduction
Membrane-associated metalloendopeptidases play a major role in the post-secretory processing of regulatory peptides. Cell surface peptidases cleave peptides in the extracellular fluid to generate biologically active forms or inactivate mature peptides. For example, angiotensin-converting enzyme-1 converts angiotensin (AT) I to ATII, which activates the ATII type 1A receptor (AT1AR), and degrades bradykinin (BK) to limit activation of the BK B2 receptor (B2R) (Yang et al., 1970, 1971). Neprilysin (NEP) degrades substance P (SP) to limit activation of the neurokinin-1 receptor (NK1R) and terminate neurogenic inflammation (Okamoto et al., 1994; Lu et al., 1997; Sturiale et al., 1999). Less is known about the role of intracellular membrane metalloendopeptidases.
Endothelin-converting enzyme 1 (ECE-1) is a metalloendopeptidase of plasma and endosomal membranes. Four ECE-1 isoforms (a–d) arise from a single gene using alternate promoters (Schmidt et al., 1994; Shimada et al., 1995; Schweizer et al., 1997; Valdenaire et al., 1999). Whereas ECE-1 isoforms share a common catalytic domain, differences in the N-terminal domains specify variable subcellular distribution (Schweizer et al., 1997; Azarani et al., 1998; Brooks et al., 2000; Muller et al., 2003; Hunter and Turner, 2006). ECE-1b and ECE-1d are mainly present in endosomal membranes (Schweizer et al., 1997; Azarani et al., 1998; Muller et al., 2003), and ECE-1a and ECE-1c are mainly at the plasma membrane, with a minor localization in endosomes (Schweizer et al., 1997; Muller et al., 2003). Cell surface ECE-1 converts big-endothelin (ET) to the pressor peptide ET-1 (Xu et al., 1994), and inactivates BK (Hoang and Turner, 1997). The function of ECE-1 in endosomes is not fully understood. However, ECE-1 can degrade neuropeptides such as SP, BK, ATI, and neurotensin at an acidic endosomal pH (Johnson et al., 1999; Fahnoe et al., 2000). Because many peptides traffic to endosomes with their receptors, we hypothesized that ECE-1 degrades peptides in endosomes to disrupt the peptide/receptor complex and to control post-endocytic sorting and signaling of receptors.
Little is known about post-endocytic sorting of G protein–coupled receptors (GPCRs). Endocytosis requires receptor phosphorylation by G protein receptor kinases, which increases the affinity of the receptor for β-arrestins. β-arrestins translocate from the cytosol to the plasma membrane, where they uncouple receptors from heterotrimeric G proteins to mediate desensitization (Lohse et al., 1990), and couple receptors to clathrin and AP2 to mediate endocytosis (Ferguson et al., 1996; Goodman et al., 1996). One determinant of the rate of recycling is the affinity of receptors for β-arrestins. “Class A” GPCRs (e.g., β2 adrenergic receptor, B2R, μ-opioid receptor, neurokinin 3 receptor) have few phosphorylation sites, interact transiently with β-arrestin2 with low affinity, and rapidly recycle (Oakley et al., 1999, 2000, 2001; Schmidlin et al., 2003). “Class B” GPCRs (e.g., AT1AR, NK1R, neurotensin receptor 1, vasopressin V2 receptor [V2R]) are highly phosphorylated, interact with both β-arrestin1 and 2 with high affinity for prolonged periods in endosomes, and slowly recycle. Although dissociation from β-arrestins is necessary for receptor recycling and resensitization, the critical event that initiates this process is unknown.
We recently reported that ECE-1 degrades SP in acidified endosomes to disrupt the SP/NK1R/β-arrestin complex, and initiate NK1R recycling and resensitization (Roosterman et al., 2007). However, it is not known whether this is a general mechanism that regulates trafficking of other GPCRs and associated proteins. The factors that specify this role for endosomal ECE-1, including peptide susceptibility to ECE-1 degradation, peptide trafficking to ECE-1–containing endosomes, and receptor affinity for β-arrestins, are unknown. To address these questions, we examined the role of ECE-1 in post-endocytic sorting of the receptor for calcitonin gene-related peptide (CGRP), a heterodimer of the calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) (McLatchie et al., 1998). CGRP induces β-arrestin–dependent endocytosis of CLR/RAMP1, which remains associated with β-arrestins in endosomes, typical of a “class B” GPCR (Hilairet et al., 2001), and then recycles (Cottrell et al., 2007). CGRP is a potent vasodilator and a major mediator of neurogenic inflammation (Brain and Grant, 2004). Given its prominent expression in the endothelium (Korth et al., 1999), ECE-1 may regulate these vasoactive actions of CGRP. However, it is not known whether ECE-1 degrades CGRP, and the role of ECE-1 in regulating CLR/RAMP1 is completely unexplored. We also examined the role of ECE-1 in regulating receptors for two other vasoactive peptides that are potential ECE-1 substrates: AT1AR, a prototypical “class B” GPCR (Oakley et al., 2000), and B2R, which transiently interacts with β-arrestins and rapidly recycles (Simaan et al., 2005).
Results
ECE-1 is endogenously expressed in HEK, SK-N-MC, and A549 cells
Using RT-PCR with isoform-specific primers, we amplified mRNA encoding ECE-1a–d from HEK, SK-N-MC, and A549 cells, and confirmed identity by sequencing (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200704053/DC1). We amplified transcripts of B2R in HEK cells, CLR and RAMP1 in SK-N-MC cells, and AT1AR in A549 cells (Fig. S1 A). We detected ECE-1 (∼120 kD) in membranes from HEK, SK-N-MC, and A549 cells by Western blotting (antibody 473–17-A to all ECE-1 isoforms) (Fig. S1 B). Thus, HEK, SK-N-MC and A549 cells express all ECE-1 isoforms.
ECE-1 isozymes are present in early endosomes
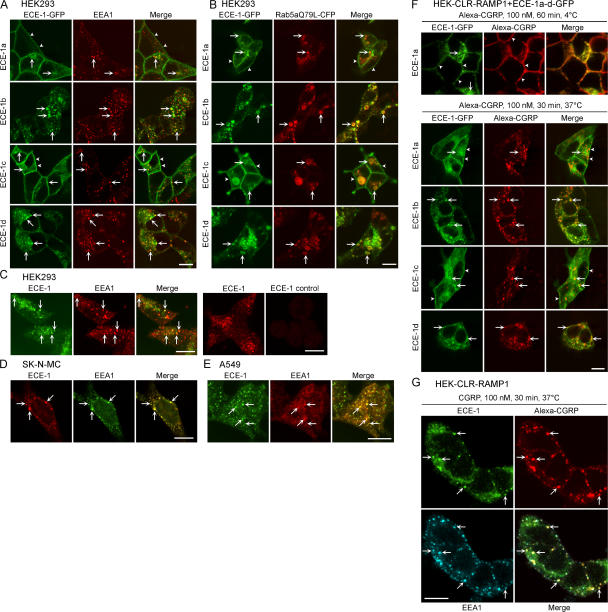
We examined the subcellular localization of ECE-1a-d-GFP expressed in HEK cells. All ECE-1 isoforms colocalized with early endosomal antigen 1 (EEA1) to varying extents. ECE-1b and ECE-1d prominently colocalized with EEA1 (Fig. 1 A, arrows). ECE-1a and ECE-1c were mainly at the plasma membrane (Fig. 1 A, arrowheads), but sometimes colocalized with EEA1 (Fig. 1 A, arrows). To confirm the endosomal localization of ECE-1a-d, we coexpressed ECE-1a-d-GFP with Rab5aQ79L-CFP, a constitutively active mutant that causes endosomal fusion, facilitating detection of proteins in endosomes (Stenmark et al., 1994). All isoforms of ECE-1 prominently colocalized with Rab5aQ79L in enlarged endosomes (Fig. 1 B, arrows), although ECE-1a and ECE-1c were also detected at the plasma membrane (Fig. 1 B, arrowheads).
Figure 1.
ECE-1 isozymes are present in early endosomes. (A) Localization of ECE-1a-d-GFP and EEA1 in HEK cells. All ECE-1 isoforms, especially ECE-1b and ECE-1d, colocalized with immunoreactive EEA1 (arrows). ECE-1a and ECE-1c were also at the plasma membrane (arrowheads). (B) Localization of ECE-1a-d-GFP and Rab5aQ79L-CFP in HEK cells. Rab5aQ79L colocalized in enlarged endosomes with ECE-1a-d (arrows). (C–E) Colocalization of endogenous ECE-1 and EEA1 (arrows) in HEK (C), SK-N-MC (D), and A549 (E) cells. (C, right panel) ECE-1 preabsorption control. (F) Trafficking of Alexa-CGRP in HEK-CLR-RAMP1 cells expressing ECE-1a-d-GFP. After 1 h at 4°C, CGRP was at the plasma membrane (arrowheads), shown in HEK-CLR-RAMP1+ ECE-1a-GFP cells. After washing and incubation for 30 min at 37°C, CGRP prominently colocalized with ECE-1b and ECE-1d, sometimes colocalized with ECE-1c, and was in proximity to intracellular ECE-1a (arrows). (G) Trafficking of Alexa-CGRP in HEK-CLR-RAMP1 cells. After 30 min at 37°C, CGRP colocalized with endogenous immunoreactive ECE-1 and EEA1 (arrows). Bar, 10 μm.
Because overexpression may alter the subcellular distribution of ECE-1, we localized endogenous ECE-1 (antibody 52–6497). This antibody recognized ECE-1b and ECE-1d, but not ECE-1a or ECE-1c expressed in KNRK cells, and untransfected KNRK cells, which do not express ECE-1 mRNA, were unstained (unpublished data). In HEK, SK-N-MC, and A549, immunoreactive ECE-1b/d colocalized with EEA1 (Fig. 1, C–E; arrows). Staining of HEK cells was abolished by preabsorption with the ECE-1 fragment used for immunization (Fig. 1 C, right). Thus, endogenous ECE-1b/d are present in early endosomes.
CGRP and CLR traffic from the plasma membrane to early endosomes containing ECE-1
To determine whether CGRP traffics to endosomes containing ECE-1, we incubated HEK-CLR-RAMP1 cells expressing ECE-1a-d-GFP with Alexa-CGRP (100 nM). After 1 h at 4°C, CGRP was at the plasma membrane, and ECE-1 isoforms were in endosomes or at the plasma membrane (Fig. 1 F). After washing and 30 min incubation at 37°C, CGRP was prominently colocalized in endosomes with ECE-1b and ECE-1d (Fig. 1 F, arrows). Some endosomes containing CGRP also contained ECE-1c and were in proximity to intracellular ECE-1a (Fig. 1 F, arrows). CGRP was also prominently detected in early endosomes with endogenous immunoreactive ECE-1b/d and EEA1 in HEK-CLR-RAMP1 cells (Fig. 1 G, arrows). In HEK-CLR-RAMP1 cells expressing ECE-1a-d-GFP, CGRP (100 nM, 30 min, 37°C) induced trafficking of immunoreactive CLR to endosomes containing ECE-1b and ECE-1d (Fig. S2, arrows; available at http://www.jcb.org/cgi/content/full/jcb.200704053/DC1). Although CLR was detected in endosomes in close proximity to intracellular ECE-1a and ECE-1c, colocalization was less apparent. Thus, CGRP and CLR traffic to early endosomes containing ECE-1 isoforms.
