Abstract
BACH1 (also known as FANCJ and BRIP1) is a DNA helicase that directly interacts with the C-terminal BRCT repeat of the breast cancer susceptibility protein BRCA1. Previous biochemical and functional analyses have suggested a role for the BACH1 homolog in Caenorhabditis elegans during DNA replication. Here, we report the association of BACH1 with a distinct BRCA1/BRCA2-containing complex during the S phase of the cell cycle. Depletion of BACH1 or BRCA1 using small interfering RNAs results in delayed entry into the S phase of the cell cycle. Such timely progression through S phase requires the helicase activity of BACH1. Importantly, cells expressing a dominant negative mutation in BACH1 that results in a defective helicase displayed increased activation of DNA damage checkpoints and genomic instability. BACH1 helicase is silenced during the G1 phase of the cell cycle and is activated through a dephosphorylation event as cells enter S phase. These results point to a critical role for BACH1 helicase activity not only in the timely progression through the S phase but also in maintaining genomic stability.
Inherited defects in the breast cancer susceptibility genes BRCA1 and BRCA2 are associated with increased risk for hereditary breast, ovarian, and other cancers (1, 9, 23, 25). BRCA1 and BRCA2 encode very large proteins with little resemblance to one another or to proteins of known function (23, 37). BRCA1 encodes a 1,863-amino-acid nuclear phosphoprotein with an N-terminal RING domain and C-terminal BRCT motifs (6, 14, 23, 30, 32).
The BRCA1 carboxyl-terminal domain, BRCT, is approximately 90 amino acids in length and plays an important role in the tumor suppressor functions of BRCA1 (2). Apart from BRCA1, more than 30 other BRCT-containing proteins have been documented in the human genome and appear to interact with proteins involved in DNA repair and checkpoint control (2, 4). Recent evidence suggests that the BRCT domain represents a new class of modules that mediate phosphorylation-dependent protein-protein interactions (29). The BRCT motif of BRCA1 plays a critical role in its ability to mediate double-strand break repair and homologous recombination (24, 33, 44). Mutations which disrupt or delete the C-terminal BRCT domain, but not other regions of BRCA1, have been shown to cause significant relocalization of BRCA1 from nucleus to cytoplasm (28). Loss of the BRCA1 BRCT domain has been attributed to tumor formation in mice (19). Cancer-causing missense mutations have been identified at the interface between the two BRCT repeats of BRCA1, which destabilize the structure (39, 40).
BACH1 (BRCA1-associated C-terminal helicase, also known as FANCJ and BRIP1), a member of the DEAH family of DNA helicases, directly interacts with the BRCT domain of the breast cancer gene product BRCA1 (5). More recently, it was shown that the BRCA1 interaction with BACH1 depends on the phosphorylation status of BACH1, and this phosphorylation-specific interaction is likely involved in the double-strand break repair function of BACH1 (41). BACH1 reveals substantial sequence similarity to the domains of the known members of the DEAH helicase family, XPD and CHL1 (22, 38). A recent report suggested that Chl1p, a DNA helicase-like protein in budding yeast that exhibits a high degree of sequence similarity to BACH1, plays a role critical for sister chromatid cohesion contributing to genome stability (35). Further, Cheung et al. have shown that a homolog of BACH1 called dog-1 (deletions of guanine-rich DNA) in Caenorhabditis elegans is required to maintain genetic stability of guanine-rich DNA in vivo (7). Disruption of dog-1 resulted in germ line as well as somatic deletions in genes containing polyguanine tracts. Taken together, these observations suggest that BACH1 could play a critical role in maintenance of genome stability in a manner dependent on its association with BRCA1. Although it has long been known that defects in the RecQ family of DNA helicases BLM, WRN, and RTS manifest perturbations in the S phase of the cell cycle indicative of their role in genomic DNA replication (10, 18, 27), little is known about the role of BACH1 in S-phase progression.
