Abstract
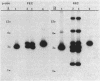
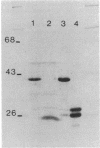
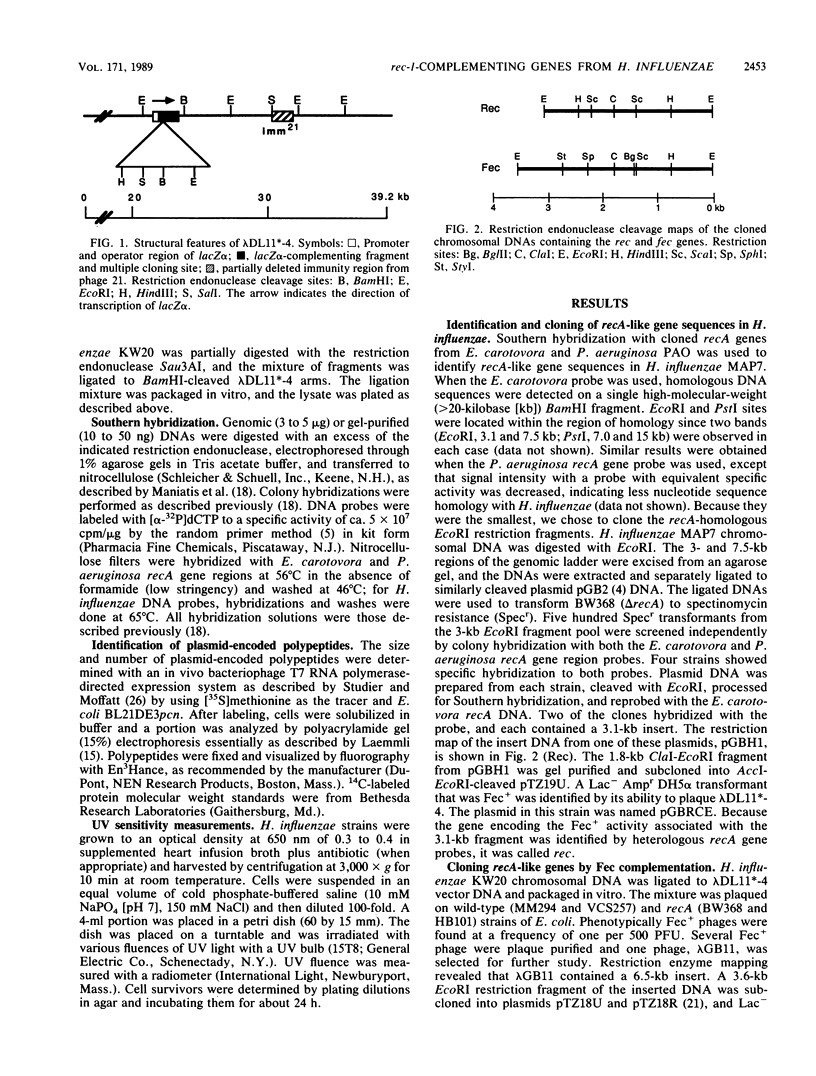
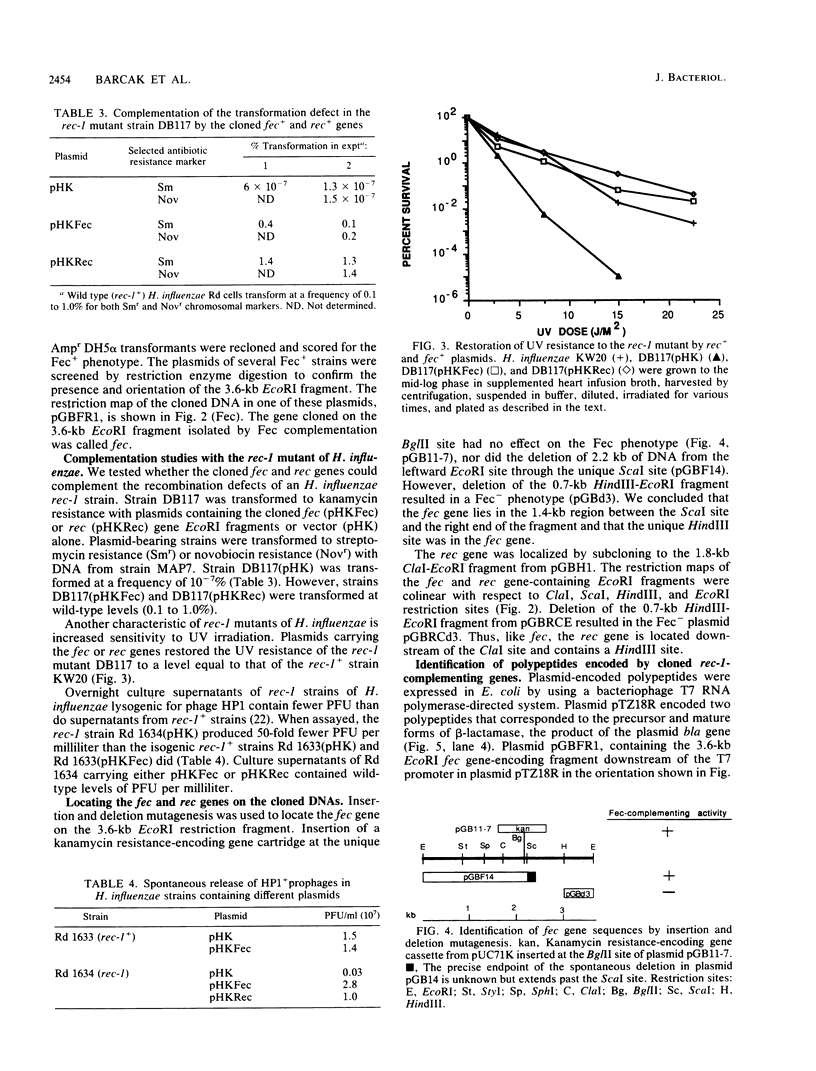
Two Haemophilus influenzae Rd genes each complemented the pleiotropic defects of the recA-like mutation rec-1. One gene, fec, was isolated on a 3.6-kilobase-pair EcoRI restriction fragment by complementation of the Fec- phenotype of bacteriophage lambda. The other gene, rec, was identified on a 3.1-kilobase-pair EcoRI fragment by Southern hybridization by using recA-like gene probes from Erwinia carotovora and Pseudomonas aeruginosa PAO. In a rec-1 strain of H. influenzae, the cloned genes restored resistance to UV irradiation, transformation by chromosomal DNA, and spontaneous release of HP1 prophage to wild-type levels. The fec and rec genes were located on the cloned segments by insertion and deletion mutagenesis and subcloning. The restriction endonuclease cleavage maps of the two DNAs were similar but not identical. Southern hybridization demonstrated that the two EcoRI restriction fragments contained homologous DNA sequences, but a fec gene-specific probe was prepared. Each gene encoded a 38,000-dalton polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barouki R., Smith H. O. Reexamination of phenotypic defects in rec-1 and rec-2 mutants of Haemophilus influenzae Rd. J Bacteriol. 1985 Aug;163(2):629–634. doi: 10.1128/jb.163.2.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Churchward G., Belin D., Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984 Nov;31(1-3):165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Smith H. O. Transformation in Haemophilus: a problem in membrane biology. J Membr Biol. 1984;81(2):89–103. doi: 10.1007/BF01868974. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Characterization of the Pseudomonas aeruginosa recA analog and its protein product: rec-102 is a mutant allele of the P. aeruginosa PAO recA gene. J Bacteriol. 1987 Apr;169(4):1499–1508. doi: 10.1128/jb.169.4.1499-1508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Setlow J. K. Similarity in properties and mapping of three Rec mutants of Haemophilus influenzae. J Bacteriol. 