ECE-1 hydrolyzes CGRP, ATI, and BK, but not ATII, at endosomal pH
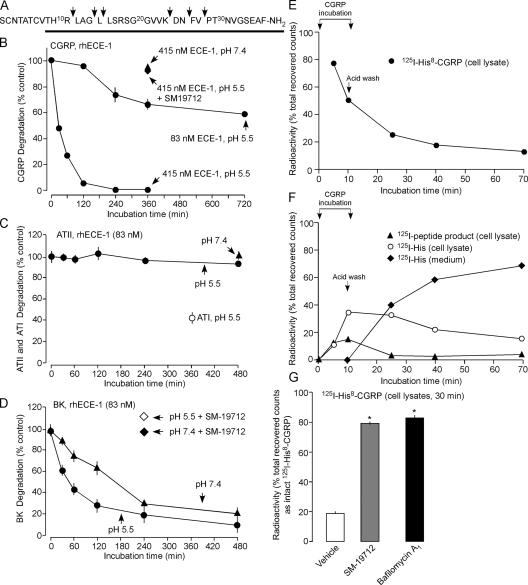
The pH optimum for ECE-1 is broad and substrate dependent (Johnson et al., 1999; Fahnoe et al., 2000). Although ECE-1 converts big-ET to ET-1 at a neutral pH optimum (pH 6.8–7.0), consistent with generating ET-1 at the cell surface, ECE-1 hydrolyzes SP and ATI at an acidic pH optimum (pH 5.6–5.8), similar to that of endosomes. To determine if ECE-1 degrades CGRP at endosomal pH, we incubated CGRP with recombinant human (rh) ECE-1 at endosomal pH 5.5 (Pelkmans et al., 2004). rhECE-1 degraded CGRP in a concentration-dependent manner (Fig. 2, A and B). The major products separated by HPLC were CGRP[15–26], CGRP[27–37], CGRP[1–24], and CGRP[1–26], indicating hydrolysis of Gly14-Leu15, Lys24-Asp25, and Asn26-Phe27 bonds (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200704053/DC1). Additional products were also identified when digests were analyzed by mass spectrometry, indicating hydrolysis of Arg11-Leu12, Leu15-Leu16, and Val28-Pro29 bonds. Because the biological activity of CGRP depends on the 8–37 sequence (Poyner et al., 1998), this hydrolysis would inactivate CGRP. CGRP degradation was minimal at extracellular fluid pH 7.4 (Fig. 2 B). The ECE-1 inhibitor SM-19712 (Umekawa et al., 2000) (100 μM) abolished rhECE-1 degradation of CGRP at pH 5.5 (Fig. 2 B, Fig. S3). Because ATI is an ECE-1 substrate (Johnson et al., 1999), we also examined hydrolysis of ATII. When ATII was incubated with 83 nM rhECE-1 at pH 5.5 or 7.4, there was no detectable degradation at up to 480 min (Fig. 2 C), although rhECE-1 degraded ATI under the same conditions (Fig. 2 C). BK is also degraded by ECE-1, but there are conflicting reports about the pH optimum (Hoang and Turner, 1997; Johnson et al., 1999; Fahnoe et al., 2000). rhECE-1 (83 nM) degraded BK at pH 5.5 and 7.4, and SM-19712 prevented this degradation, but degradation was faster at pH 5.5 (Fig. 2 D). Thus, ECE-1 degrades some (CGRP, ATI, BK) but not all (ATII) vasoactive peptides. The pH optimum favors degradation in acidified endosomes and prevents degradation at the cell surface.
Figure 2.
ECE-1 degrades neuropeptides at endosomal pH. (A–D) Degradation of peptides by rhECE-1 at pH 5.5 and 7.4 assessed byHPLC. (A) CGRP cleavage sites; solid line shows bioactive region. (B–D) Time course of degradation of GCRP (B), ATI and II (C), and BK (D). (E–G) Degradation of 125I-His8-CGRP by HEK-CLR-RAMP1 cells. 125I-His8-CGRP was incubated with cells for 5 or 10 min, cells were acid-washed, and incubated for 0, 15, 30, or 60 min. The percentage of recovered counts eluting as intact CGRP or metabolites in cell lysates or medium was determined by HPLC; standard errors within symbols. (E) Time course of degradation of endocytosed 125I-His8-CGRP. (F) Time course of appearance of metabolites in lysates and medium. (G) Effects of SM-19712 and bafilomycin A1 on degradation of 125I-His8-CGRP at 30 min after internalization. *, P < 0.05 compared with vehicle.
ECE-1 degrades endocytosed CGRP
To examine the contribution of ECE-1 to the degradation of endocytosed CGRP, we incubated HEK-CLR-RAMP1 cells with 125I-His8-CGRP and fractionated cell lysates and medium by HPLC. Internalized 125I-His8-CGRP was rapidly degraded to products with HPLC retention times of 3 min (125I-His) and 15 min (unidentified peptide) (Fig. 2, E and F; Fig. S3). SM-19712 and bafilomycin A1, an inhibitor of vacuolar-type H+-ATPase, prevented degradation of 125I-His8-CGRP (Fig. 2 G). Thus, ECE-1 degrades CGRP in endosomes, and endosomal acidification is necessary for degradation.
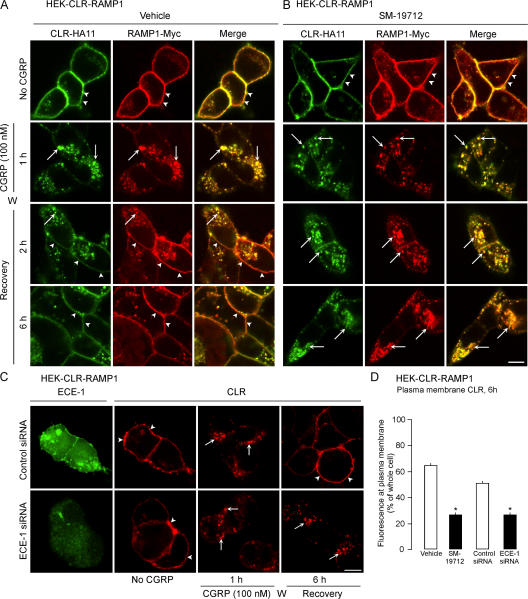
ECE-1 inhibition and knockdown slows CLR and RAMP1 recycling
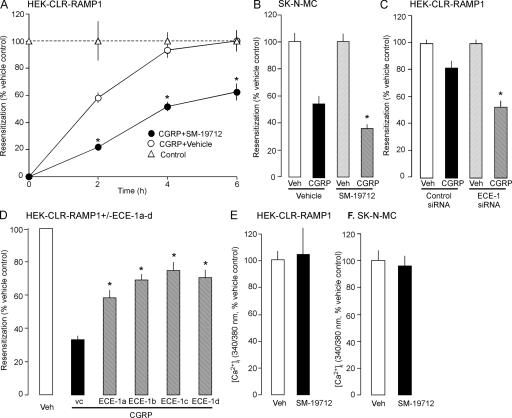
Acidotropic agents suppress recycling and resensitization of many GPCRs, including CLR/RAMP1 and NK1R (Grady et al., 1995; Garland et al., 1996; Cottrell et al., 2007). We determined whether ECE-1, by degrading CGRP in acidified endosomes, promotes dissociation of CGRP from CLR/RAMP1 to accelerate recycling. Cell surface receptors were labeled by incubating HEK-CLR-RAMP1 cells with antibodies to extracellular CLR (HA11) and RAMP1 (Myc) epitopes. Cells were exposed to CGRP (100 nM, 1 h, 37°C), washed, and recovered in CGRP-free medium. In unstimulated cells, CLR and RAMP1 colocalized at the plasma membrane (Fig. 3, A and B; arrowheads). In vehicle-treated cells (DMSO), CGRP caused internalization of CLR and RAMP1 into the same endosomes after 1 h (Fig. 3 A, arrows). CLR and RAMP1 recycled from 2–6 h (Fig. 3 A, arrowheads). SM-19712 did not affect endocytosis of CLR/RAMP1 (Fig. 3 B, arrows), but caused their retention in endosomes even at 6 h (Fig. 3 B, arrows). To confirm the role of ECE-1 in CLR recycling, we used siRNA to knockdown ECE-1. ECE-1 siRNA suppressed levels of ECE-1 detected by Western blotting by 71 ± 7% after 72 h, whereas control siRNA had a minimal effect (10 ± 6% reduction) compared with untransfected cells (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200704053/DC1). ECE-1 siRNA also down-regulated ECE-1 detected by immunofluorescence (Fig. 3 C). In cells transfected with control siRNA, CGRP induced CLR endocytosis and recycling (Fig. 3 C). In cells transfected with ECE-1 siRNA, CLR was retained in endosomes after 6 h. Quantification of CLR at the plasma membrane after 6 h revealed that SM-19712 and ECE-1 siRNA inhibited recycling of CLR by >50% (Fig. 3 D). Thus, active ECE-1 is necessary for CLR and RAMP1 recycling.
Figure 3.
ECE-1 inhibition or knockdown suppresses CLR and RAMP1 recycling. Cells were incubated with CGRP, washed (W), and recovered in CGRP-free medium. (A) In unstimulated vehicle-treated cells, CLR and RAMP1 colocalized at the plasma membrane (arrowheads). After incubation with CGRP for 1 h, CLR and RAMP1 colocalized in endosomes (arrows). After 2–6 h recovery, CLR and RAMP1 had recycled (arrowheads). (B) SM-19712 caused retention of CLR and RAMP1 in endosomes and prevented recycling. (C) In cells transfected with control siRNA, endogenous ECE-1 was readily detected, and CGRP induced endocytosis (arrows) and recycling (arrowheads) of CLR. In cells transfected with ECE-1 siRNA, endogenous ECE-1 was barely detectable, and CLR internalized and was retained in endosomes (arrows). Bar, 10 μm. (D) Quantification of the effect of SM-19712 and ECE-1 siRNA on CLR recycling at 6 h, showing the percentage of total cellular fluorescence at the plasma membrane. *, P < 0.05.