Recently, we described the isolation of a multiprotein complex termed BRCC, which contains BARD1, BRCA1, and BRCA2 along with novel subunits with homology to the signalosome and proteasome complexes (8). A recent report indicated that this complex also contains RAP80, a subunit involved in targeting BRCA1 to DNA damage sites (36). In order to gain insight into the biological function of BACH1, we isolated a BACH1-containing complex that also contains BRCA1, BRCA2, and BARD1. The BACH1 complex is distinct from that of the BRCC complex in that it does not contain BRCC36 or BRCC45/BRE. Interestingly, we show that the DNA-dependent ATPase activity of the complex is negligible in the G1 phase of the cell cycle and increases dramatically as cells enter S phase. Furthermore, depletion of BACH1 by RNA interference or mutations in the helicase domain of BACH1 resulted in delayed G1/S transition, suggesting an important role of BACH1 in S-phase progression.
MATERIALS AND METHODS
Plasmids.
Full-length BACH1 cDNA was constructed by reverse transcription-PCR from human testis mRNA. The PCR product was ligated into the mammalian expression vector pFLAG-CMV2 (Sigma) to produce a full-length clone. The clone was sequenced completely and verified to be intact. The mutant clones of BACH1 were generated with the QuikChange XL site-directed mutagenesis kit (Stratagene) using wild-type (WT) FLAG-BACH1 as template. For K52R, the lysine residue at position 52 was changed to arginine by using primers 5′-CCC ACA GGA AGT GGA AGG AGC TTA GCC TTA GCC-3′ and 5′-GGC TAA GGC TAA GCT CCT TCC ACT TCC TGT GGG-3′.
Purification of FLAG-BACH1.
FLAG-BACH1 was cotransfected along with a puromycin-resistant selection marker into 293 human embryonic kidney cells. After 48 h of transfection, the cells were grown in the presence of 5 μg/ml puromycin, and individual clones were screened for FLAG-BACH1 expression. For purification of the FLAG-BACH1 complex, nuclear extract prepared from FLAG-BACH1-expressing cells was incubated with anti-FLAG-M2 agarose (Sigma). The beads were washed extensively with 500 mM KCl in buffer A (20 mM Tris-HCl [pH 7.9], 0.2 mM EDTA, 10% glycerol, 5 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride) containing 0.5% NP-40 and 1 μg/ml aprotinin, leupeptin, and pepstatin. The bound fractions were eluted with 400 μg/ml FLAG peptide (Sigma) in 0.1 M KCl in buffer A. The FLAG-containing mutant BACH1 and other complexes were affinity purified using a procedure similar to that described for wild-type BACH1.
Antibodies.
BACH1 antibodies were raised against peptides corresponding to the last 20 amino acids at the C terminus. BRCA1 antibodies were from Oncogene. BARD1 antibody was a gift from Junjie Chen. Anti-BRCC36 and anti-BRE antibodies were raised against peptides corresponding to the last 20 amino acids of BRCC36 and BRE, respectively. BRCA2 antibody was as described elsewhere (20). γ-H2AX antibody was from Upstate Biotechnology. pATM and anti-53BP1 (clone W11) were gifts from Thanos Halazonetis. ORC2 antibody was a gift from Paul Lieberman.
Cell culture, DNA transfections, and cell synchronization.
HeLa or 293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum under standard conditions. 293 cells were transfected with vectors expressing wild-type and mutant BACH1 using Fugene 6 (Roche) according to the manufacturer's protocol. For synchronization at the G1/S boundary, cells were plated in a 15-cm dish to 40% confluence, and 24 hours after plating, the cells were incubated in 3 μg/ml aphidicolin for 18 h and then released into aphidicolin-free medium. The cells were harvested at various time points (0 to 10 h) after release and treated as indicated below. The harvested cells were fixed as a single-cell suspension at −20°C in 70% ethanol for 30 min, incubated in 100 μg/ml RNase (Roche), and then stained with 5 μg/ml propidium iodide (Sigma, St. Louis, MO) DNA stain and analyzed on the basis of their DNA content by flow cytometry. The BACH1 complex was purified from synchronous cells by immunoprecipitation using anti-FLAG antibody and used for Western blotting or enzyme assays as indicated.
