Abstract
Polysialic acid, which is synthesized by two polysialyltransferases, ST8SiaII and ST8SiaIV, plays an essential role in brain development by modifying the neural cell adhesion molecule (NCAM). It is currently unclear how polysialic acid functions in different processes of neural development. Here we generated mice doubly mutant in both ST8SiaII and ST8SiaIV to determine the effects of loss of polysialic acid on brain development. In contrast to NCAM-deficient, ST8SiaII-deficient, or ST8SiaIV-deficient single mutant mice, ST8SiaII and ST8SiaIV double mutants displayed severe defects in anatomical organization of the forebrain associated with apoptotic cell death. Loss of polysialic acid affected both tangential and radial migration of neural precursors during cortical development, resulting in aberrant positioning of neuronal and glial cells. Glial cell differentiation was aberrantly increased in vivo and in vitro in the absence of polysialic acid. Consistent with these findings, polysialic acid-deficient mice exhibited increased expression of the glial cell marker glial fibrillary acidic protein and a decrease in expression of Pax6, a transcription factor regulating neural cell migration. These results indicate that polysialic acid regulates cell migration and differentiation of neural precursors crucial for brain development.
Addition of polysialic acid, a homopolymer of α-2,8-linked sialic acid, is a unique posttranslational modification primarily of the neural cell adhesion molecule (NCAM). Polysialylated NCAM (PSA-NCAM) is widely expressed on neural cells in the brain during embryonic and neonatal stages, and expression is significantly reduced in adult stages (3, 28, 38). In adults, however, PSA-NCAM persists in areas of active neurogenesis and synapse formation, such as the subventricular zone (SVZ), migration pathways to the olfactory bulb, and the hippocampus (6, 18, 30, 31, 39). Polysialic acid is synthesized by two polysialyltransferases, ST8SiaII (also called STX) and ST8SiaIV (also called PST), which are expressed in a spatiotemporally regulated manner in the central nervous system (3, 23, 35). Due to its polyanionic nature and extended helical structure, polysialic acid can block both cis-interactions between NCAM molecules on the same cell and trans-interactions between adjacent cells. Polysialic acid also influences interaction of NCAM with other molecules, such as heparan sulfate proteoglycans and brain-derived neurotrophic factor (BDNF) (17, 27, 42, 45). These observations suggest that polysialic acid modifications on NCAM affect molecular interactions involved in neural development and potentially synaptic plasticity.
Studies of NCAM-deficient mice (NCAM−/−) (11, 43) or enzymatic removal of polysialic acid by endoneuraminidase show that PSA-NCAM plays diverse roles in promoting neurogenesis, neuronal pathfinding, defasciculation, and synapse formation (3, 28, 38). For example, PSA-NCAM functions in cell-cell interactions necessary for chain migration of neural precursors from the SVZ to the olfactory bulb (10, 24, 25, 36). However, NCAM−/− mice do not exhibit clear defects in other types of cell migration required for cerebrum and cerebellum formation in vivo. Interestingly, ST8SiaII and ST8SiaIV single mouse mutants exhibit a normal rostral migratory stream (RMS) forming the olfactory bulb (4, 13), indicating that olfactory interneuron precursors from the SVZ can use polysialic acid synthesized by either polysialyltransferase. Inactivation of either ST8SiaII or ST8SiaIV differentially impairs learning and memory and/or fear-associated conditioning associated with hippocampal function (4, 13, 41), suggesting that ST8SiaII and ST8SiaIV play distinct roles in neuronal plasticity. Studies also show that single knockout mice do not lose all polysialic acid expression, since ST8SiaII and ST8SiaIV expression likely overlaps (4, 13). Therefore, to determine the role of polysialic acid in vivo, it was necessary to inactivate both ST8SiaII and ST8SiaIV.
Recently, Weinhold et al. reported that ST8SiaII and ST8SiaIV double knockout mice exhibit postnatal lethality and morphological brain anomalies, such as hydrocephalus, reduction in size of the internal capsule, and malformation of the anterior commissure and corticospinal tract (46). These phenotypes were not observed in mice deficient in NCAM, ST8SiaII, or ST8SiaIV alone. Interestingly, many of these phenotypes were rescued in mice lacking NCAM, ST8SiaII, and ST8SiaIV (46), indicating that NCAM protein in polysialic acid-deficient mice is responsible for the severe phenotypes seen in ST8SiaII/ST8SiaIV double knockout mice. However, it is still unclear how polysialic acid deficiency impairs neural cell function. It is also important to determine whether specific deficiencies are NCAM dependent or polysialic acid dependent. Several transcription factors, growth factors, and cell adhesion molecules are required for neural cell migration and differentiation, and mutations in the genes encoding these proteins are associated with brain malformations observed in human neurological disorders (7, 32, 34). It is important then to determine if loss of polysialic acid affects function of these molecules and corresponding diseases.
Here we have undertaken cellular and molecular analyses of ST8SiaII and ST8SiaIV double mutant mice, which completely lack polysialic acid. We employed immunohistochemistry to detect specific cell types, such as glial cells, pyramidal cells, and dividing and migrating neural precursors, in both wild-type (WT) and double knockout mice. We identified a new role for polysialic acid in migration of both neurons and glial cells during cortex formation. Many neural cells lacking polysialic acid underwent apoptosis, which was rarely detected in mice deficient in NCAM, ST8SiaII, and ST8SiaIV alone. In vitro differentiation assays using neurosphere culture indicated that polysialic acid rather than NCAM regulates differentiation of glial precursors. Reverse transcription-PCR (RT-PCR) experiments analyzing genes associated with neural cell migration and differentiation showed decreased transcription of Pax6, which is required for cell migration in the cortex, and increased expression of glial fibrillary acidic protein (GFAP) in polysialic acid-deficient mice. These studies collectively show that polysialic acid plays critical roles in regulating cell migration, neural cell differentiation, and establishment of the glial cell lineage.
MATERIALS AND METHODS
Targeting of the ST8SiaIV gene.
Genomic DNA encoding mouse ST8SiaIV was cloned from a mouse129/SvJ genomic DNA library (Stratagene) and used to construct a targeting vector in which a SacI-BglII fragment containing the initiation methionine and transmembrane domain in exon 1 was cloned between two loxP sequences of a pflox vector (33). ST8SiaIV cDNA lacking a signal peptide and transmembrane domain cannot generate the protein to synthesize polysialic acid in HeLa cells transfected with NCAM cDNA (HeLa-NCAM cells), confirming inactivation of ST8SiaIV gene by this targeting. Embryonic stem (ES) cell clones with homologous recombination were identified by Southern hybridization using a DNA probe (see Fig. S1A, probe 1, in the supplemental material) recognizing sequences adjacent to the genomic sequence in the targeting vector. Two ES clones, B2 and C2, were chosen after Cre recombinase expression to establish ST8SiaIV-deficient (ST8IV−/−) mouse lines (see Fig. S1B in the supplemental material).