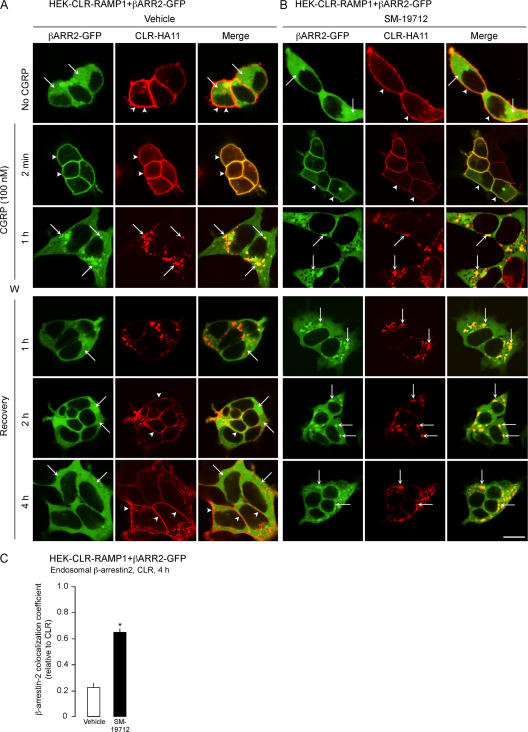
Inhibition of ECE-1 and endosomal acidification prolongs the interaction between β-arrestin2 and CLR
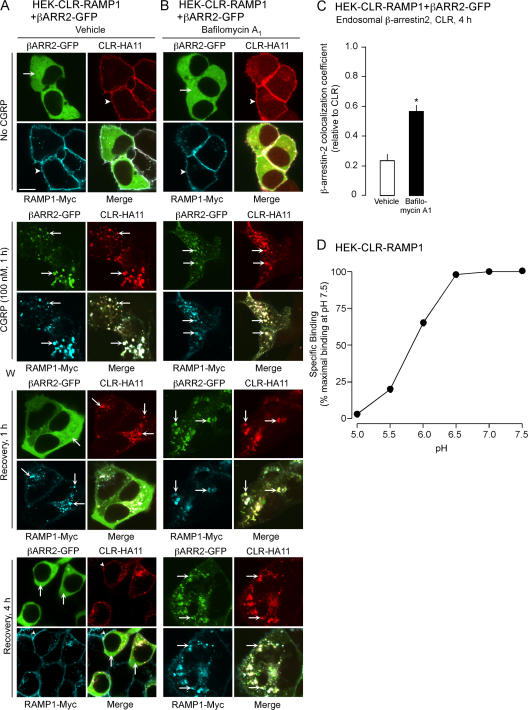
We similarly determined if ECE-1 is necessary for the dissociation of CLR and β-arrestins in endosomes of HEK-CLR-RAMP1 cells expressing β-arrestin2-GFP. In unstimulated cells, β-arrestin2 was cytosolic and CLR was at the plasma membrane (Fig. 4, A and B). In vehicle-treated cells, CGRP induced the translocation of β-arrestin2 to the plasma membrane after 2 min (Fig. 4 A, arrowheads) and caused endocytosis of CLR and β-arrestin2 into the same endosomes after 1 h (Fig. 4 A, arrows). After 1 h recovery, β-arrestin2 had returned to the cytosol and CLR was in endosomes. After 2–4 h, CLR was at the plasma membrane. SM-19712 did not affect CGRP-induced membrane translocation of β-arrestin2 or internalization of CLR and β-arrestin2, but caused their retention in endosomes (Fig. 4 B, arrows). The colocalization coefficient of CLR and β-arrestin2 at 4 h was approximately threefold higher in cells treated with SM-19712 compared with controls (Fig. 4 C). Thus, ECE-1 is necessary for the dissociation of β-arrestins from the receptor in endosomes, and the return of β-arrestins to the cytosol.
Figure 4.
ECE-1 inhibition suppresses post-endocytic trafficking of β-arrestin2 (βARR2)-GFP and CLR. HEK-CLR-RAMP1+βARR2-GFP cells were incubated with CGRP, washed, and recovered in CGRP-free medium. (A) In unstimulated vehicle-treated cells, β-arrestin2 was cytosolic and CLR was at the plasma membrane. After 2 min with CGRP, β-arrestin2 was at the plasma membrane (arrowheads), and after 1 h β-arrestin2 and CLR colocalized in endosomes (arrows). After 1 h recovery, β-arrestin2 was mostly cytosolic and CLR remained in endosomes, and after 2–4 h β-arrestin2 was cytosolic and CLR was at the plasma membrane. (B) SM-19712 caused retention of β-arrestin2 and CLR in endosomes (arrows). Bar, 10 μm. (C) Colocalization coefficient of β-arrestin2 and CLR at 4 h recovery, showing approximately threefold increase in colocalization in cells treated with SM-19712. *, P < 0.05.
To examine the role of endosomal acidification in the trafficking of CLR, RAMP1, and β-arrestin2, we treated cells with bafilomycin A1. In vehicle-treated cells, CGRP induced trafficking of CLR, RAMP1, and β-arrestin2 into the same endosomes after 1 h (Fig. 5 A, arrows). At 1 h recovery, CLR and RAMP1 remained in endosomes, and β-arrestin2 was cytosolic, and at 4 h CLR and RAMP1 were at the plasma membrane (Fig. 5 A). Bafilomycin A1 did not affect CGRP-induced trafficking of CLR, RAMP1, and β-arrestin2 to endosomes (Fig. 5 B, arrows), but caused retention of CLR, RAMP1, and β-arrestin2 in endosomes for up to 4 h recovery (Fig. 5 B, arrows). The colocalization coefficient of CLR and β-arrestin2 at 4 h was more than twofold higher in cells treated with bafilomycin A1 compared with control cells (Fig. 5 C). Thus, endosomal acidification is necessary for the redistribution of β-arrestin2 to the cytosol and for CLR and RAMP1 recycling.
Figure 5.
Acidification regulates post-endocytic trafficking of CLR, RAMP1, and β-arrestin2-GFP. (A–C) Role of endosomal acidification on trafficking of CLR, RAMP1 and β-arrestin2-GFP. HEK-CLR-RAMP1+βARR2-GFP cells were incubated with CGRP, washed, and recovered in CGRP-free medium. (A) In unstimulated vehicle-treated cells, β-arrestin2 was cytosolic and CLR and RAMP1 were at the plasma membrane. After incubation with CGRP for 1 h, β-arrestin2, CLR, and RAMP1 colocalized in endosomes (arrows). After 1 or 4 h recovery, β-arrestin2 was mostly cytosolic and CLR and RAMP1 recycled at 4 h (arrowheads). (B) Bafilomycin A1 caused retention of β-arrestin2, CLR and RAMP1 in endosomes, preventing the return of β-arrestin2 to the cytosol and CLR/RAMP1 recycling. Bar, 10 μm. (C) Colocalization coefficient of β-arrestin2 and CLR at 4 h recovery, showing greater than twofold increase in colocalization in cells treated with bafilomycin A1. *, P < 0.05. (D) 125I-CGRP binding to membranes from HEK-CLR-RAMP1 at different pH, expressed as the percentage binding at pH 7.5 (100%); standard errors within the symbols.
Acidification promotes the dissociation of CGRP from CLR/RAMP1
By inducing the dissociation of CGRP from CLR and RAMP1, endosomal acidification may promote recycling and resensitization of CLR and RAMP1 and the return of β-arrestin2 to the cytosol. Therefore, we examined binding of 125I-CGRP to membranes from HEK-CLR-RAMP1 in buffers of graded pH (7.5–5.0). Although binding was similar from pH 7.5 to pH 6.5, it sharply declined at pH <6.5, with a >30% reduction at pH 6.0 and a >75% reduction at pH 5.5 (Fig. 5 D). At endosomal pH 5.5, ∼20% of CGRP was bound to CLR/RAMP1. By degrading CGRP in endosomes to forms that are unable to rebind CLR/RAMP1, ECE-1 may promote the further dissociation of CGRP from CLR/RAMP1.
Endosomal ECE-1 regulates resensitization of CGRP signaling
To determine if ECE-1-induced recycling of CLR/RAMP1 mediates resensitization, we examined the effect of ECE-1 inhibition, knockdown, or overexpression on resensitization of CGRP-induced Ca2+ signaling.
HEK-CLR-RAMP1 cells were preincubated with CGRP (100 nM, 1 h, 37°C) or vehicle (control), washed, and recovered in CGRP-free medium for 0–6 h. The change in [Ca2+]i to a second CGRP challenge (30 nM) was measured. Preincubation with CGRP abolished CGRP-induced Ca2+ signals at 0 h recovery, indicating complete desensitization (Fig. S5 A, available at http://www.jcb.org/cgi/content/full/jcb.200704053/DC1). In cells with active ECE-1, responses to CGRP fully resensitized at 4–6 h recovery (Fig. 6 A). SM-19712 did not affect desensitization but strongly inhibited resensitization at all times (Fig. 6 A). We obtained similar results in SK-N-MC cells. Preincubation with CGRP (100 nM, 1 h, 37°C) completely desensitized responses to CGRP challenge (100 nM) (Fig. S5 B). After 4 h recovery, responses to CGRP were resensitized by 54 ± 5% (Fig. 6 B). SM-19712 did not affect desensitization, but strongly inhibited resensitization at 4 h (33% inhibition; Fig. 6 B). We used ECE-1 siRNA to confirm the role of ECE-1 in regulating resensitization in HEK-CLR-RAMP1 cells. In cells treated with control siRNA, after 1 h exposure to CGRP (100 nM) and 2 h recovery, CGRP (30 nM)-induced Ca2+ signaling was resensitized by 82 ± 5% (Fig. 6 C). ECE-1 siRNA strongly inhibited resensitization at 2 h (>35% inhibition, Fig. 6 C).
Figure 6.
ECE-1 regulates the resensitization of CLR and RAMP1. (A–D) Resensitization of CGRP-induced Ca2+ signaling. HEK-CLR-RAMP1 (A, C, and D) or SK-N-MC (B) cells were incubated with CGRP (100 nM) or vehicle for 1 h, washed, and challenged with CGRP (30 nM HEK, 100 nM SK-N-MC) at 0–6 h recovery. The change in [Ca2+]i to CGRP challenge was determined, and expressed as % response in vehicle-treated cells (100%). SM-19712 inhibited resensitization in HEK-CLR-RAMP1 cells at 0–6 h (A) and in SK-N-MC cells at 4 h (B). (C) ECE-1 siRNA inhibited resensitization in HEK-CLR-RAMP1 cells at 2 h. (D) Overexpression of ECE-1a-d-GFP in HEK-CLR-RAMP1 cells enhanced resensitization after 2 h. (E and F) Effects of ECE-1 inhibition on CGRP-induced Ca2+ signaling. SM-19712 did not affect the magnitude of response to a single challenge with CGRP (30 nM in HEK-CLR-RAMP1 [panel E], 100 nM in SK-N-MC [panel F]). *, P < 0.05 compared with SM-19712 vehicle (DMSO) (A and B), control siRNA (C), or ECE-1 vector control (vc) (D).
Conversely, we determined whether ECE-1 overexpression accelerated resensitization of CGRP-induced Ca2+ signaling. In HEK cells expressing CLR/RAMP1 alone (control), CGRP-induced Ca2+ signaling was resensitized by 33 ± 2% after 2 h (Fig. 6 D). Overexpression of ECE-1a-d accelerated resensitization of CGRP-induced Ca2+ signaling (Fig. 6 D). The most pronounced effect was in cells overexpressing ECE-1c, where there was 75 ± 5% resensitization at 2 h.