Chromatin isolation.
MCF7 cells were synchronized with aphidicolin and at different time points after release were analyzed by flow cytometry for cell cycle distribution. The cells at different points in the cell cycle were subjected to a biochemical fractionation method to isolate chromatin-bound proteins as described elsewhere (21). Immunoblot assays were carried out with antibodies against BRCA2, BRCA1, BACH1, and ORC2 on the chromatin-enriched fractions.
siRNA transfections and cell synchronization.
The small interfering RNAs (siRNAs) were chemically synthesized by Dharmacon Inc. The sequence of BRCA1 siRNA was AA-CUUAGGUGAAGCAGCAUCU, the BACH1 siRNA was AA-ACAGCAAGCAACAUUGUUU, and the control siRNA was AA-GUUACUCAGCCAAGAACGA. siRNA transfections were done using Lipofectamine 2000 (Life Technologies, Inc.) according to the manufacturer's protocol. The cells were plated to 40% confluence in 10-cm dishes 24 h prior to transfection. A 1.6-nmol aliquot of siRNA was mixed with 20 μl Lipofectamine 2000 in 3 ml OPTI-MEM. The mixture was added, and the cells were incubated for 6 h. After 24 hours, a second transfection was performed similarly. Seventy-two hours after the initial transfection, the cells were harvested or treated as indicated. The cells were synchronized at the G1/S boundary as described earlier, and at various time points (0 to 10 h) after release they were treated as indicated. The cell cycle profile was monitored for control and BACH1- and BRCA1-depleted cells by analyzing the DNA content by propidium iodide staining.
BrdU labeling and staining.
HeLa cells were transfected with control, BACH1, and BRCA1 siRNAs and synchronized at the G1/S boundary with aphidicolin as described above. After release from the G1/S block into S phase, the cells were labeled with 10 μM bromodeoxyuridine (BrdU) for 30 min at the indicated times after release. The cells were harvested at each time point and fixed in 70% ethanol for 30 min, and DNA was denatured by treating with 2 M HCl for 30 min and neutralized by washing with phosphate-buffered saline (PBS) followed by PBS containing Tween 20. The cells were then stained with 1 μg of monoclonal antibody against BrdU (Zymed) in 200 μl PBS containing 1% bovine serum albumin at 4°C overnight, followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG; Vector Laboratories Inc., Burlingame, CA) for 1 h at room temperature. The cells were washed and stained with propidium iodide (PI), and DNA synthesis (FITC) and DNA content (PI) were analyzed by flow cytometry.
Phosphatase reaction.
Nuclear extracts from FLAG-BACH1 cells were immunoprecipitated with anti-FLAG antibody. The FLAG immunoprecipitates were extensively washed with buffer containing 500 mM KCl with protease inhibitors. The beads were resuspended in phosphatase buffer and incubated with 300 units of lambda phosphatase or equivalent amounts of heat-treated enzyme (New England Biolabs) at 30°C for 1 h in the presence of MnCl2. The beads were washed again with buffer containing 500 mM KCl followed by one wash with buffer containing 100 mM KCl. The treated samples were eluted with FLAG peptide and used for the ATPase assay.
ATPase assay.
An ATPase activity assay was performed as follows. The reaction mixture contained 20 mM Tris-HCl, 60 mM KCl, 4% glycerol, 4 mM MgCl2, 1 mM cold ATP, 1μCi [γ-32P]ATP, and BACH1 enzyme in a 10-μl volume. A 100-ng amount of double-stranded DNA, pcDNA3.1, was used in the reaction mixture wherever indicated. The reactions were performed at 30°C for 1 h. Free phosphate and ATP were separated by thin-layer chromatography (TLC) on PEI-cellulose plates (J.T. Baker). A 1-μl aliquot of the reaction mixture was spotted onto the plate, and TLC was carried out in 1 M formic acid and 0.5 M LiCl. Plates were allowed to dry and exposed to a phosphorimager cassette (Molecular Dynamics, Sweden).
Ionizing radiation sensitivity.