Animals.
ST8SiaIV heterozygotes were back-crossed to C57BL/6 mice for more than eight generations. ST8SiaII-deficient (ST8II−/−) mice were reported previously (4). Inactivation of ST8SiaII was confirmed by expressing truncated ST8SiaII cDNA isolated from mutant mice in HeLa-NCAM cells (4). NCAM-deficient (NCAM−/−) mice (11) were purchased from Jackson Laboratory. Transgenic mice expressing Thy1-YFP in pyramidal cell populations of cortical layer V (16) were purchased from Jackson Laboratory and crossed with the ST8SiaII-deficient line to generate double knockout mice expressing the yellow fluorescent protein (YFP) marker. All protocols for animal use were approved by the Animal Research Committee at the Burnham Institute for Medical Research in accordance with NIH guidelines.
RT-PCR and Western blot analysis.
RT-PCR was performed to analyze expression levels of ST8SiaII (mX), ST8SiaIV (mP), NCAM (mNC), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; mGA) in brains of wild-type and heterozygote and homozygote ST8SiaIV knockout mice as described elsewhere (4). Total RNA treated with DNase was used to synthesize cDNA by using SuperScript II and oligo(dT) primers (Invitrogen). The number of cycles for PCR was determined by analyzing amplified products at 20, 25, and 30 cycles. RT-PCR primers are listed in Table 1. The RT-PCR product of the deleted ST8SiaIV gene amplified by mP-5C and mP-3 primers was subcloned and sequenced to confirm that the transmembrane domain was removed and that no methionines were present up to the 134th amino acid, indicating that any potentially expressed protein would lack the domain required for enzymatic activity, as shown previously (2).
TABLE 1.
RT-PCR primers
| Gene | Primer | Sequence (5′-3′) | Positiona |
|---|---|---|---|
| ST8SiaII | mX-5 | GAATTCTGGAGGCAGAGGTACAATCAGATC | 99-128 |
| mX-3 | AAGGTCCTCAAAGGCCCGCTGGATGACAGA | 651-622 | |
| ST8SiaIV | mP-5A | CTGAGGAGCACCAAGAGACGCAACTCATCG | 83-122 |
| mP-5B | TCCGGAAGGCTGGCTCCACCATCTTCCAAC | 167-196 | |
| mP-5C | CCGCCACCTCCAATGCACAAGGTGTCACAT | −296-−267 | |
| mP-3 | TTTCTCTGTCACTCTCATTCCGAAAGCCTC | 628-599 | |
| NCAM | mNC-5 | GCCAAGGAGAAATCAGCGTTGGAGAGTC | 81-107 |
| mNC-3 | ATGCTCTTCAGGGTCAAGGAGGACACAC | 1127-1100 | |
| GAPDH | mGA-5 | CAGCAATGCATCCTGCACCACCAACTGC | 435-462 |
| mGA-3 | TTACTCCTTGGAGGCCATGTAGGCCATG | 1002-975 | |
| Nestin | mNes-5 | GACCTGGAACATGAATCTGTGGGTG | 5116-5140 |
| mNes-3 | CTAATCTTCCCCTGAGGACCAGGAG | 5595-5571 | |
| Notch | mNot-5 | AGGTGGATGCAGGCAATAAGGTCTG | 4364-4388 |
| mNot-3 | AAGCAGTGAAGAGGTGGCCCAACCC | 4932-4908 | |
| Dlx1 | mDlx1-5 | CCGGCTGGAATCCGAACTCCTCATC | 641-665 |
| mDlx1-3 | CTGTCTCCAGTGGCTGTGCCTGCGC | 1065-1041 | |
| Emx2 | mEmx2-5 | ATTGCTACCAAGCAGGCGAGTCCGG | 709-732 |
| mEmx2-3 | TCACTCAGCTGCCTCTTTAGACGAG | 1036-1012 | |
| Pax6 | mPax6-5 | TCGAAGGGCCAAATGGAGAAGAGAA | 822-846 |
| mPax6-3 | TTACTGTAATCGAGGCCAGTACTGA | 1311-1287 | |
| Olig2 | mOlig-5 | CTTCACAGGAGGGACTGTGTCCTCG | 99-123 |
| mOlig-3 | TCACCAGTCGCTTCATCTCCTCCAG | 520-496 | |
| NSE | mNse-5 | TTGTCCGGAACTATCCTGTGGTCTC | 848-872 |
| mNes-3 | TCACAGCACACTGGGATTTCGGAAA | 1305-1281 | |
| GFAP | mGfap-5 | AACGCAGAGCTGCTCCGCCAAGCCA | 802-826 |
| MGfap-3 | TCACATCACCACGTCCTTGTGCTCC | 1293-1269 | |
| NG2 | mNg2-5 | CATGCGGCCAAATCCTACAGTGTGG | 6511-6535 |
| MNg2-3 | TCACACCCAGTACTGGCCATTCCTG | 6984-6960 | |
| GAPDH | mGapd-5 | ATCATCCCTGCATCCACTGGTGCTG | 610-634 |
| mGapd-3 | CCTTGGAGGCCATGTAGGCCATGAG | 997-973 |
Nucleotides 1 to 3 encode the initiation methionine of each gene.
Comparative RT-PCR amplification of Nestin, Notch, Dlx1, Emx2, Pax6, Olig2, NSE, GFAP, NG2, and GAPDH (Table 1) was carried out using total RNA of neonatal forebrain prepared from double mutants, NCAM knockouts, and control littermates of each. Two samples of each genotype were used, and PCR was undertaken twice for quantitative analysis. PCR products were separated on 1% agarose gels and analyzed densitometrically using the AlphaImager system (Alpha Innotech Corporation).
Protein samples were prepared from various brain regions and subjected to immunoblot analysis as described previously (4). Aliquots of tissue extracts were incubated with endoneuraminidase for 1 h at 37°C prior to gel loading. Mouse anti-PSA-NCAM 5A5 (University of Illinois Hybridoma Bank) and rat anti-NCAM H28 (Immunotech) were used to detect polysialic acid and NCAM, respectively. All NCAM isoforms including PSA-NCAM were detected using a polyclonal anti-NCAM antibody (Chemicon).
Immunohistochemistry.
Brain sections were prepared and examined by hematoxylin and eosin staining and immunostaining as described elsewhere (4, 5). To compare corresponding brain areas between littermates, brains from each animal were cut in half at the midline and sectioned laterally for a sagittal view. For coronal sections, cutting was started from the anterior cerebrum after removing the olfactory bulbs. NIH Image software was used for area measurements and quantification. The following antibodies were used for immunohistochemistry: polysialic acid (12F8; BD Biosciences); β-III tubulin (Tuj1; BAbCo); calbindin D-28K (CaBP), glutamic acid decarboxylase, myelin basic protein, NCAM, nestin, and synapsin I (all from Chemicon); Ki67 (DAKO); GFAP (Roche Applied Science); calretinin (Swant); and neurofilament (SMI-32; Sternberger Monoclonal). Binding of primary antibodies was visualized with Alexa Fluor-labeled secondary antibodies (Invitrogen), nuclei were labeled with Hoechst 33342 (Sigma), and NeuroTracer was used to detect Nissl substances, which correspond to nuclear RNA in neurons (Invitrogen). Apoptotic cells were labeled using the ApopTag kit (Chemicon), and slides were counterstained with cresyl violet, which stains nuclei. To analyze YFP-expressing cells, brains fixed in 4% paraformaldehyde were cut with a Vibratome (Capital) at 80-μm thickness.