To determine if ECE-1 degrades CGRP at the cell surface to attenuate signaling, we examined the effects of ECE-1 inhibition on responses to a single challenge with CGRP. HEK-CLR-RAMP1 or SK-N-MC cells were treated with vehicle or SM-19712 and then challenged with CGRP (30 nM for HEK-CLR-RAMP1, 100 nM for SK-N-MC). SM-19712 did not affect magnitude or duration of CGRP-induced increases in [Ca2+]i in HEK-CLR-RAMP1 cells (Fig. 6 F) or SK-N-MC cells (Fig. 6 F). ECE-1 siRNA did not affect the response to an initial challenge with CGRP (unpublished data). Thus, ECE-1 is a major determinant of CLR/RAMP1 resensitization.
ECE-1 isoforms co-internalize with CGRP
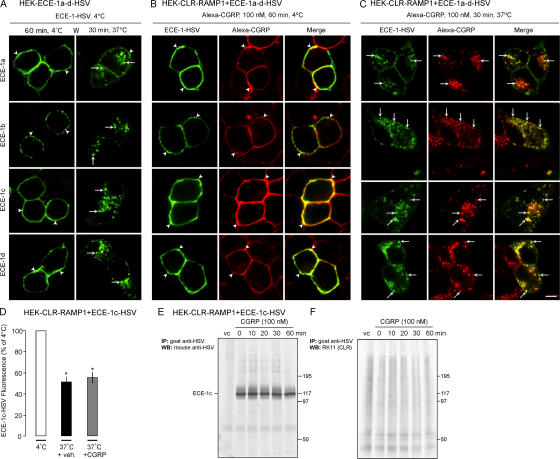
Although ECE-1c was mainly at the cell surface, overexpression of ECE-1c had the largest effect on resensitization of CGRP-induced Ca2+ signaling. The observation that overexpression of Rab5aQ79L caused a pronounced localization of ECE-1c in endosomes suggests that this isoform constitutively internalizes, becoming trapped in enlarged endosomes. To examine trafficking of ECE-1 from the plasma membrane, we incubated HEK cells expressing ECE-1a-d-HSV with FITC-labeled antibody to the extracellular HSV epitope. When incubated with HSV antibody for 1 h at 4°C, ECE-1a-d-HSV were detected at the plasma membrane (Fig. 7 A, arrowheads). At 30 min after washing and warming to 37°C, all isoforms were prominently detected in endosomes (Fig. 7 A, arrows). By expressing ECE-1a-d-HSV in HEK-CLR-RAMP1 cells, we determined whether ECE-1a-d-HSV co-internalize with Alexa-CGRP. After incubation with HSV antibody and Alexa-CGRP at 4°C, ECE-1a-d-HSV colocalized with CGRP at the plasma membrane (Fig. 7 B, arrowheads). After 30 min at 37°C, ECE-1a-d-HSV colocalized with CGRP in endosomes (Fig. 7 C, arrows). Thus, ECE-1 isoforms constitutively internalize and traffic with CGRP and CLR/RAMP1 to the same endosomes.
Figure 7.
ECE-1 and CGRP co-internalize into the same endosomes. (A–C) ECE-1 trafficking. (A) HEK cells expressing ECE-1a-d-HSV were incubated with FITC-HSV antibody for 1 h at 4°C, washed, and incubated for 30 min at 37°C. After warming, ECE-1a-d trafficked from the plasma membrane (arrowheads) to endosomes (arrows). (B and C) Trafficking of ECE-1 and Alexa-CGRP. HEK-CLR-RAMP1 cells expressing ECE-1a-d-HSV were incubated with Alexa-CGRP, and ECE-1 was localized as in A. (B) After 1 h at 4°C, ECE-1a-d-HSV and CGRP colocalized at the plasma membrane (arrowheads). (D) After 30 min at 37°C, ECE-1a-d-HSV and CGRP colocalized in endosomes (arrows). Bar, 10 μm. (D) Quantification of cell surface ECE-1c-HSV by flow cytometry. HEK-CLR-RAMP1 cells expressing ECE-1c-HSV were incubated with HSV antibody, and incubated with vehicle or CGRP (100 nM) for 30 min. Surface ECE-1c-HSV was quantified by flow cytometry. In vehicle-treated cells, warming resulted in loss of surface ECE-1c, which was not affected by CGRP. *, P < 0.05 to 4°C. (E) Immunoprecipitation of ECE-1c and CLR. HEK-CLR-RAMP1 cells expressing ECE-1c or vector control were incubated with CGRP. ECE-1c was immunoprecipitated and blots probed for ECE-1c and CLR. ECE-1c but not CLR was detected.
To determine whether CGRP promoted endocytosis of ECE-1, we used flow cytometry to quantify ECE-1c-HSV at the cell surface of HEK-CLR-RAMP1 cells. In cells incubated with vehicle for 30 min at 37°C, ECE-1c-HSV at the cell surface was 55.9 ± 5.0% of that at 4°C (100%), consistent with constitutive endocytosis (Fig. 7 D). CGRP (100 nM, 30 min, 37°C) did not affect this endocytosis. Thus, activation of CLR/RAMP1 with CGRP does not promote ECE-1c endocytosis.
We used immunoprecipitation and Western blotting to determine whether CLR interacts with ECE-1c. HEK-CLR-RAMP1 cells expressing ECE-1c-HSV or empty vector were incubated with CGRP (100 nM, 0–30 min, 37°C). ECE-1c was immunoprecipitated and blots were probed for ECE-1c and CLR. Although ECE-1c was successfully immunoprecipitated and detected by Western blotting, CLR was not detectable in the immunoprecipitates before or after treatment with CGRP (Fig. 7 E). There were no signals in cells expressing vector control. Thus, ECE-1c and CLR do not detectably associate.
AT1AR and B2R resensitize and recycle independently of ECE-1
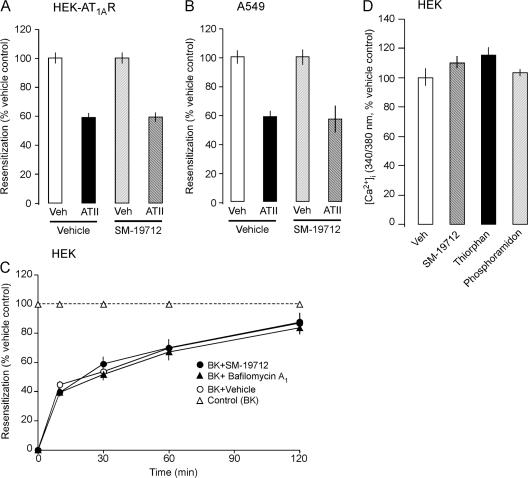
To investigate the selectivity with which ECE-1 controls receptor recycling and resensitization, we examined resensitization of the class B AT1AR. HEK-AT1AR cells were preincubated with ATII (100 nM, 1 h, 37°C) to desensitize AT1AR or vehicle (control), washed, recovered for 20 min or 2 h, and challenged with ATII (100 nM). In cells with active ECE-1, ATII-induced Ca2+ signaling was resensitized to 59 ± 3% at 20 min (Fig. 8 A) and 96 ± 3% at 2 h (unpublished data). SM-19712 did not affect resensitization at either time. SM-19712 also did not affect resensitization of responses to ATII in A549 cells expressing endogenous AT1AR and ECE-1 (Fig. 8 B). Thus, ECE-1 does not regulate resensitization of AT1AR, consistent with its inability to degrade ATII at endosomal pH.
Figure 8.
ECE-1 does not regulate ATII or BK Ca2+ signaling. (A and B) Resensitization of ATII-induced Ca2+ signaling in HEK-AT1AR cells (A) and A549 cells (B). Cells were incubated with ATII (100 nM for HEK, 1 μM for A549) or vehicle (veh, control) for 1 h at 37°C, washed and recovered for 20 min (HEK) or 1 h (A549). The change in [Ca2+]i to a second ATII challenge (100 nM for HEK, 1 μM for A549) was determined and expressed as % response in the vehicle-treated cells (100%). SM19712 did not affect resensitization. (C) Resensitization of BK-induced Ca2+ signaling in HEK cells. Cells were incubated with BK (100 nM) or vehicle (veh, control) for 10 min at 37°C, washed and recovered for 0–120 min. Resensitization was identical in cells treated with vehicle (DMSO), SM-19712, or bafilomycin A1. (D) Effects of peptidase inhibitors on BK-induced Ca2+ signaling in HEK cells. SM-19712, thiorphan, and phosphoramidon did not affect responses to a single BK challenge (100 nM).
We examined the role of ECE-1 and endosomal acidification in regulating resensitization of the class A B2R. HEK cells, which naturally express B2R and ECE-1, were preincubated with BK (100 nM, 10 min, 37°C) or vehicle (control), washed, and challenged with BK (100 nM) after a 0–120-min recovery. Preincubation with BK abolished BK-induced Ca2+ signals at 0 min recovery, indicating complete desensitization (Fig. S5 C). BK-induced Ca2+ signaling rapidly resensitized to 45 ± 2% at 10 min and 87 ± 6% at 120 min recovery (Fig. 8 C). SM-19712 or bafilomycin A1 had no effect on desensitization (Fig. S5 C) or resensitization (Fig. 8 C). Thus, although ECE-1 rapidly degrades BK at endosomal pH, ECE-1 and endosomal acidification do not affect B2R resensitization. Moreover, neither SM-19712, thiorphan (NEP inhibitor), nor phosphoramidon (NEP and ECE-1 inhibitor) affected responses to a single BK (100 nM) challenge of HEK cells (Fig. 8 D). Thus, cell surface ECE-1 and NEP do not regulate BK-induced activation of the B2R in HEK cells.
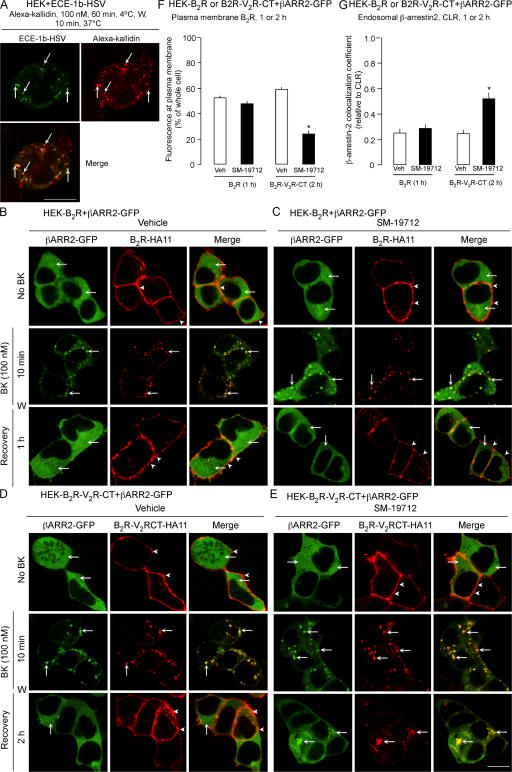
One explanation of the lack of involvement of ECE-1 in B2R resensitization is that B2R recycles without trafficking to ECE-1– containing endosomes. To examine this possibility, we incubated HEK cells expressing ECE-1a-d-HSV with Alexa-kallidin (100 nM, BK with N-terminal Lys labeled with Alexa), an agonist of the B2R, and localized HSV-FITC and Alexa-kallidin. After 1 h at 4°C, kallidin and ECE-1a-d-HSV colocalized to the plasma membrane (unpublished data). After 10 min at 37°C, kallidin prominently colocalized with ECE-1a-d-HSV in endosomes (Fig. 9 A for ECE-1b). Kallidin also internalized to localize with endogenous immunoreactive ECE-1 in HEK cells (unpublished data). Thus, kallidin and presumably BK and B2R traffic to endosomes containing ECE-1a-d.