About 2 × 104 293, FLAG WT BACH1, and FLAG K52R cells were exposed to varying doses of γ-irradiation from a 137Cs source and incubated at 37°C for 4 days. The ionizing radiation sensitivity was assayed as described elsewhere (8).
Immunofluorescence.
Cells grown on coverslips were stained for 53BP1 (monoclonal primary antibody followed by anti-mouse IgG conjugated to Texas red secondary antibody) and γ-H2AX (polyclonal primary followed by anti-rabbit IgG conjugated to FITC secondary antibody) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) according to the protocol described previously (31).
RESULTS
BACH1 is a component of a distinct BRCA1/BRCA2-containing complex.
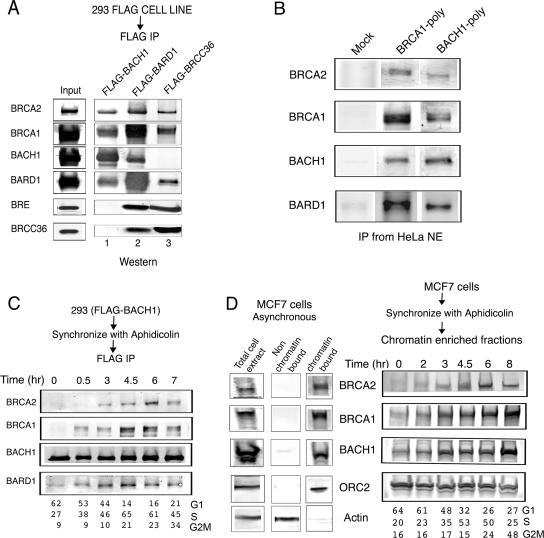
In order to gain insight into the biological function of BACH1 and its possible role in S-phase progression, we developed a 293-derived cell line expressing FLAG-BACH1. FLAG-BACH1 was affinity purified from nuclear extract by using anti-FLAG antibodies followed by elution of the bound material with FLAG peptide. We compared the polypeptide composition of the BACH1-containing complex to the previously characterized BRCC complex (8). Although BRCC contained BRCA1, BRCA2, and BARD1, it was distinguished by the presence of two novel subunits, BRCC36 and BRCC45/BRE. We examined whether the BACH1 complex contained the BRCC36 and BRCC45/BRE proteins or whether it may represent a distinct complex from that of BRCC. Western blot analysis of affinity eluates derived from FLAG-BACH1, FLAG-BRCC36, or FLAG-BARD1 indicated that both BRCC and the BACH1 complex share BRCA1, BRCA2, and BARD1 subunits (Fig. 1A). However, while BRCC does not contain BACH1, the BACH1 complex is devoid of BRCC36 and BRCC45 (Fig. 1A). Similar results were obtained following immunoprecipitation of the BACH1 complex using anti-BACH1 or anti-BRCA1 antibodies (Fig. 1B). These results indicate that while BARD1, BRCA1, and BRCA2 are shared subunits of both complexes, BRCC36 and BACH1 define two distinct complexes.
FIG. 1.
Purification of the BACH1-BRCA1 complex. (A) FLAG-BACH1, FLAG-BARD1, and FLAG-BRCC36 were immunoprecipitated from nuclear extracts of the corresponding cells with anti-FLAG-M2 agarose beads. The purified proteins were resolved on 4-12% Tris-glycine gels followed by Western blot analysis using the antibodies shown to the right of the panel. (B) Endogenous BACH1-BRCA1 complex was immunoprecipitated from HeLa nuclear extracts with polyclonal antibodies against BRCA1 and BACH1. The purified proteins were immunoblotted with antibodies against the proteins shown to the right of the panel. (C) Association of BACH1 and BRCA1 during cell cycle progression: the BACH1-BRCA1 interaction is cell cycle dependent. FLAG-BACH1 cells were synchronized with aphidicolin as described in Materials and Methods. The cells were released at the indicated times, followed by immunoprecipitation of nuclear extracts with anti-FLAG antibody. Immunoblot assays were carried out with the antibodies mentioned. Distribution of cells in the cell cycle as examined by flow cytometry is included for each time point. (D) Chromatin association of BRCA2, BRCA1, and BACH1 is cell cycle regulated. MCF7 cells were synchronized with aphidicolin and, at different time points after release, subjected to biochemical fractionation to isolate chromatin-bound proteins as described in Materials and Methods. Immunoblot assays of the chromatin-enriched fractions were carried out with the antibodies indicated. The cell cycle distribution at each time point is shown. Western blot analysis using the total lysate or the chromatin-bound and non-chromatin-bound amounts of BRCA2, BRCA1, BACH1, ORC2, and actin is shown on the right of the panel.