BrdU labeling.
5-Bromo-2′-deoxyuridine (BrdU; 100 mg/kg of body weight) was intraperitoneally injected into pregnant mice at embryonic day 13.5 (E13.5) or E16.5. BrdU-labeled brains were collected from pups at postnatal day 0 (P0) or P10 and stained with BrdU antibody (Roche Applied Science) as described previously (4). The area of the somatosensory cortex was divided equally into five parts, and BrdU-positive cells were counted in each bin. The number of BrdU-positive cells seen in four WT samples was compared with the number derived from four double mutant mice and analyzed statistically using Student's t test.
Neurosphere culture and in vitro assay.
Neurosphere cells were established in Dulbecco's modified Eagle's-F-12/B27 medium (Invitrogen) with 20 ng/ml FGF2 (Sigma) from neural precursor cells in striatum and in ganglionic eminences (20). After genotyping, data were obtained from cells from four double mutants and four controls. For migration assays, neurospheres were plated in 12-well plates coated with laminin alone or plus polyethylenimine and cultured in neurobasal/B27 medium (Invitrogen) without FGF2. After 48 h, cells were fixed and the distance between the migrated cell and the edge of aggregated neurosphere cells (500 cells, 25 cells from each of 20 neurospheres) was measured and analyzed statistically. To analyze differentiation of neurosphere cells, cells were further cultured without FGF2 for a total of 6 days and then stained using markers for neurons (β-III tubulin) and astrocytes (GFAP). BDNF (40 ng/ml; Invitrogen) or platelet-derived growth factor AA (PDGF-AA; 10 ng/ml; ICN) was added to the culture medium, and differentiation of neurosphere cells was evaluated.
RESULTS
Generation of polysialic acid-deficient mice through ablation of ST8SiaII and ST8SiaIV.
To analyze the role of polysialic acid in neural processes during brain development, we generated mice lacking all polysialic acid expression. To generate polysialic acid-deficient mice, we first generated ST8SiaIV-deficient (ST8IV−/−) mice (see Fig. S1 in the supplemental material) and then crossed them with previously described ST8SiaII-deficient mice (ST8II−/−) (4). We found that NCAM polysialylation was significantly decreased in adult brain of our newly developed ST8IV−/− mice compared to ST8II−/− and WT mice, similar to what has been reported elsewhere (13) (see Fig. S2A in the supplemental material). No compensatory increase in NCAM or ST8SiaII transcripts was observed in ST8IV−/− mice (see Fig. S1C in the supplemental material).
Mice doubly mutant in ST8SiaII and ST8SiaIV (II−/−/IV−/−) expressed NCAM unmodified with polysialic acid (see Fig. S2A, right, in the supplemental material). Immunohistochemical analysis revealed that polysialic acid expression was completely ablated in these mice, as seen in the hippocampus and RMS (see Fig. S2B and S3L and N in the supplemental material). These results indicate that ST8SiaII and ST8SiaIV are solely responsible for polysialic acid synthesis in mouse brain.
Gross anatomy of ST8SiaII and ST8SiaIV double knockout mice.
Double mutant mice expressing NCAM but no polysialic acid exhibited more severe phenotypes than did mice singly mutant in NCAM, ST8SiaII, or ST8SiaIV, as reported elsewhere (46). Phenotypically, II−/−/IV−/− mice were smaller than WT littermates and showed slow, weak, and uncoordinated movements. Most II−/−/IV−/− mice died during the early postnatal period (from P0 to P10), although some survived until 2 months of age. Gross histological analyses of mutant brains revealed a size reduction in the cerebellum and olfactory bulb relative to WT littermates (see Fig. S3A and B in the supplemental material). Coronal and sagittal brain sections showed an increase in the size of the lateral ventricle accompanied by a drastically thinner cortex (Fig. 1A; see also Fig. S3G to I in the supplemental material). In addition, the striatum and diencephalon were less thick than in WT mice, and commissures such as the corpus callosum were thin or partly absent in double mutant mice (Fig. 1A; see also Fig. S3G to I in the supplemental material). These phenotypes were not observed in NCAM-deficient mice (11, 43). These results indicate that polysialic acid is widely required for formation of various brain structures and suggest that it plays much more important roles than what was suggested by analysis of NCAM-deficient mice.
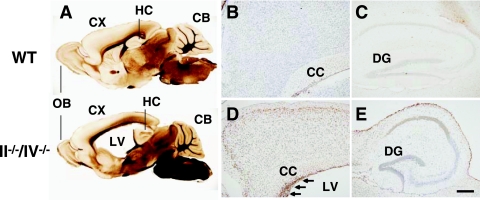
FIG. 1.
Apoptotic cell death in the cortex of polysialic acid-deficient mice. (A) Vibratome sections showing sagittal views of 1-month-old WT (upper) and II−/−/IV−/− (lower) mice. CB, cerebellum; CX, cerebral cortex; HC, hippocampus; LV, lateral ventricle; OB, olfactory bulb. (B to E) Apoptotic cells (brown) in sagittal sections of 1-month-old WT (B and C) and II−/−/IV−/− (D and E) mice were detected. Nuclei were stained by cresyl violet. The cerebral cortex (B and D) and hippocampus (C and E) are shown. Many cells undergo apoptosis in the SVZ of double mutant mice (arrows in panel D). CC, corpus callosum; DG, dentate gyrus. Bar, 0.2 mm.
Forebrain defects in polysialic acid-deficient mice.
To investigate defects underlying abnormal brain development in polysialic acid-deficient mice, we focused on development of the forebrain, since polysialic acid is highly expressed there in early developmental stages (see Fig. S4J to L in the supplemental material) (39). Polysialic acid is continuously expressed by neuroblasts in the RMS (see Fig. S3K in the supplemental material), where polysialic acid-expressing cells form chain migration from the SVZ to the olfactory bulb (30). The RMS at P0 was thicker in double knockout compared to WT mice (see Fig. S4A and B in the supplemental material), probably due to slower migration of neuroblasts. By P10, the RMS of some double knockout mice was thinner, and mice exhibited small olfactory bulbs (see Fig. S4C and D in the supplemental material), likely due to impaired movement of neuroblasts from the SVZ to the olfactory bulb.