Figure 9.
ECE-1 does not regulate recycling of the B2R. (A) Localization of Alexa-kallidin and ECE-1b-HSV in HEK cells expressing ECE-1b-HSV. Cells were incubated with Alexa-kallidin and FITC-HSV antibody for 1 h at 4°C, washed, and incubated at 37°C for 10 min. Kallidin colocalized with ECE-1b-HSV (arrows). (B–E) Effects of ECE-1 inhibition on BK-induced translocation of β-arrestin2-GFP in HEK cells. HEK-B2R cells (B and C) or HEK-B2R-V2RCT cells (D and E) were incubated with BK (100 nM) for 0–10 min at 37°C, washed and recovered in BK-free medium for 1–2 h. (B and D) In unstimulated vehicle-treated cells, β-arrestin2 was cytosolic (arrows) and B2R and B2R-V2RCT were at the plasma membrane (arrowheads). After 10 min with BK, β-arrestin2 colocalized in endosomes with B2R and B2R-V2RCT (arrows). After 1 h (B2R) or 2 h (B2R-V2RCT) recovery, β-arrestin2 was cytosolic and B2R and B2R-V2RCT were at the plasma membrane. (C and E) SM-19712 did not affect trafficking of B2R or β-arrestin2 (C), but caused retention of β-arrestin2 and B2R-V2RCT in endosomes (E) (arrows). Bar, 10 μm. (F) Quantification of the effect of SM-19712 on recycling of B2R at 1 h recovery and B2R-V2RCT at 2 h recovery. SM-19712 caused an ∼50% reduction in B2R-V2RCT recycling. (G) Colocalization coefficient of β-arrestin2 and B2R at 1 h recovery and B2R-V2RCT at 2 h recovery. SM-19712 did not affect colocalization with B2R but caused an approximately twofold increase in colocalization with B2R-V2RCT. *, P < 0.05.
Alternatively, because B2R interacts only transiently with β-arrestins (Simaan et al., 2005), B2R may rapidly dissociate from β-arrestins to recycle by a mechanism that does not require ECE-1 degradation of BK. To assess this possibility, HEK-B2R cells expressing β-arrestin2-GFP were incubated with antibody to the extracellular B2R HA11 epitope, and then stimulated with BK (100 nM, 10 min). After 10 min, B2R and β-arrestin2 colocalized in endosomes, and after 60 min recovery B2R had recycled and β-arrestin2 was cytosolic (Fig. 9 B). SM-19712 did not affect this trafficking (Fig. 9 C). Substitution of the C terminus of the B2R with that of the class B V2R generates a functional receptor (B2R-V2RCT) that shows sustained interactions with β-arrestins (Simaan et al., 2005). BK induced sustained colocalization of B2R-V2RCT and β-arrestin2 in endosomes, and B2R-V2RCT recycled more slowly (2 h) than B2R (1 h) (Fig. 9 D). SM-19712 inhibited recycling of B2R-V2RCT, causing it retention in endosomes with β-arrestin2 (Fig. 9 E). Quantitative analysis confirmed that SM-19712 inhibited recycling of B2R-V2RCT (>50% reduction) but not B2R (<10% reduction) (Fig. 9 F), and caused a twofold increase in the colocalization coefficient of B2R-V2RCT with β-arrestin2 (Fig. 9 G). Thus, ECE-1 does not control B2R recycling because this receptor interacts transiently with β-arrestins.
Discussion
We have defined a novel role for endosomal ECE-1 in the post-endocytic sorting of GPCRs and β-arrestins. Our results show that CGRP, CLR, RAMP1, β-arrestins, and ECE-1 traffic to early endosomes (Fig. 10, steps 1–4). ECE-1 degrades CGRP in acidified endosomes to fragments that are unable to rebind CLR/RAMP1. This degradation promotes the dissociation of CGRP and β-arrestins from CLR/RAMP1, inducing redistribution of β-arrestins to the cytosol and recycling and resensitization of CLR/RAMP1. This mechanism is specific, because ECE-1 does not degrade ATII and thus does not regulate recycling or resensitization of the AT1AR. Moreover, ECE-1 has little effect on resensitization of the rapidly recycling B2R that interacts only transiently with β-arrestins.
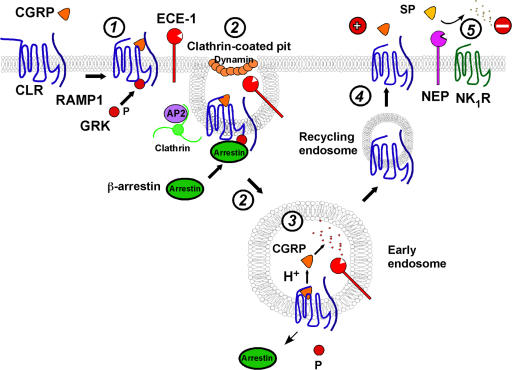
Figure 10.
Metalloendopeptidases regulation of peptide signaling. (1) CGRP binding to CLR and RAMP1 induces G protein receptor kinase (GRK) phosphorylation (P) of CLR. β-arrestins translocate to the plasma membrane to interact with phosphorylated CLR. (2) The CGRP/CLR/RAMP1/β-arrestin complex undergoes clathrin-, AP2- and dynamin-dependent endocytosis; ECE-1 co-internalizes with the complex. (3) Endosomal acidification promotes CGRP dissociation from CLR/RAMP1. ECE-1 degrades CGRP in acidic endosomes, preventing CGRP rebinding and promoting dissociation of CGRP and β-arrestin from CLR/RAMP1, which allows dephosphorylation of CLR. (4) CLR and RAMP1, freed from CGRP and β-arrestins, recycle and resensitize. (5) NEP degrades SP in extracellular fluid to limit NK1R activation.
ECE-1 shows isoform-specific localization in early endosomes containing CGRP, CLR, RAMP1, and β-arrestins
ECE-1b and ECE-1d were prominently detected in EEA1-positive endosomes. Although ECE-1a and ECE-1c were mainly at the plasma membrane, they also colocalized with EEA1. All ECE-1 isoforms, including ECE-1a and ECE-1c, were prominently detected in enlarged endosomes expressing the constitutively active Rab5aQ79L, which stimulates homotypic early endosome fusion (Stenmark et al., 1994). These results are consistent with reports of the presence of ECE-1b in early, recycling, and late endosomes, ECE-1d in recycling endosomes, and ECE-1a and ECE-1c at the plasma membrane (Schweizer et al., 1997; Azarani et al., 1998; Muller et al., 2003).
CGRP, CLR, and RAMP trafficked to endosomes containing ECE-1. Because CLR/RAMP1 and β-arrestin2 prominently colocalized in endosomes, β-arrestin2 also colocalizes with ECE-1 in endosomes. CGRP and CLR most prominently colocalized with ECE-1b and ECE-1d, and there was limited colocalization with ECE-1a and ECE-1c in endosomes. By labeling ECE-1 at the cell surface with antibody to an extracellular epitope, we observed constitutive endocytosis of all ECE-1 isoforms. Although we cannot exclude the possibility that antibody binding caused ECE-1 endocytosis, the prominent localization of ECE-1a and ECE-1c in Rab5aQ79QL-containing endosomes supports the suggestion of dynamic regulation of these isozymes. Indeed, ECE-1c, and to a lesser extent ECE-1b and ECE-1d, constitutively internalize by a dynamin-dependent mechanism and recycle (Muller et al., 2003). Notably, all ECE-1 isoforms co-internalized with Alexa-CGRP. Although flow cytometry indicated that CGRP did not affect endocytosis of ECE-1c, the possibility that CGRP induces trafficking of ECE-1 from the plasma membrane to endosomes requires further evaluation because the subcellular distribution of ECE-1 is regulated by second messenger kinases (Jafri and Ergul, 2006). Thus, receptor-activated second messenger kinases may stimulate the redistribution of ECE-1 isoforms to endosomes, promoting neuropeptide degradation.
ECE-1 degrades CGRP in acidified early endosomes
ECE-1 hydrolyzed six bonds within the biologically active C-region of CGRP (8–37), which would inactivate the peptide. ECE-1 degraded CGRP at pH 5.5 but not at pH 7.4, in accord with the narrow acidic pH optimum reported for ECE-1 degradation of other neuropeptides (Fahnoe et al., 2000). The lack of degradation at pH 7.4 suggests that ECE-1 present at the plasma membrane is unlikely to degrade CGRP in the extracellular fluid to regulate activation of CLR/RAMP1. Indeed, ECE-1 inhibition/knockdown did not affect CGRP-induced Ca2+ signaling in HEK or SK-N-MC cells, which expressed ECE-1a and ECE-1c that are known to be at the cell surface. The inability of ECE-1 to degrade CGRP and SP in the extracellular fluid contrasts with NEP, which degrades SP at the cell surface to limit activation of the NK1R (Okamoto et al., 1994) (Fig. 10, step 5).
The observation that SM-19712 prevented degradation of internalized CGRP in HEK-CLR-RAMP1 cells indicates that ECE-1 degrades CGRP in endosomes. The isoform of ECE-1 that is mainly responsible for the degradation of endocytosed CGRP remains to be identified because all isoforms prominently colocalized with Alexa-CGRP. The vacuolar H+-ATPase inhibitor bafilomycin A1 also prevented degradation of endocytosed CGRP, indicating a requirement for endosomal acidification. Endosomal acidification may promote CGRP degradation by enhancing ECE-1 activity or by causing CGRP dissociation from CLR/RAMP1. ECE-1 probably degrades receptor-dissociated CGRP, but it remains to be determined whether ECE-1 degrades receptor-associated peptide. Although we did not detect interaction of ECE-1c with CLR/RAMP1, another metalloendopeptidase, endopeptidase 3.4.24.15, interacts with the C-tails of the AT1AR and B2R (Shivakumar et al., 2005). Although principally cytosolic, endopeptidase 24.15 has been detected in endosomes and degrades BK (Norman et al., 2003). Cathepsin B degrades EGF and insulin-like growth factor-I in endosomes (Authier et al., 1999, 2005). Thus, several proteases may degrade peptides in endosomes.