The BACH1 complex displays increased association with chromatin at S phase.
To examine the dynamic regulation of the BACH1 complex during the cell cycle, we synchronized the FLAG-BACH1 cells by aphidicolin treatment, and at various time points following release from the block, cells were harvested and nuclear extract was prepared. Affinity purification was performed using anti-FLAG antibodies, and the FLAG-eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blot analysis. As Fig. 1C indicates, BACH1 association with BRCA2, BRCA1, and BARD1 predominates as cells progress through S phase. We next analyzed the association of the BACH1 complex with chromatin at different stages of the cell cycle following a protocol established by Mendez and Stillman (21). Consistent with the predominance of the BACH1 complex during the S phase of the cell cycle, these experiments revealed the enhanced association of the BACH1 complex with chromatin as cells progress through the S phase (Fig. 1D). Taken together, these results suggest a role for the BRCA1-BACH1 complex in S-phase progression.
Depletion of BACH1 or BRCA1 by siRNA disrupts timely progression through S phase.
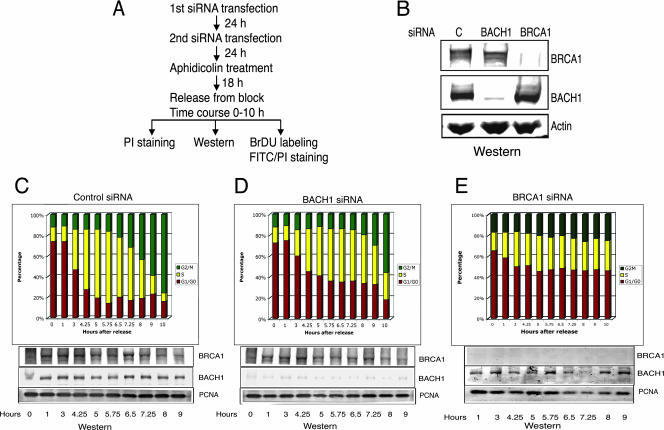
To assess the role of BACH1 and its partner BRCA1 in S-phase progression, we used RNA interference to deplete BACH1 and BRCA1. HeLa cells were treated with siRNA against BACH1, BRCA1, or a control siRNA for 48 h, after which they were treated with the DNA polymerase inhibitor aphidicolin to synchronize them prior to their entry into S phase (Fig. 2A and B). Analysis of BACH1- or BRCA1-depleted cells using flow cytometry following their release from aphidicolin revealed a pronounced delay in entry into S phase (Fig. 2C to E). This effect was manifested by an increased number of cells at the G1 phase of the cell cycle about 6 hours after release from aphidicolin (Fig. 2C to E; nearly 40% of cells remained in G1 following treatment with siRNA against BACH1 or BRCA1, while approximately less than 20% of cells were in G1 for cells treated with control siRNA).
FIG. 2.
Depletion of BACH1 and BRCA1 results in delayed G1/S progression and accumulation of cells in G1. (A) Flow chart depicting the sequence of siRNA transfections and S-phase analysis. (B) Western blot analysis of whole-cell lysate of HeLa cells treated with control, BACH1, or BRCA1 siRNA. Equal amounts of extract were loaded and probed with antibodies against BACH1 and BRCA1. Actin was used as a loading control. (C, D, and E) Cells transfected with control, BACH1, and BRCA1 siRNAs were synchronized at G1/S and released into S phase, and the cell cycle progression was monitored at the indicated time points after release by flow cytometric analysis of the total DNA content. The percentage distribution of cells in the G1, S, and G2/M phases of the cell cycle in control and BACH1- and BRCA1-depleted cells, as determined by PI staining, is shown. Western blot analysis results of BACH1 and BRCA1 in the corresponding cells at different time points after release are shown under each panel. PCNA is shown as a loading control.