As double mutant mice grew postnatally, their cortex became thinner and the lateral ventricle became larger (Fig. 1A; see also Fig. S3B and G to I and S4A to H in the supplemental material), indicating that the mice had fewer cortical cells than did WT mice. Analysis of DNA fragmentation indicated that neural cells and precursors in the cortex and SVZ were undergoing apoptosis (Fig. 1D) in greater numbers than in WT mice. On the other hand, hippocampal cells did not appear to undergo apoptosis, except for those facing the lateral ventricle (Fig. 1E), consistent with the observation that the hippocampus of double knockout mice is similar in size to the hippocampus of WT mice (see Fig. S3B in the supplemental material). These results indicate that loss of polysialic acid causes cell death of precursors and neural cells in the forebrain, likely due to apoptosis. They also suggest that polysialic acid is necessary to form and maintain forebrain structures during mouse brain development.
Requirement of polysialic acid for cellular migration in the cerebral cortex.
We next stained brain sections with neural markers to determine which cells were affected by polysialic acid loss. Studies of NCAM-deficient mice suggest that polysialic acid is required for tangential migration of neural precursors toward the olfactory bulb (10, 24, 25, 36). We observed that neurons positive for calretinin, a marker of olfactory interneurons, accumulate near the lateral ventricle in polysialic acid-deficient mice at P10 (Fig. 2D), indicating that loss of polysialic acid affects tangential migration of interneurons toward the olfactory bulb. To analyze potential defects in other types of tangential migration during cortex formation, we determined the number of GABAergic interneurons in cortical sections using the marker calbindin (CaBP) in adult double mutant and WT mice, since polysialic acid is expressed in those neurons of WT mice. The number of CaBP-positive cells was decreased in the cerebral cortex of adult double knockout mice (∼20% of WT) (Fig. 2B and E), consistent with the decrease in the number of glutamic acid decarboxylase-positive cells (data not shown), indicating that tangential migration of GABAergic interneurons from the ganglionic eminences to the dorsal cerebral cortex (22, 29, 32) is impaired in the absence of polysialic acid.
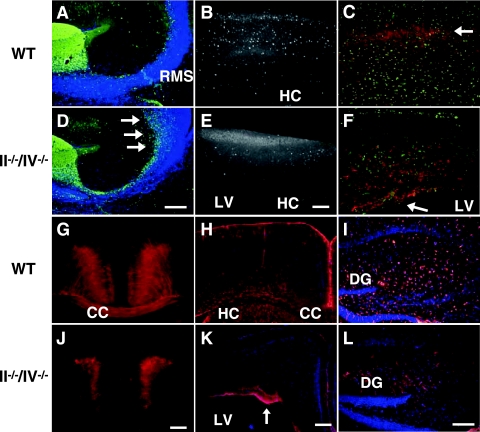
FIG. 2.
Polysialic acid is required for cerebral cortex development. (A and D) Calretinin expression in olfactory neuron precursors (green) of P10 mice. Arrows indicate accumulated cells near the SVZ. (B and E) CaBP (white) marks GABAergic interneurons in a 1-month-old cortex. (C, F, G, and J) Nonphosphorylated neurofilament (red) marks pyramidal cells in cortical layers II and III and V and VI in sagittal sections (C and F) and coronal sections (G and J) of 1-month-old mice. In II−/−/IV−/− mice, neurofilament-expressing cells remained near the ventricular zone (arrow in panel F). Nissl substance staining (green) shows the distribution of neurons (C and F). (H, I, K, and L) Distribution of glial cells expressing GFAP (red) in coronal sections of the cortex (H and K) and hippocampal sagittal sections (I and L) of 1-month-old mice. Note that the number of astrocyte-like cells was decreased in the hippocampus of II−/−/IV−/− mice (L). Panels A to C and G to I show WT mice, and panels D to F and J to L show II−/−/IV−/− mice. CC, corpus callosum; DG, dentate gyrus; HC, hippocampus; LV, lateral ventricle. Bars, 0.2 mm.
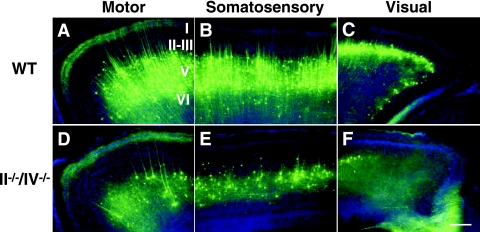
The expression of nonphosphorylated neurofilament, a marker of pyramidal cells in layers II/III and IV of the neocortex, was decreased, and staining was aberrantly localized in the SVZ of polysialic acid-deficient cortex (Fig. 2F and J), suggesting that radial migration of pyramidal cells is also impaired. To further investigate these defects, we crossed transgenic Thy1-YFP mice expressing YFP in layer V pyramidal cells into a II−/−/IV−/− background (Fig. 3). In the resulting mice, we observed that not only was the number of pyramidal cells decreased (to ∼25% of WT) but also the projection of dendrites and axons from pyramidal cells was disorganized compared to WT mice, particularly in the visual cortex (Fig. 3F). Taken together these results indicate perturbations in pyramidal cell migration in the cerebral cortex in the absence of polysialic acid, an observation suggesting defects in establishment of excitatory neural networks.
FIG. 3.
Lack of polysialic acid affects lamination of pyramidal cells in the cerebral cortex. Images show YFP expression (green) in Thy1-YFP mice and Hoechst staining (blue) in 1-month-old WT (A to C) and II−/−/IV−/− (D to F) mice. Pyramidal neurons in layer V of the motor (A and D), somatosensory (B and E), and visual (C and F) cortex are shown. Bars, 0.2 mm. Note that in polysialic acid-deficient mice the number of pyramidal cells was decreased and the number of neuronal projections was decreased and not as extensive as in WT mice.
Slow migration of II−/−/IV−/− neural precursors in embryonic cerebral cortex.
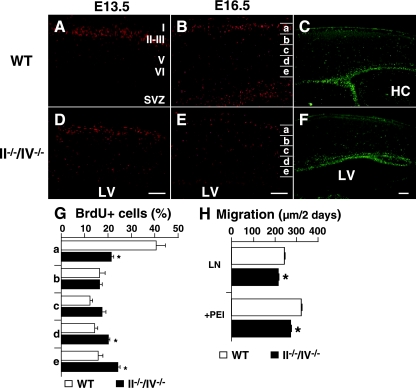
The above findings suggest that polysialic acid may be broadly required for neural cell migration or cell growth and survival in the developing brain. To determine which developmental steps require polysialic acid, we performed birth date (cell migration) analysis using BrdU. In WT mice, most BrdU-labeled cells at E13.5 and E16.5 migrated to cortical layer II/III at P0 (Fig. 4A and B). In II−/−/IV−/− mice, many BrdU-positive cells remained scattered in the lower layers V and VI and in the SVZ close to the lateral ventricle (Fig. 4D and E). To evaluate cell migration quantitatively, BrdU-positive cells were counted in divided areas (bins a to e) as shown in Fig. 4B and E, and the ratio of BrdU-positive cells in each area to the total number of BrdU-positive cells in all areas was determined. Since BrdU-positive cells in the SVZ migrate to the upper layer of the cortex during this period, cells in area a are further away from the labeling point than are those in areas d and e. The results showed that the number of BrdU-positive cells in area a was significantly lower in double mutant mice, while the number of BrdU-positive cells seen in areas d and e was higher compared to WT mice (Fig. 4G). Importantly, the total number of BrdU-positive cells did not differ between WT and II−/−/IV−/− mice. In addition, the number of cells positive for Ki67, a marker of proliferating cells, was comparable between WT and double knockout mice at P0 (Fig. 4C and F). Taken together, these results indicate that polysialic acid deficiency results in impaired migration but not impaired proliferation in the forebrain during embryonic development.