Endosomal ECE-1 promotes dissociation of CLR/RAMP1 and β-arrestins in endosomes, and induces recycling and resensitization of CLR/RAMP1
Several observations suggest that ECE-1 promotes dissociation of GPCRs from β-arrestins in endosomes, thereby allowing receptors to recycle. First, ECE-1 inhibition/knockdown prevented CLR/RAMP1 recycling from endosomes. Second, ECE-1 inhibition prevented the translocation of β-arrestin from endosomes to the cytosol. Third, inhibition of vacuolar H+-ATPase prevented CLR/RAMP1 recycling and the return of β-arrestin to the cytosol. Thus, although we did not directly examine the interactions between CLR/RAMP and β-arrestins, our data suggest that ECE-1, by degrading CGRP in acidified endosomes, promotes dissociation of β-arrestins from CLR/RAMP1, accelerating the return of β-arrestins to the cytosol and of CLR/RAMP1 to the cell surface. ECE-1–induced dissociation of β-arrestins from CLR/RAMP1 may promote the dephosphorylation of CLR by endosomal phosphatases, permitting recycled receptors to resensitize (Fig. 10, step 3).
Our results suggest that ECE-1 accelerates resensitization of CGRP signaling. In HEK-CLR-RAMP1 and SK-N-MC cells, ECE-1 inhibition and knockdown markedly delayed resensitization of CGRP signaling. Thus, the mechanism is not unique to cells overexpressing ECE-1, CLR, and RAMP1, but also occurs in cells that endogenously express these proteins, and inhibition of existing ECE-1 and suppression of ECE-1 synthesis have similar effects. Conversely, overexpression of all ECE-1 isoforms in HEK-CLR-RAMP1 cells markedly amplified resensitization of CGRP signaling. Although Alexa-CGRP colocalized most prominently with ECE-1b and ECE-1d in endosomes, overexpression of ECE-1c had the greatest effect on resensitization. A likely explanation of this discrepancy is that CGRP prominently co-internalizes with ECE-1c, which constitutively internalizes and presumably recycles.
β-arrestins recruit components of the MAPK pathway, including src and raf-1, to endosomes, allowing endocytosed receptors to continue to signal (DeFea et al., 2000a,b; Tohgo et al., 2003). ECE-1, by controlling the subcellular distribution of β-arrestins, may thereby affect mitogenic signaling of receptors, a possibility that requires further investigation. In support of this possibility, inhibitors of cathepsin B, which degrades insulin-like growth factor-I in endosomes, markedly influence insulin-like growth factor-I signaling (Navab et al., 2001).
Specificity of regulation of post-endocytic trafficking and signaling of GPCRs by endosomal ECE-1
ECE-1 is expressed by endothelial, epithelial, smooth muscle, and neuronal cells, which are regulated by neuropeptides that are potential ECE-1 substrates (Barnes and Turner, 1997, 1999; Korth et al., 1999). Endosomal ECE-1 may regulate receptors in these cells provided the peptide and its receptor traffic to ECE-1– containing endosomes, and the peptide is an ECE-1 substrate at endosomal pH. Our finding that ECE-1 regulates recycling and resensitization of CLR/RAMP1 supports the report that ECE-1 also degrades tachykinins in endosomes to control recycling and resensitization of the NK1R (Roosterman et al., 2007). ECE-1 may also control post-endocytic trafficking of the neurotensin 1 receptor because ECE-1 degrades neurotensin at acidic pH (Johnson et al., 1999). CLR/RAMP1, NK1R, and neurotensin receptor 1 are class B GPCRs that colocalize for prolonged periods with β-arrestins in endosomes containing ECE-1 (Oakley et al., 1999, 2000, 2001; Hilairet et al., 2001; Schmidlin et al., 2003). AT1AR is also a class B GPCR that slowly recycles after activation by ATII. However, ECE-1 did not degrade ATII and ECE-1 inhibition did not affect resensitization of ATII- induced Ca2+ signaling. Thus, ECE-1 does not regulate all class B GPCRs, and further studies are required to determine whether this is a general mechanism.
Although ECE-1 rapidly degraded BK at pH 5.5 and the B2R agonist kallidin trafficked to ECE-1–containing endosomes, SM-19712 did not affect B2R recycling or resensitization. An explanation for this discrepancy is that B2R is a class A GPCR that interacts with low affinity with β-arrestins and rapidly recycles without a requirement for endosomal acidification or BK degradation. Indeed, bafilomycin A1 did not affect resensitization of the B2R. Moreover, SM-19712 strongly inhibited recycling of B2R-V2RCT, which shows sustained interactions with β-arrestins (Simaan et al., 2005). Thus, the lack of effect of an ECE-1 inhibitor on B2R recycling and resensitization signaling may be due to its low affinity interactions with β-arrestins. Whether ECE-1 regulates trafficking and signaling of class A GPCRs is unknown.
In summary, we have identified a new role for endosomal ECE-1. ECE-1 co-internalizes with and degrades neuropeptides in acidified endosomes. By degrading peptides to fragments that are unable to rebind receptors, ECE-1 destabilizes the peptide/receptor/β-arrestin complex, and thereby initiates receptor recycling and resensitization. The mechanism requires that peptides are substrates for ECE-1 in acidified endosomes, and that receptors show sustained interactions with β-arrestins which are disrupted by endosomal acidification and peptide degradation. The observation that ECE-1 inhibitors prevent resensitization of responses to CGRP and SP, mediators of inflammation and pain, suggests that inhibitors of endosomal ECE-1, by attenuating receptor resensitization, may provide a novel therapy for peptide-mediated diseases.
Materials and methods
Antibodies
Source of antibodies were: rabbit antibody to the catalytic domain common to all isoforms of human ECE-1 (Korth et al., 1997); goat anti–human ECE-1 (R&D Systems); rabbit antibody to the N terminus of human ECE-1 (Invitrogen); rabbit antibodies to rat CLR and rat RAMP1 (Cottrell et al., 2005); rat high affinity anti-HA11 (Roche); rabbit anti-Myc (Sigma-Aldrich); goat anti-HSV and anti-HSV-FITC (AbCam); goat anti-Myc (Santa Cruz Biotechnology, Inc.); mouse anti-early endosomal antigen 1 (EEA1) (BD Transduction Laboratories); mouse anti-Na+K+-ATPase and mouse anti-HSV (Novus Biologicals); goat or donkey anti–mouse, –rat, –rabbit, or –goat IgG coupled to FITC, rhodamine red-X, or Cy5 (Jackson ImmunoResearch Laboratories); goat and donkey anti–rabbit IgG coupled to AlexaFluor 680 (Invitrogen); and donkey anti–mouse IRDye 800 (Rockland Immunochemicals, Inc.).
Reagents
Rat αCGRP, ATI, ATII, BK and kallidin were from Bachem. CGRP and kallidin were fluorescently labeled with an AlexaFluor 594 labeling kit (Invitrogen) (Cottrell et al., 2005). 125I-His8 human CGRP was from GE Healthcare. rhECE-1 was from R&D Systems. The ECE-1 inhibitor SM-19712 (4-chloro-N-[(4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino]carbonyl] benzenesulfonamide, monosodium salt) (Umekawa et al., 2000) was from Sigma-Aldrich. Other reagents were from Sigma-Aldrich or have been described (Cottrell et al., 2007; Roosterman et al., 2007).
cDNAs
cDNAs encoding ECE-1a-d with intracellular N-terminal GFP have been described (Roosterman et al., 2007). Constructs of ECE-1a-d with extracellular C-terminal HSV epitope were generated by PCR (primer sequences available on request). Constructs of rat CLR and RAMP1 with extracellular N-terminal HA11 and Myc epitopes, respectively, have been described (Cottrell et al., 2007). To create a vector expressing CLR, RAMP1, and ECE-1a-d-GFP, the CLR-RAMP1 vector was digested with BsmI and each ECE-1 isoform was subcloned into this site with its own CMV promoter and bovine growth hormone polyA tail. Human AT1AR was cloned from A549 cells by RT-PCR and subcloned into pcDNA5/FRT. Human B2R and B2R-V2RCT with N-terminal HA11 epitopes were from S.A. Laporte (McGill University, Montreal, Canada). Rab5aQ79L-GFP was from M. von Zastrow (University of California, San Francisco, San Francisco, CA), and a CFP tag was added by subcloning. Human β-arrestin2 was cloned from HEK cells using RT-PCR, and subcloned into pEGFP-N1. Constructs were sequenced.
Generation and maintenance of cell lines
Sources and maintenance of human embryonic kidney (HEK293) cells, sarcoma virus-transformed rat kidney epithelial cells (KNRK), human neuroblastoma cells SK-N-MC, and human lung adenocarcinoma cells A549 have been described (Schmidlin et al., 2003; Cottrell et al., 2007; Roosterman et al., 2007). HEKFLP cells stably expressing CLR and RAMP1 have been described (Cottrell et al., 2007). In some experiments, cells were transiently transfected with CLR/RAMP1, CLR/RAMP1/ECE-1a-d-GFP, ECE-1a-d-GFP, ECE-1a-d-HSV, Rab5aQ79L-CFP, or β-arrestin2-GFP using Lipofectamine 2000 (Invitrogen). Cells were plated 24–48 h before use.
RT-PCR
Total RNA from HEK, SK-N-MC, and A549 cells was analyzed by RT-PCR using primers specific for ECE-1 isoforms, and sequenced (Roosterman et al., 2007).
SDS-PAGE and Western blotting
Membranes proteins from HEK, SK-N-MC, and A549 cells (15 μg) or rhECE-1 (10 ng) were separated by SDS-PAGE, transferred to PVDF membranes, and analyzed by Western blotting using antibodies to ECE-1 (1:2,000 or 1:1,000) or Na+K+-ATPase (1:5,000) (overnight, 4°C) (Cottrell et al., 2007; Roosterman et al., 2007). Membranes were incubated with secondary antibody conjugated to AlexaFluor 680 (1:20,000, 1 h, room temperature), and blots were analyzed with the Odyssey Infrared Imaging System (Li-COR Biosciences).
Immunoprecipitation
Proteins were cross-linked using dithiobis(succinimidyl)propionate (Thermo Fisher Scientific), lysed with 1 ml RIPA buffer and centrifuged (10 min, 15,000 g, 4°C). ECE-1c was immunoprecipitated using goat anti-HSV (2 μg/ml, 1 h, 4°C). Protein A/G PLUS beads (Santa Cruz Biotechnology, Inc.) were added (30 μl, 1 h, 4°C). Immunoprecipitates were analyzed by Western blotting using mouse anti-HSV (1:5,000) and CLR (RK11; 1:5,000) (overnight, 4°C) (Cottrell et al., 2007).