1976 Jul;127(1):327–333. doi: 10.1128/jb.127.1.327-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Properties of Haemophilus influenzae mutants that are slightly recombination deficient and carry a mutation in the rec-1 gene region. J Bacteriol. 1980 Jun;142(3):829–835. doi: 10.1128/jb.142.3.829-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., van Boxel T., Venema G. Characterization of a conditionally transformation-deficient mutant of Haemophilus influenzae that carries a mutation in the rec-1 gene region. J Bacteriol. 1983 Feb;153(2):852–860. doi: 10.1128/jb.153.2.852-860.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., van Boxel T., Venema G. Inhibition of DNA replication by transformation in a Haemophilus influenzae mutant carrying an altered Rec-1 protein. J Bacteriol. 1983 May;154(2):686–692. doi: 10.1128/jb.154.2.686-692.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. Plasmid recombination in Haemophilus influenzae. J Mol Biol. 1982 Jun 5;157(4):577–596. doi: 10.1016/0022-2836(82)90500-9. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Spikes D., Griffin K. Characterization of the rec-1 gene of Haemophilus influenzae and behavior of the gene in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3876–3881. doi: 10.1128/jb.170.9.3876-3881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Stuy J. H., Walter R. B. Effect of glycerol on plasmid transfer in genetically competent Haemophilus influenzae. Mol Gen Genet. 1986 May;203(2):296–299. doi: 10.1007/BF00333969. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Smith H. O. Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5'-triphosphate-dependent deoxyribonuclease activity. J Bacteriol. 1975 May;122(2):443–453. doi: 10.1128/jb.122.2.443-453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle B. E. Phage lambda and plasmid expression vectors with multiple cloning sites and lacZ alpha-complementation. Gene. 1986;45(1):95–99. doi: 10.1016/0378-1119(86)90136-8. [DOI] [PubMed] [Google Scholar]