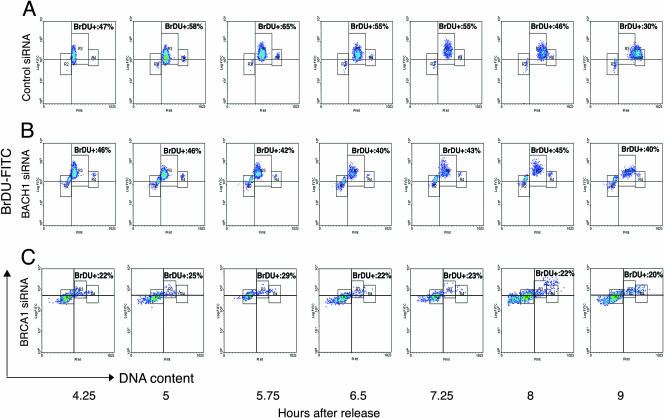
To further assess the role of BACH1 in S-phase progression, we analyzed the effects of BACH1 and BRCA1 depletion by incorporation of the thymidine analog BrdU following release from aphidicolin. As Fig. 3 delineates, treatment of cells with siRNA against BACH1 or BRCA1 not only resulted in a marked delay in entry into the S phase of the cell cycle but also reduced the number of cells incorporating BrdU (Fig. 3A through C; approximately 5 h after aphidicolin release). These results attest to the importance of BACH1 and BRCA1 in the timely progression through S phase. Depletion of BRCA1 resulted in a more pronounced effect than BACH1 depletion, consistent with the presence of BRCA1 in multiple complexes (i.e., BRCC).
FIG. 3.
Progression of cells through S phase in control and BACH1- and BRCA1-depleted cells. Control (A) and BACH1-depleted (B) and BRCA1-depleted (C) cells after release from G1/S arrest were labeled with BrdU at the indicated times and stained with anti-BrdU antibody followed by FITC-conjugated secondary antibody. The cells were stained with PI, and results were analyzed by two-color flow cytometry. Cell cycle populations are characterized as R2 (G1 cells with 2N DNA content), R3 (S phase cells with variable DNA content) and R4 (G2/M cells with 4N DNA content). The times after release from G1/S arrest are indicated. Cells in R3 above the quadrant are BrdU positive and represent the percentage of replicating cells in the S phase of the cell cycle. Percentage incorporation of BrdU at each time point in control, BACH1, and BRCA1 siRNA-treated cells is shown, indicating DNA synthesis. Cells begin to enter S phase at 4 h after release from aphidicolin in the control population, while the BACH1- and BRCA1-depleted cells show a delay in entering S phase with considerable accumulation of cells in G1. Loss of BACH1 and BRCA1 also led to a delay in exit from S to G2 (compare results at 8 h and 9 h after release).
The DNA-dependent ATPase of BACH1 is activated at S phase.
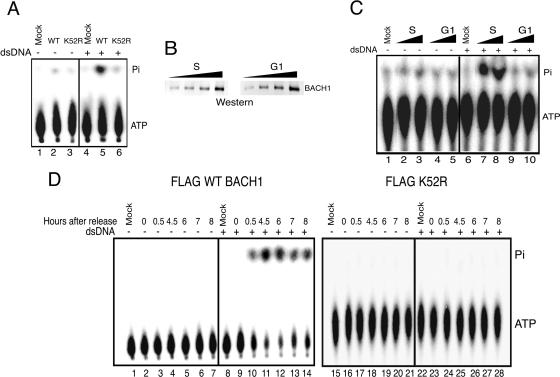
To gain insight into the functional activity of BACH1, we examined the FLAG-BACH1 affinity eluate in assays measuring DNA-dependent ATPase activity. The activity of Flag-BACH1 was compared to that of mutant FLAG-BACH1 (K52R), which was predicted to abolish the ATPase activity. As Fig. 4A indicates, while FLAG-BACH1 isolated from asynchronous cells displays robust DNA-dependent ATPase activity, a single point mutation in the ATPase domain of BACH1 (K52R) abolishes the ATPase activity.