FIG. 4.
Effect of loss of polysialic acid on cell migration. (A, B, D, and E) BrdU-positive cells (red) at P0 are shown after BrdU injection at E13.5 (A and D) or E16.5 (B and E). Results obtained from littermates including both WT and mutant mice in each experiment were compared. Note that the number of BrdU-positive cells in the cerebral cortex was almost equivalent between wild-type and knockout mice. (C and F) P0 sections were stained with Ki67. (G) Distribution of BrdU-positive cells at P0 from the E16.5 injection was analyzed in four mice of each genotype. The cortex was equally divided into five bins (a to e in panels B and E), and BrdU-positive cells were counted in each bin. Forty percent of BrdU-labeled cells in the cortex reached bin a in WT mice, while 20% reached the same destination in II−/−/IV−/− mice, a statistically significant difference. Error bars indicate the standard deviations. *, P < 0.002. (H) Migration of neurosphere cells cultured for 2 days in 12-well plates coated with laminin (LN) alone or plus polyethylenimine (PEI). Neurosphere cells from II−/−/IV−/− mice migrated significantly more slowly than did wild-type cells. Error bars indicate the standard errors of the means. **, P < 0.001. Panels A, B, and C show WT mice, and panels D, E, and F show II−/−/IV−/− mice. HC, hippocampus; LV, lateral ventricle. Bars, 0.2 mm.
To determine whether the lack of polysialic acid slows neural precursor migration, we prepared neurosphere cells from II−/−/IV−/− embryos for an in vitro migration assay. Cells from double knockout mice migrated 8 to 15% more slowly than those from WT mice when plated on laminin (Fig. 4H), supporting the conclusion that polysialic acid itself promotes neural cell migration.
Impaired glial cell migration due to lack of polysialic acid.
Polysialic acid is expressed in precursors of both neuronal and glial cells, but it is not known whether polysialic acid is required for glial cell migration in vivo. To answer this question, we stained brain sections of double knockout mice with antibodies to GFAP, an astrocytic marker. GFAP-positive cells in the cerebral cortex were evenly distributed in 1-month-old WT mice (Fig. 2H). By contrast, in double mutants GFAP-positive cells were confined to the SVZ close to the lateral ventricle (Fig. 2K), indicating that their migration is impaired. The number of GFAP-positive astrocytes was also decreased in the hippocampus of double knockout mice (∼30% of WT hippocampus) (Fig. 2L and I). These results suggest that polysialic acid is required for migration of glial cells in the forebrain.
Glial cell differentiation of neurosphere cells from II−/−/IV−/− mice.
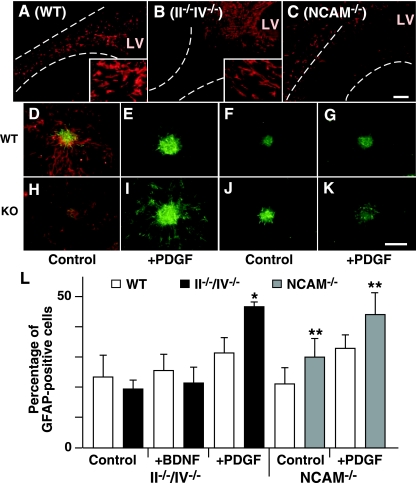
In the RMS, polysialic acid-positive neuroblasts migrate as a chain and are supported by GFAP-positive cells (30). Hoechst staining revealed that the RMS of double knockout mice was poorly organized structurally, while polysialic acid-positive cells in WT mice were well aligned (see Fig. S3K and L in the supplemental material). Consistently, WT mice exhibited GFAP-positive cells localized to an organized RMS (Fig. 5A), but in double mutants GFAP-positive cells primarily remained close to the lateral ventricle in the SVZ and were less dense in the broader RMS (Fig. 5B). Since NCAM-deficient mice also develop a wider RMS, we analyzed NCAM−/− mice to determine if this phenotype requires NCAM or polysialic acid. In the NCAM−/− mice, the number of GFAP-positive cells was increased along the RMS, but most of these cells were not in the RMS directed to the olfactory bulb (Fig. 5C) (10). These results indicate that loss of polysialic acid does not decrease GFAP expression in cells derived from the SVZ but rather decreases the number of GFAP-positive cells in the RMS, suggesting that glial cell organization in the RMS is polysialic acid dependent in the RMS.
FIG. 5.
Roles of polysialic acid in cell differentiation. (A to C) Distribution of glial cells expressing GFAP (red) in the RMS of wild-type (A), II−/−/IV−/− (B), and NCAM−/− (C) mice. The RMS defined by Hoechst staining (not shown) is indicated by dotted lines. Enlarged images of GFAP-positive cells are shown in insets in panels A and B. LV, lateral ventricle. (D to K) Neurosphere cells regrown after dissociation were induced to differentiate by culturing without FGF2 (D, F, H, and J) but with PDGF-AA (E, G, I, and K) and stained for markers of neurons (β-III tubulin; red in panels D and H) and astrocytes (GFAP; green). Panels D to G show WT mice, panels H and I show II−/−/IV−/− mice, and panels J and K show NCAM−/− mice. (L) The proportion of astrocytes in the total number of differentiated cells is shown (four for each neurosphere clone for II−/−/IV−/− mice and two for each clone for NCAM−/− mice). Error bars indicate the standard deviations. *, P < 0.005; **, P < 0.01. Bar, 0.2 mm.