Subcellular localization of ECE-1, CLR, RAMP1, B2R, B2R-V2RCT, Rab5aQ79L, β-arrestin2, Alexa-CGRP, and Alexa-kallidin
Cells were fixed in 4% paraformaldehyde, 100 mM PBS, pH 7.4 (20 min at 4°C), and washed for 15 min with PBS, 0.1% saponin, and 1% normal goat serum or 2% normal donkey serum. Cells were incubated with primary antibodies to CLR (1:4,000–8,000), RAMP1 (1:2,000 or goat anti-Myc, 1:100), ECE-1 (1:500), or EEA1 (1:250) (overnight, 4°C). Cells were washed and incubated with fluorescent secondary antibodies (1:200–500, 2 h, room temperature; overnight, 4°C). As a control for specificity, the ECE-1 antibody was incubated with antigen used for immunization (25 μg/ml, 72 h). In some experiments, ECE-1 isoforms, Rab5aQ79L, and β-arrestin2 were detected using GFP or CFP. To examine trafficking of fluorescent peptides, cells were incubated with Alexa-CGRP or Alexa-kallidin (100 nM) for 1 h at 4°C, washed, incubated at 37°C for specified times, and fixed. To examine receptor recycling, CLR, RAMP1, B2R, or B2R-V2RCT were labeled at the cell surface by incubating cells with rat anti-HA11 (to detect CLR, B2R, or B2R-V2RCT; 1:100) and rabbit or goat anti-Myc (to detect RAMP1; 1:100) for 30 min at 37°C (Cottrell et al., 2007). Cells were washed, incubated with CGRP (100 nM, 1 h) or BK (100 nM, 10 min), washed, and recovered in agonist-free medium for 0–6 h. To examine ECE-1 endocytosis, HEK cells expressing ECE-1a-d-HSV were incubated with goat anti-HSV-FITC (1:1,000, 1 h, 4°C) with or without fluorescent peptides. Cells were washed and incubated at 37°C for 0–30 min
Confocal microscopy
Cells were observed by using a LSM 510 Meta confocal microscope (Carl Zeiss, Inc.) with Plan Apo 100× (NA 1.4) or 63× (NA 1.4) objectives (Carl Zeiss, Inc.) (Cottrell et al., 2007; Roosterman et al., 2007). Images were collected at a zoom of 1–3 and an iris of <3 μm, and 5–10 optical sections were taken at intervals of 0.5 μm. Slides were mounted on Vectashield mounting medium with DAPI (Vector Laboratories) and observed at room temperature.
Image analysis
The proportion of the total cellular fluorescence at the plasma membrane and the colocalization coefficient of proteins in endosomes (0, no colocalization; 1, complete colocalization) were determined using LSM 510 software (Carl Zeiss, Inc.) (Hasdemir et al., 2007). More than 20 cells were analyzed per experiment.
Peptide degradation by ECE-1
Peptides (250 μM) were incubated with rhECE-1 (83 or 415 nM) at 37°C in 50 mM MES-KOH, pH 5.5, or 50 mM Tris-HCl, pH 7.4, for 0–720 min (Roosterman et al., 2007). The ECE-1 inhibitor SM-19712 (100 μM) was preincubated with ECE-1 on ice for 30 min at pH 5.5 or 7.4 before addition of substrate. Products were separated by reversed-phase HPLC and identified using an ABI 4700 MALDI TOF/TOF mass spectrometer. Mass spectrometry data were provided by the UCSF Mass Spectrometry Facility (A.L. Burlingame, Director) supported by the Biomedical Research Technology Program of the National Center for Research Resources, National Institutes of Health (NCRR BRTP 01614).
Endocytosis and degradation of internalized 125I-CGRP
HEK-CLR-RAMP1 cells (3 × 105 cells per 35-mm dish) were incubated in HBSS, pH 7.4, 0.1% BSA with CGRP (10 nM unlabeled CGRP, 300,000 cpm 125I-His8-CGRP) for 5 or 10 min at 37°C (Roosterman et al., 2007). Cells were washed with acidified HBSS (pH 4.75, acetic acid), 0.1% BSA to remove surface-bound label, and incubated in HBSS, pH 7.4, 0.1% BSA for 0, 15, 30, or 60 min at 37°C. The medium was collected and acidified with 10% TFA in water. Cells were lysed with 0.1% TFA in water. Samples were fractionated by HPLC (Roosterman et al., 2007).
pH dependency of 125I-CGRP binding to CLR/RAMP1
Membranes from HEK-CLR-RAMP1 cells (20 μl, ∼200 μg protein) were incubated (90 min, 4°C) with 100,000 cpm 125I-CGRP in buffers of graded acidity: pH 5.0, acetic acid; pH 5.5 and 6.0 MES; pH 6.5 and 7.0 Pipes; pH 7.5 Tris (all with 150 mM NaCl, 0.1% BSA, protease inhibitor cocktail, 20 mM buffer, 300 μl total volume) (Roosterman et al., 2007). Membranes were isolated by filtration, washed with corresponding buffer (4°C), and the membrane-bound radioactivity was counted. Non-specific binding was determined in the presence of 0.1 mM unlabeled CGRP and was subtracted to give specific binding. Results are expressed as the percent binding at pH 7.5 (100%)
Measurement of [Ca2+]i
[Ca2+]i was measured in cells on glass coverslips or in suspension at 37°C using Fura-2AM as described (Cottrell et al., 2007; Roosterman et al., 2007). To examine responses to a single stimulus, cells were challenged with CGRP or BK (30 or 100 nM). To assess desensitization and resensitization, cells were incubated with CGRP, ATII, or BK (100–1,000 nM, 1 h, 37°C) or vehicle (control), washed, and recovered in agonist-free medium for 20 min to 6 h at 37°C. Cells were challenged with CGRP, ATII, or BK (30–1,000 nM), and [Ca2+]i was measured.
Drug treatments
SM-19712 (10 μM), phosphoramidon (1 μM), thiorphan (1 μM), bafilomycin A1 (0.15 or 1 μM), or vehicle (DMSO, control) was preincubated with cells for 20–60 min, and included in buffers throughout the experiment.
siRNA
siRNA reagents were from Dharmacon. ON-TARGET plus SMART pool (-005857-00) consisted of four distinct siRNA duplexes targeted to knockdown of human ECE-1 mRNA. siCONTROL Non-Targeting siRNA pool (D-001206) consisted of four off-target siRNA duplexes. HEK-CLR-RAMP1 cells (0.5 × 106 cells per well of 6-well plate in antibiotic-free medium) were transfected with 200 pmol siRNA and 5 μl of DharmaFECT 1 according to the manufacturer's instructions. Cells were incubated in the transfection medium for 72 h, and then used for experiments.
Flow cytometry
HEK-CLR-RAMP1 cells expressing ECE-1c-HSV were incubated with goat anti-HSV (1 mg/ml, 1 h, 4°C). Cells were washed, stimulated with CGRP (100 nM, 30 min, 37°C), and incubated with donkey anti–goat-FITC (1:200, 1 h, 4°C). Cells were analyzed using a FACSort (Becton Dickinson) and CELLQuest 3.3 software (BD Biosciences). Background fluorescence was measured in cells treated with secondary antibody only and was subtracted from the fluorescence histograms of the treatment groups.
Statistics
Results are expressed as mean ± SE from n ≥ 3 experiments. Results are compared by t test for two comparisons or ANOVA and Student-Newman-Kuel's test for multiple comparisons, with P < 0.05 considered significant. Representative images of cells and gels are shown from n ≥ 3 experiments.
Online supplemental material
Fig. S1 shows endogenous expression of ECE-1a-d, B2R, CLR, RAMP1, and AT1AR in HEK, SK-N-MC, and A549 cell lines. Fig. S2 shows that CGRP induces trafficking of CLR to endosomes containing ECE-1. The chromatograms in Fig. S3 show that rhECE-1 degrades CGRP, and that endogenous ECE-1 degrades endocytosed 125I-His8-CGRP in HEK cells. Fig. S4 shows that ECE-1 siRNA effectively suppresses levels of ECE-1 in HEK cells. Fig. S5 shows that SM-19712 does not affect CGRP-induced [Ca2+]i signaling or desensitization of [Ca2+]I signaling in HEK-CLR-RAMP1 and SK-N-MC cells. The figure also shows that SM-19712 and bafilomycin A1 do not affect BK-induced [Ca2+]i signaling or desensitization in HEK cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200704053/DC1.
Acknowledgments
This work was supported by National Institutes of Health grants DK39957, DK43207 (N.W. Bunnett), and Interdisziplinäres Zentrum für Klinische Forschung (STE), Sonderforschungsbereiche, Deutsche Forschungsgemeinschaft (M. Steinhoff).
Abbreviations used in this paper: AT, angiotensin; AT1AR, angiotensin type 1A receptor; B2R, bradykinin 2 receptor; BK, bradykinin; CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like receptor; ECE-1, endothelin-converting enzyme-1; ET, endothelin; GPCR, G protein–coupled receptor; NEP, neprilysin; NK1R, neurokinin 1 receptor; RAMP1, receptor activity-modifying protein 1; rhECE-1, recombinant human ECE-1; SP, substance P; V2R, vasopressin 2 receptor.