FIG. 4.
DNA-dependent ATPase activity of BACH1 is cell cycle regulated. (A) FLAG-BACH1 hydrolyzes ATP in a DNA-dependent manner. Equivalent amounts of purified WT BACH1 and K52R proteins were incubated with [γ-32P]ATP in reaction mixtures containing 100 ng of double-stranded DNA (dsDNA) pcDNA3.1 and incubated at 30°C for 1 h. The reaction products were subjected to TLC on PEI-cellulose, and the inorganic phosphate (Pi) separated from ATP was analyzed by autoradiography of TLC plates (lanes 1 to 6). (B) Increasing amounts of BACH1 purified from FLAG-BACH1 cells at the S phase and G1 phase of the cell cycle were subjected to immunoblot analysis with anti-BACH1 antibody. (C) BACH1 purified from the G1 phase of the cell cycle has negligible ATPase activity. FLAG-BACH1 purified from nuclear extracts of cells synchronized in S and G1 phases of the cell cycle was analyzed for ATPase activity as described for panel A. This is a representative of five independent experiments resulting in similar outcomes. (D) FLAG-BACH1 and FLAG-K52R cells were synchronized at G1/S with aphidicolin and released into S phase, and at various times after release, BACH1 was immunoprecipitated from nuclear extracts with anti-FLAG antibody. The eluted fractions from each time point were assayed for ATPase activity as mentioned above.
We next assessed whether BACH1 DNA-dependent ATPase activity is regulated throughout the cell cycle. BACH1 was isolated from FLAG-BACH1 cells at either the G1 or S phase of the cell cycle, and the affinity eluate was used to analyze the activity of the complex in a DNA-dependent ATPase assay. We ensured that similar amounts of BACH1 at the G1 or S phase of the cell cycle were used for the assays by performing quantitative Western blot analysis (Fig. 4B). Interestingly, while FLAG-BACH1 affinity eluate prepared from cells at S phase displayed a robust DNA-dependent ATPase activity, the affinity eluate corresponding to cells at the G1 phase of the cell cycle was nearly devoid of any DNA-dependent ATPase activity (Fig. 4C).
To rigorously examine the activation of BACH1 DNA-dependent ATPase activity during cell cycle progression, we synchronized the wild type or the helicase mutant BACH1 (K52R) using aphidicolin. Analysis of affinity eluate at different time points following release from aphidicolin revealed the specific activation of wild-type BACH1 ATPase as cells entered the S phase of the cell cycle (Fig. 4D; approximately 4 hours after release from aphidicolin). The activation of BACH1 helicase is not a consequence of association of an unidentified DNA-dependent ATPase with BACH1, since no ATPase activity was detected following analysis of the mutant BACH1 (K52R) through the cell cycle (Fig. 4D). Taken together, these experiments provide support for the activation of BACH1 helicase as cells progress through the S phase.
BACH1 DNA-dependent ATPase is activated through a dephosphorylation event.
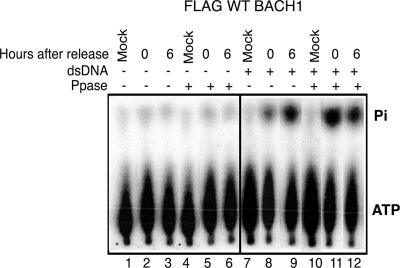
To address the mechanism by which BACH1 helicase is inactivated at the G1 phase of the cell cycle, Flag-BACH1 affinity eluate corresponding to the G1 or the S phase of the cell cycle was treated with λ-protein phosphatase. Dephosphorylation of FLAG-BACH1 eluate derived from cells harvested at G1 resulted in an enhanced ATPase activity (Fig. 5, compare lanes 8 and 11). In contrast, treatment of FLAG-BACH1 eluate derived from cells harvested at 6 h after aphidicolin release (predominantly at S phase) did not display any change in ATPase activity. Taken together, these experiments uncover a phosphorylation/dephosphorylation signaling pathway by which the activity of the BACH1 helicase is silenced at the G1 phase of the cell cycle and is reactivated as cells progress through S phase.