In WT mice, most GFAP-positive cells in the RMS have multiple short branches, while in double mutants many GFAP-positive cells exhibited an elongated morphology (Fig. 5A and B, insets). The altered distribution and morphology of GFAP-positive astrocytes in the forebrain suggested that polysialic affects glial cell differentiation. Thus, we analyzed astrocyte differentiation using in vitro culture of neurospheres. When neurosphere cells were induced to differentiate into neuronal or glial lineages by removing mitogen (FGF2), differentiation of II−/−/IV−/− neurosphere cells into GFAP-positive cells, which were mostly astrocyte-like in morphology, was comparable to that of WT neurosphere cells (Fig. 5D and H). Strikingly, however, lack of polysialic acid significantly increased the number of GFAP-expressing cells in the presence of PDGF (47.0 ± 1.5%) (Fig. 5I) but not of BDNF (21.6 ± 5.8%) (Fig. 5L) compared to controls of WT neurosphere cells (31.6 ± 4.8% by PDGF and 25.6 ± 5.2% by BDNF). These results demonstrate that polysialic acid inhibits or delays the onset of PDGF-induced differentiation of neurosphere cells to glial cell lineages. PDGF-induced differentiation of neurosphere cells from NCAM-deficient mice, which lack PSA-NCAM, was also enhanced (44.1 ± 7.5%) (Fig. 5K and L) compared to WT controls (33.0 ± 4.5%) (Fig. 5G and L). These results suggest that polysialic acid rather than NCAM inhibits glial cell differentiation.
Polysialic acid deficiency affects expression of Pax6 and GFAP during forebrain formation.
The above results demonstrate that the lack of polysialic acid expression in neural cells affects cell migration, differentiation, and survival. To reveal how polysialylation loss causes these deficiencies, we examined mRNA expression of various markers in double mutants. Since most II−/−/IV−/− mice die at early postnatal stages, we analyzed forebrains at P0, although abnormalities in brain morphology are more apparent at P10 (see Fig. S4 in the supplemental material).
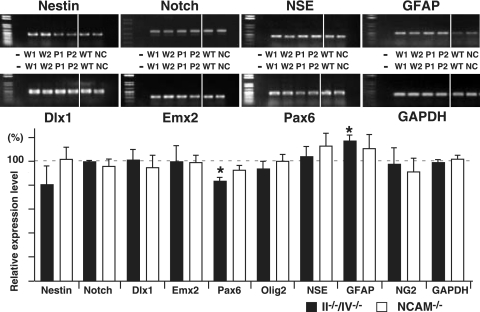
Nestin and Notch are expressed in dividing cells, including neural precursors (8), and expression of both was comparable among wild-type, II−/−/IV−/−, and NCAM-deficient mice by RT-PCR (Fig. 6). We also analyzed expression of transcription factors that play important roles in neural cell differentiation and migration (32, 34). Pax6 is required for tangential and radial cell migration and is involved in differentiation of neural precursors during cortex development (21, 32). Pax6 expression was decreased in a statistically significant manner in polysialic acid-deficient mice (83.6 ± 3.8% of control mice). Some migrating cells in the cortex express both polysialic acid and Pax6 (21, 26), suggesting that the numbers of Pax6-positive cells were decreased by loss of polysialic acid. Expression of Dlx1, Emx2, and Olig2 transcription factors was unchanged in double mutant mice.
FIG. 6.
Comparative RT-PCR analysis of NCAM- and polysialic acid-deficient mice. (Upper) Total RNA from forebrain was prepared at P0 from WT and mutant mice from the same litter, and expression levels of various genes were analyzed using specific primers by RT-PCR. Photographs of amplified fragments are shown. −, without reverse transcriptase; W1, W2, and WT are WT mice, P1 and P2 are II−/−/IV−/− mice, and NC are NCAM−/− mice. (Lower) Amplified fragments were analyzed densitometrically and compared between WT and knockout mice in the same litter. At least two different mice of each genotype were used for statistical analysis. Error bars indicate standard deviations. *, P < 0.002; **, P < 0.005.
RT-PCR analysis for markers of neuronal or glial differentiation (15) also showed that GFAP expression in II−/−/IV−/− mice was significantly increased (118 ± 4.7% of WT mice) (Fig. 6), a finding consistent with immunohistochemical analyses and results from the in vitro differentiation assay of neurosphere cells (Fig. 5).
DISCUSSION
In this study, we generated polysialic acid-deficient mice by inactivating ST8SiaII and ST8SiaIV in order to analyze the role of polysialic acid in vivo and observed severely impaired brain development leading to lethality 2 months after birth. PSA-NCAM has been suggested to play crucial roles in numerous neural cell functions in studies of NCAM-deficient mice and in vitro cell analyses (3, 28, 38). Here, we specifically focused on the role of polysialic acid, rather than NCAM, in developmental processes. Our results demonstrate the following: (i) loss of polysialic acid increases neural cell death during brain development; (ii) polysialic acid is necessary for coordinated tangential and radial cell migration required for cortical formation; (iii) lack of polysialic acid increases glial cell differentiation before cells reach their proper destination; and (iv) loss of polysialic acid decreases Pax6 expression. It is likely that these anomalies underlie the malformations seen in wide areas of the brain of polysialic acid-deficient mice. These phenotypes are in marked contrast to phenotypes of NCAM−/−, ST8II−/−, or ST8IV−/− single mutant mice in which polysialic acid is expressed at varying levels (4, 11, 13, 43). The availability of II−/−/IV−/− mice provides an important tool to determine the role of polysialic acid in mammalian brain development.
Weinhold et al. found that some defects in the cortex and anterior commissure seen in the II−/−/IV−/− double mutants are rescued by loss of NCAM (46), indicating that an important role of polysialic acid is to restrict NCAM interactions during brain development. However, in that study defects in the olfactory bulb and hippocampus of double mutant mice were not rescued by NCAM loss (46), suggesting that multiple mechanisms underlie the varied phenotypes seen in neurons and glial cells. In the RMS leading to the olfactory bulb, we found that loss of polysialic acid apparently blocks migration of neural precursors from the SVZ, resulting in a thicker RMS and smaller olfactory bulbs compared to WT mice. We also observed the RMS phenotype in NCAM-deficient mice, which lack most polysialic acid (11, 43), suggesting that chain migration of olfactory neurons requires polysialic acid, not NCAM. It is likely that in chain migration, polysialic acid presented by NCAM prevents precursor-glia interactions mediated by molecules other than NCAM.
As development progressed in double mutant mice, the cerebral cortex became progressively thinner and the lateral ventricle progressively became larger compared to WT and NCAM-deficient mice. This could be caused by multiple defects. In the developing cortex, two distinct types of neuronal precursors derived from two different proliferative areas (the ganglionic eminences and the ventricular zone) tangentially and radially migrate to establish complex neuronal layers (22, 29, 32). We found that polysialic acid deficiency reduces both tangential migration of GABAergic interneurons from the ganglionic eminences to the dorsal cerebrum and radial migration of pyramidal cells from the SVZ at embryonic and neonatal stages. In contrast to II−/−/IV−/− mutant mice, NCAM-deficient mice do not exhibit cell migration defects in cortex, suggesting that a different microenvironment for migrating cells exists in the cortex compared to the RMS. It is possible that abundant NCAM molecules lacking polysialic acid promote either strong cell adhesion or signaling that prevents both tangential and radial migration. This could explain why triple knockouts of NCAM, ST8SiaII, and ST8SiaIV rescue many phenotypes exhibited by ST8SiaII and ST8SiaIV double mutants, including cortical defects (46). Further studies are necessary to understand NCAM-dependent and NCAM-independent mechanisms underlying migration defects seen in double knockouts.