References
- Authier, F., M. Metioui, A.W. Bell, and J.S. Mort. 1999. Negative regulation of epidermal growth factor signaling by selective proteolytic mechanisms in the endosome mediated by cathepsin B. J. Biol. Chem. 274:33723–33731. [DOI] [PubMed] [Google Scholar]
- Authier, F., M. Kouach, and G. Briand. 2005. Endosomal proteolysis of insulin-like growth factor-I at its C-terminal D-domain by cathepsin B. FEBS Lett. 579:4309–4316. [DOI] [PubMed] [Google Scholar]
- Azarani, A., G. Boileau, and P. Crine. 1998. Recombinant human endothelin-converting enzyme ECE-1b is located in an intracellular compartment when expressed in polarized Madin-Darby canine kidney cells. Biochem. J. 333:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, K., and A.J. Turner. 1997. The endothelin system and endothelin-converting enzyme in the brain: molecular and cellular studies. Neurochem. Res. 22:1033–1040. [DOI] [PubMed] [Google Scholar]
- Barnes, K., and A.J. Turner. 1999. Endothelin converting enzyme is located on alpha-actin filaments in smooth muscle cells. Cardiovasc. Res. 42:814–822. [DOI] [PubMed] [Google Scholar]
- Brain, S.D., and A.D. Grant. 2004. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 84:903–934. [DOI] [PubMed] [Google Scholar]
- Brooks, S.C., L. Fernandez, and A. Ergul. 2000. Secretion of endothelin converting enzyme-1a: the hydrophobic signal anchor domain alone is not sufficient to promote membrane localization. Mol. Cell. Biochem. 208:45–51. [DOI] [PubMed] [Google Scholar]
- Cottrell, G.S., D. Roosterman, J.C. Marvizon, B. Song, E. Wick, S. Pikios, H. Wong, C. Berthelier, Y. Tang, C. Sternini, et al. 2005. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J. Comp. Neurol. 490:239–255. [DOI] [PubMed] [Google Scholar]
- Cottrell, G.S., B. Padilla, S. Pikios, D. Roosterman, M. Steinhoff, E.F. Grady, and N.W. Bunnett. 2007. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J. Biol. Chem. 282:12260–12271. [DOI] [PubMed] [Google Scholar]
- DeFea, K.A., Z.D. Vaughn, E.M. O'Bryan, D. Nishijima, O. Dery, and N.W. Bunnett. 2000. a. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. USA. 97:11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea, K.A., J. Zalevsky, M.S. Thoma, O. Dery, R.D. Mullins, and N.W. Bunnett. 2000. b. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148:1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnoe, D.C., J. Knapp, G.D. Johnson, and K. Ahn. 2000. Inhibitor potencies and substrate preference for endothelin-converting enzyme-1 are dramatically affected by pH. J. Cardiovasc. Pharmacol. 36:S22–S25. [DOI] [PubMed] [Google Scholar]
- Ferguson, S.S., W.E. Downey III, A.M. Colapietro, L.S. Barak, L. Menard, and M.G. Caron. 1996. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 271:363–366. [DOI] [PubMed] [Google Scholar]
- Garland, A.M., E.F. Grady, M. Lovett, S.R. Vigna, M.M. Frucht, J.E. Krause, and N.W. Bunnett. 1996. Mechanisms of desensitization and resensitization of G protein-coupled neurokinin1 and neurokinin2 receptors. Mol. Pharmacol. 49:438–446. [PubMed] [Google Scholar]
- Goodman, O.B., Jr., J.G. Krupnick, F. Santini, V.V. Gurevich, R.B. Penn, A.W. Gagnon, J.H. Keen, and J.L. Benovic. 1996. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 383:447–450. [DOI] [PubMed] [Google Scholar]
- Grady, E.F., A.M. Garland, P.D. Gamp, M. Lovett, D.G. Payan, and N.W. Bunnett. 1995. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol. Biol. Cell. 6:509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasdemir, B., N.W. Bunnett, and G.S. Cottrell. 2007. Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) mediates post-endocytic trafficking of protease-activated receptor 2 (PAR2) and calcitonin receptor-like receptor. J. Biol. Chem. 282:29646–29657. [DOI] [PubMed] [Google Scholar]
- Hilairet, S., C. Belanger, J. Bertrand, A. Laperriere, S.M. Foord, and M. Bouvier. 2001. Agonist-promoted internalization of a ternary complex between calcitonin receptor-like receptor, receptor activity-modifying protein 1 (RAMP1), and beta-arrestin. J. Biol. Chem. 276:42182–42190. [DOI] [PubMed] [Google Scholar]
- Hoang, M.V., and A.J. Turner. 1997. Novel activity of endothelin-converting enzyme: hydrolysis of bradykinin. Biochem. J. 327:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, A.R., and A.J. Turner. 2006. Expression and localization of endothelin-converting enzyme-1 isoforms in human endothelial cells. Exp. Biol. Med. (Maywood). 231:718–722. [PubMed] [Google Scholar]
- Jafri, F., and A. Ergul. 2006. Phosphorylation of endothelin converting enzyme-1 isoforms: relevance to subcellular localization. Exp. Biol. Med. (Maywood). 231:713–717. [PubMed] [Google Scholar]
- Johnson, G.D., T. Stevenson, and K. Ahn. 1999. Hydrolysis of peptide hormones by endothelin-converting enzyme-1. A comparison with neprilysin. J. Biol. Chem. 274:4053–4058. [DOI] [PubMed] [Google Scholar]
- Korth, P., G. Egidy, C. Parnot, J.M. LeMoullec, P. Corvol, and F. Pinet. 1997. Construction, expression and characterization of a soluble form of human endothelin-converting-enzyme-1. FEBS Lett. 417:365–370. [DOI] [PubMed] [Google Scholar]
- Korth, P., R.M. Bohle, P. Corvol, and F. Pinet. 1999. Cellular distribution of endothelin-converting enzyme-1 in human tissues. J. Histochem. Cytochem. 47:447–462. [DOI] [PubMed] [Google Scholar]
- Lohse, M.J., J.L. Benovic, J. Codina, M.G. Caron, and R.J. Lefkowitz. 1990. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 248:1547–1550. [DOI] [PubMed] [Google Scholar]
- Lu, B., M. Figini, C. Emanueli, P. Geppetti, E.F. Grady, N.P. Gerard, J. Ansell, D.G. Payan, C. Gerard, and N. Bunnett. 1997. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat. Med. 3:904–907. [DOI] [PubMed] [Google Scholar]
- McLatchie, L.M., N.J. Fraser, M.J. Main, A. Wise, J. Brown, N. Thompson, R. Solari, M.G. Lee, and S.M. Foord. 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 393:333–339. [DOI] [PubMed] [Google Scholar]
- Muller, L., A. Barret, E. Etienne, R. Meidan, O. Valdenaire, P. Corvol, and C. Tougard. 2003. Heterodimerization of endothelin-converting enzyme-1 isoforms regulates the subcellular distribution of this metalloprotease. J. Biol. Chem. 278:545–555. [DOI] [PubMed] [Google Scholar]
- Navab, R., E. Chevet, F. Authier, G.M. Di Guglielmo, J.J. Bergeron, and P. Brodt. 2001. Inhibition of endosomal insulin-like growth factor-I processing by cysteine proteinase inhibitors blocks receptor-mediated functions. J. Biol. Chem. 276:13644–13649. [DOI] [PubMed] [Google Scholar]
- Norman, M.U., R.A. Lew, A.I. Smith, and M.J. Hickey. 2003. Metalloendopeptidases EC 3.4.24.15/16 regulate bradykinin activity in the cerebral microvasculature. Am. J. Physiol. Heart Circ. Physiol. 284:H1942–H1948. [DOI] [PubMed] [Google Scholar]
- Oakley, R.H., S.A. Laporte, J.A. Holt, L.S. Barak, and M.G. Caron. 1999. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 274:32248–32257. [DOI] [PubMed] [Google Scholar]
- Oakley, R.H., S.A. Laporte, J.A. Holt, M.G. Caron, and L.S. Barak. 2000. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 275:17201–17210. [DOI] [PubMed] [Google Scholar]
- Oakley, R.H., S.A. Laporte, J.A. Holt, L.S. Barak, and M.G. Caron. 2001. Molecular determinants underlying the formation of stable intracellular g protein-coupled receptor-beta -arrestin complexes after receptor endocytosis. J. Biol. Chem. 276:19452–19460. [DOI] [PubMed] [Google Scholar]
- Okamoto, A., M. Lovett, D.G. Payan, and N.W. Bunnett. 1994. Interactions between neutral endopeptidase (EC 3.4.24.11) and the substance P (NK1) receptor expressed in mammalian cells. Biochem. J. 299:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans, L., T. Burli, M. Zerial, and A. Helenius. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 118:767–780. [DOI] [PubMed] [Google Scholar]
- Poyner, D.R., U. Soomets, S.G. Howitt, and U. Langel. 1998. Structural determinants for binding to CGRP receptors expressed by human SK-N-MC and Col 29 cells: studies with chimeric and other peptides. Br. J. Pharmacol. 124:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosterman, D., G.S. Cottrell, B.E. Padilla, L. Muller, C.B. Eckman, N.W. Bunnett, and M. Steinhoff. 2007. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc. Natl. Acad. Sci. USA. 104:11838–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin, F., D. Roosterman, and N.W. Bunnett. 2003. The third intracellular loop and carboxyl tail of neurokinin 1 and 3 receptors determine interactions with beta-arrestins. Am. J. Physiol. Cell Physiol. 285:C945–C958. [DOI] [PubMed] [Google Scholar]
- Schmidt, M., B. Kroger, E. Jacob, H. Seulberger, T. Subkowski, R. Otter, T. Meyer, G. Schmalzing, and H. Hillen. 1994. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1). FEBS Lett. 356:238–243. [DOI] [PubMed] [Google Scholar]
- Schweizer, A., O. Valdenaire, P. Nelbock, U. Deuschle, J.B. Dumas Milne Edwards, J.G. Stumpf, and B.M. Loffler. 1997. Human endothelin-converting enzyme (ECE-1): three isoforms with distinct subcellular localizations. Biochem. J. 328:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, K., M. Takahashi, M. Ikeda, and K. Tanzawa. 1995. Identification and characterization of two isoforms of an endothelin-converting enzyme-1. FEBS Lett. 371:140–144. [DOI] [PubMed] [Google Scholar]
- Shivakumar, B.R., Z. Wang, T.G. Hammond, and R.C. Harris. 2005. EP24.15 interacts with the angiotensin II type I receptor and bradykinin B2 receptor. Cell Biochem. Funct. 23:195–204. [DOI] [PubMed] [Google Scholar]
- Simaan, M., S. Bedard-Goulet, D. Fessart, J.P. Gratton, and S.A. Laporte. 2005. Dissociation of beta-arrestin from internalized bradykinin B2 receptor is necessary for receptor recycling and resensitization. Cell. Signal. 17:1074–1083. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., R.G. Parton, O. Steele-Mortimer, A. Lutcke, J. Gruenberg, and M. Zerial. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturiale, S., G. Barbara, B. Qiu, M. Figini, P. Geppetti, N. Gerard, C. Gerard, E.F. Grady, N.W. Bunnett, and S.M. Collins. 1999. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc. Natl. Acad. Sci. USA. 96:11653–11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgo, A., E.W. Choy, D. Gesty-Palmer, K.L. Pierce, S. Laporte, R.H. Oakley, M.G. Caron, R.J. Lefkowitz, and L.M. Luttrell. 2003. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 278:6258–6267. [DOI] [PubMed] [Google Scholar]
- Umekawa, K., H. Hasegawa, Y. Tsutsumi, K. Sato, Y. Matsumura, and N. Ohashi. 2000. Pharmacological characterization of a novel sulfonylureid-pyrazole derivative, SM-19712, a potent nonpeptidic inhibitor of endothelin converting enzyme. Jpn. J. Pharmacol. 84:7–15. [DOI] [PubMed] [Google Scholar]
- Valdenaire, O., D. Lepailleur-Enouf, G. Egidy, A. Thouard, A. Barret, R. Vranckx, C. Tougard, and J.B. Michel. 1999. A fourth isoform of endothelin- converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. Eur. J. Biochem. 264:341–349. [DOI] [PubMed] [Google Scholar]
- Xu, D., N. Emoto, A. Giaid, C. Slaughter, S. Kaw, D. deWit, and M. Yanagisawa. 1994. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 78:473–485. [DOI] [PubMed] [Google Scholar]
- Yang, H.Y., E.G. Erdos, and Y. Levin. 1970. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim. Biophys. Acta. 214:374–376. [DOI] [PubMed] [Google Scholar]
- Yang, H.Y., E.G. Erdos, and Y. Levin. 1971. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J. Pharmacol. Exp. Ther. 177:291–300. [PubMed] [Google Scholar]