Polysialic acid is expressed in precursors of both neurons and glia, and its level decreases during their differentiation (19, 44). Our data suggest that lack of polysialic acid in mouse brain changes the fate of neurons and glial cells, demonstrating an important role for polysialic acid in the microenvironment in terms of differentiation. When PSA-NCAM is absent, myelination is disorganized in vivo and accelerated in vitro (9, 10), as we observed aberrantly increased glial differentiation of neurosphere cells from both double mutants and NCAM single mutant mice. NCAM-deficient mice show increased numbers of migrating precursors adopting an oligodendroglial fate when demyelination of the corpus callosum was induced by lysolecithin, compared to WT mice (12). It has also been shown that NCAM-induced cell contact inhibits proliferation and promotes neuronal differentiation of hippocampal progenitor cells or SVZ explants (1, 37), suggesting that an additional function of polysialic acid is to prevent premature cellular interactions with surrounding cells. In agreement, we found that glial precursors differentiated into astrocyte-like cells before they reached their destination in polysialic acid-deficient mice, consistent with an increase in GFAP expression. In addition, we found that immature neural cells in the cortex undergo early cell death in polysialic acid-deficient mice, indicating that polysialic acid promotes their survival. Further studies are necessary to reveal molecular mechanisms underlying signals for differentiation or cell death in polysialic acid-expressing cells.
Pax6, a paired box gene, is required for tangential cell migration of interneuron precursors from the ganglionic eminences, and Pax6 mutant (Sey) mice exhibit an unusual migration pattern of polysialic acid-expressing cells (26). Mutation of Pax6 in humans causes the disease aniridia and results in forebrain malformation, such as thin cerebral hemispheres widely separated with a single open ventricular system, reduced olfactory bulb size, and hypoplasia of the anterior commissure (14, 40; www.aniridia.org). These phenotypes are similar to those observed in polysialic acid-deficient mice. We show here that loss of polysialic acid decreases Pax6 expression but not that of other transcription factors analyzed, suggesting a specific Pax6 function in polysialic acid-dependent cell migration during cortical formation. These results also suggest that cell surface modification by polysialic acid could regulate Pax6 expression essential for neural cell migration and differentiation. It will be of interest to determine if NCAM interaction with itself or other molecules regulates Pax6 expression. Since defects in polysialic acid expression cause morphological changes in the brain, it is crucial to determine whether anomalies in polysialic acid synthesis occur in human disease characterized by cortical malformation and neurological disorders (7, 32, 34).
Supplementary Material
Acknowledgments
We thank Yu Yamaguchi, Keith Murai, Junya Mitoma, Paola Bonfanti, and Ruchi Bajpai for discussions and technical assistance, Elise Lamar and Jun Nakayama for critical reading of the manuscript, and Aleli Morse for organizing the manuscript.
This work was supported by NIH grants CA33895 (M.F.), HD25938 (B.R.), HL57345 (J.D.M.), and NS47351 (A.T.). J.D.M. is supported as an Investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 6 August 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Amoureux, M. C., B. A. Cunningham, G. M. Edelman, and K. L. Crossin. 2000. N-CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype. J. Neurosci. 15:3631-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angata, K., D. Chan, J. Thibault, and M. Fukuda. 2004. Molecular dissection of the ST8Sia IV polysialyltransferase. Distinct domains are required for neural cell adhesion molecule recognition and polysialylation. J. Biol. Chem. 279:25883-25890. [DOI] [PubMed] [Google Scholar]
- 3.Angata, K., and M. Fukuda. 2003. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 85:195-206. [DOI] [PubMed] [Google Scholar]
- 4.Angata, K., J. M. Long, O. Bukalo, W. Lee, A. Dityatev, A. Wynshaw-Boris, M. Schachner, M. Fukuda, and J. D. Marth. 2004. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 279:32603-32613. [DOI] [PubMed] [Google Scholar]
- 5.Berglund, E. O., K. K. Murai, B. Fredette, G. Sekerkova, B. Marturano, L. Weber, E. Mugnaini, and B. Ranscht. 1999. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron 24:739-750. [DOI] [PubMed] [Google Scholar]
- 6.Bernier, P. J., A. Bedard, J. Vinet, M. Levesque, and A. Parent. 2002. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA 99:11464-11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielas, S., H. Higginbotham, H. Koizumi, T. Tanaka, and J. G. Gleeson. 2004. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu. Rev. Cell Dev. Biol. 20:593-618. [DOI] [PubMed] [Google Scholar]
- 8.Cai, J., Y. Wu, T. Mirua, J. L. Pierce, M. T. Lucero, K. H. Albertine, G. J. Spangrude, and M. S. Rao. 2002. Properties of a fetal multipotent neural stem cell (NEP cell). Dev. Biol. 251:221-240. [DOI] [PubMed] [Google Scholar]
- 9.Charles, P., M. P. Hernandez, B. Stankoff, M. S. Aigrot, C. Colin, G. Rougon, B. Zalc, and C. Lubetzki. 2000. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 97:7585-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazal, G., P. Durbec, A. Jankovski, G. Rougon, and H. Cremer. 2000. Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J. Neurosci. 20:1446-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremer, H., R. Lange, A. Christoph, M. Plomann, G. Vopper, J. Roes, R. Brown, S. Baldwin, P. Kraemer, S. Scheff, D. Barthels, K. Rajewsky, and W. Wille. 1994. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367:455-459. [DOI] [PubMed] [Google Scholar]
- 12.Decker, L., P. Durbec, G. Rougon, and A. B. Evercooren. 2002. Loss of polysialic residues accelerates CNS neural precursor differentiation in pathological conditions. Mol. Cell. Neurosci. 19:225-238. [DOI] [PubMed] [Google Scholar]
- 13.Eckhardt, M., O. Bukalo, G. Chazal, L. Wang, C. Goridis, M. Schachner, R. Gerardy-Schahn, H. Cremer, and A. Dityatev. 2000. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 20:5234-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison-Wright, Z., I. Heyman, I. Frampton, K. Rubia, X. Chitnis, I. Ellison-Wright, S. C. Williams, J. Suckling, A. Simmons, and E. Bullmore. 2004. Heterozygous PAX6 mutation, adult brain structure and fronto-striato-thalamic function in a human family. Eur. J. Neurosci. 19:1505-1512. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa-Jeffrey, A., S. G. Becker-Catania, P. M. Zhao, R. Cole, J. Edmond, and J. de Vellis. 2002. Selective specification of CNS stem cells into oligodendroglial or neuronal cell lineage: cell culture and transplant studies. J. Neurosci. Res. 69:810-825. [DOI] [PubMed] [Google Scholar]
- 16.Feng, G., R. H. Mellor, M. Bernstein, C. Keller-Peck, Q. T. Nguyen, M. Wallace, J. M. Nerbonne, J. W. Lichtman, and J. R. Sanes. 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28:41-51. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto, I., J. L. Bruses, and U. Rutishauser. 2001. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J. Biol. Chem. 276:31745-31751. [DOI] [PubMed] [Google Scholar]
- 18.Gage, F. H. 2000. Mammalian neural stem cells. Science 287:1433-1438. [DOI] [PubMed] [Google Scholar]
- 19.Gago, N., V. Avellana-Adalid, A. B. Evercooren, and M. Schumacher. 2003. Control of cell survival and proliferation of postnatal PSA-NCAM+ progenitors. Mol. Cell. Neurosci. 22:162-178. [DOI] [PubMed] [Google Scholar]
- 20.Geschwind, D. H., J. Ou, M. C. Easterday, J. D. Dougherty, R. L. Jackson, Z. Chen, H. Antoine, A. Terskikh, I. L. Weissman, S F. Nelson, and H. I. Kornblum. 2001. A genetic analysis of neural progenitor differentiation. Neuron 29:325-339. [DOI] [PubMed] [Google Scholar]
- 21.Hack, M. A., M. Sugimori, C. Lundberg, M. Nakafuku, and M. Gotz. 2004. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol. Cell. Neurosci. 25:664-678. [DOI] [PubMed] [Google Scholar]
- 22.Hatten, M. E. 2002. New directions in neuronal migration. Science 297:1660-1663. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt, H., C. Becker, M. Murau, R. Gerardy-Schahn, and H. Rahmann. 1998. Heterogeneous expression of the polysialyltransferases ST8Sia II and ST8Sia IV during postnatal rat brain development. J. Neurochem. 71:2339-2348. [DOI] [PubMed] [Google Scholar]
- 24.Hu, H. 2000. Polysialic acid regulates chain formation by migrating olfactory interneuron precursors. J. Neurosci. Res. 61:480-492. [DOI] [PubMed] [Google Scholar]
- 25.Hu, H., H. Tomasiewicz, T. Magnuson, and U. Rutishauser. 1996. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron 16:735-743. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez, D., L. Lopez-Mascaraque, J. A. de Carlos, and F. Valverde. 2002. Further studies on cortical tangential migration in wild type and Pax-6 mutant mice. J. Neurocytol. 31:719-728. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, C. P., I. Fujimoto, U. Rutishauser, and D. E. Leckband. 2005. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J. Biol. Chem. 280:137-145. [DOI] [PubMed] [Google Scholar]
- 28.Kleene, R., and M. Schachner. 2004. Glycans and neural cell interactions. Nat. Rev. Neurosci. 5:195-208. [DOI] [PubMed] [Google Scholar]
- 29.Kriegstein, A. R., and S. C. Noctor. 2004. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 27:392-399. [DOI] [PubMed] [Google Scholar]
- 30.Lois, C., J. M. Garcia-Verdugo, and A. Alvarez-Buylla. 1996. Chain migration of neuronal precursors. Science 271:978-981. [DOI] [PubMed] [Google Scholar]
- 31.Luzzati, F., P. Peretto, P. Aimar, G. Ponti, A. Fasolo, and L. Bonfanti. 2003. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc. Natl. Acad. Sci. USA 100:13036-13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin, O., and J. L. Rubenstein. 2003. Cell migration in the forebrain. Annu. Rev. Neurosci. 26:441-483. [DOI] [PubMed] [Google Scholar]
- 33.Marth, J. D. 1996. Recent advances in gene mutagenesis by site-directed recombination. J. Clin. Investig. 97:1999-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monuki, E. S., and C. A. Walsh. 2001. Mechanisms of cerebral cortical patterning in mice and humans. Nat. Neurosci. 4:1199-1206. [DOI] [PubMed] [Google Scholar]
- 35.Ong, E., J. Nakayama, K. Angata, L. Reyes, T. Katsuyama, Y. Arai, and M. Fukuda. 1998. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology 8:415-424. [DOI] [PubMed] [Google Scholar]
- 36.Ono, K., H. Tomasiewicz, T. Magnuson, and U. Rutishauser. 1994. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 13:595-609. [DOI] [PubMed] [Google Scholar]
- 37.Petridis, A. K., A. El-Maarouf, and U. Rutishauser. 2004. Polysialic acid regulates cell contact-dependent neuronal differentiation of progenitor cells from the subventricular zone. Dev. Dyn. 230:675-684. [DOI] [PubMed] [Google Scholar]
- 38.Rutishauser, U., and L. Landmesser. 1996. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 19:422-427. [DOI] [PubMed] [Google Scholar]
- 39.Seki, T., and Y. Arai. 1993. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci. Res. 17:265-290. [DOI] [PubMed] [Google Scholar]
- 40.Sisodiya, S. M., S. L. Free, K. A. Williamson, T. N. Mitchell, C. Willis, J. M. Stevens, B. E. Kendall, S. D. Shorvon, I. M. Hanson, A. T. Moore, and V. van Heyningen. 2001. PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nat. Genet. 28:214-216. [DOI] [PubMed] [Google Scholar]
- 41.Stoenica, L., O. Senkov, R. Gerardy-Schahn, B. Weinhold, M. Schachner, and A. Dityatev. 2006. In vivo synaptic plasticity in the dentate gyrus of mice deficient in the neural cell adhesion molecule NCAM or its polysialic acid. Eur. J. Neurosci. 23:2255-2264. [DOI] [PubMed] [Google Scholar]
- 42.Storms, S. D., and U. Rutishauser. 1998. A role for polysialic acid in neural cell adhesion molecule heterophilic binding to proteoglycans. J. Biol. Chem. 273:27124-27129. [DOI] [PubMed] [Google Scholar]
- 43.Tomasiewicz, H., K. Ono, D. Yee, C. Thompson, C. Goridis, U. Rutishauser, and T. Magnuson. 1993. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron 11:1163-1174. [DOI] [PubMed] [Google Scholar]
- 44.Vitry, S., V. Avellana-Adalid, F. Lachapelle, and A. B. Evercooren. 2001. Migration and multipotentiality of PSA-NCAM+ neural precursors transplanted in the developing brain. Mol. Cell. Neurosci. 17:983-1000. [DOI] [PubMed] [Google Scholar]
- 45.Vutskits, L., Z. Djebbara-Hannas, H. Zhang, J. P. Paccaud, P. Durbec, G. Rougon, D. Muller, and J. Z. Kiss. 2001. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur. J. Neurosci. 13:1391-1402. [DOI] [PubMed] [Google Scholar]
- 46.Weinhold, B., R. Seidenfaden, I. Röckle, M. Mühlenhoff, F. Schertzinge, S. Conzelmann, J. D. Marth, R. Gerardy-Schahn, and H. Hildebrandt. 2005. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 280:42971-42977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.