FIG. 5.
Dephosphorylation of BACH1 positively regulates G1 ATPase activity. FLAG-BACH1 cells were synchronized at G1/S with aphidicoiln and released into S phase, and at various times after release, cells were harvested followed by nuclear extract preparation and immunoprecipitation with M2-anti-FLAG antibody. The beads were treated with λ-protein phosphatase (+; lanes 4 to 6 and 10 to 12) or the heat-treated enzyme (−; lanes 1 to 3 and 7 to 9) and washed extensively, and the FLAG-eluted fractions from each time point were assayed for ATPase activity.
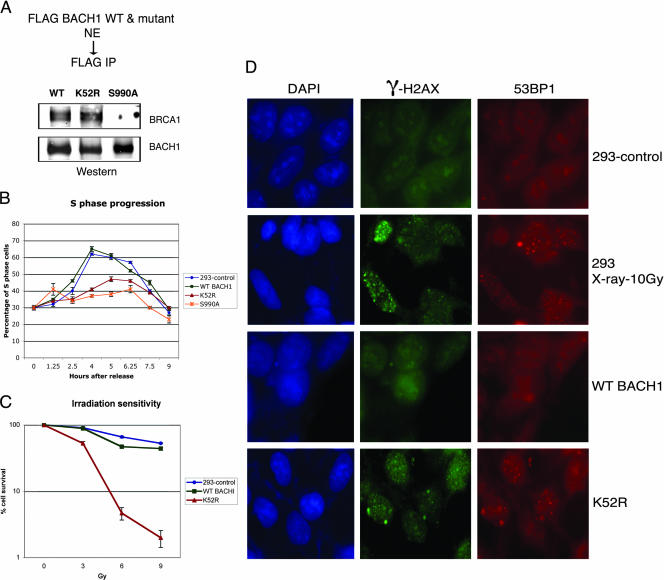
Inactivation of BACH1 helicase leads to delayed entry into S phase, increased ionizing radiation sensitivity, and activation of the DNA damage checkpoint.
We assessed the role of the BACH1 ATPase domain in S-phase progression by analyzing cell lines expressing the mutant form of BACH1 helicase (K52R) known to abolish DNA-dependent ATPase. We also examined whether cells expressing a mutant of BACH1 (S990A), which can no longer associate with BRCA1, also display defects in S-phase progression (Fig. 6A). Both mutations are predicted to act as dominant negative mutations. While the helicase mutant of BACH1 would be expected to poison the wild-type helicase-containing complexes, the S990A mutant of BACH1 will compete with complexes containing BRCA1. Analysis of cell lines expressing BACH1 (K52R) or BACH1 (S990A) revealed a similar defect in S-phase progression as that observed following the knockdown of BACH1 or BRCA1 by siRNAs (Fig. 6B). A likely scenario for participation of BACH1 helicase in S-phase progression may involve a role for the helicase activity in resolving structural barriers to DNA replication. In such a scenario, a defect in the helicase may result in replication stress and the consequent formation of DNA breaks. To assess whether mutation of BACH1 helicase results in phenotypes consistent with increased DNA damage, we measured the ionizing radiation sensitivity of BACH1 mutant cell lines. As Fig. 6C attests, cell lines carrying mutant BACH1 helicase (K52R) display a potent increase in ionizing radiation sensitivity. To further assess whether expression of BACH1 carrying the mutation in the helicase domain leads to the activation of DNA damage checkpoint machinery, we analyzed 53BP1-mediated foci formation, which has been proposed to function as a DNA damage sensor (31, 43), as well as the phosphorylation status of H2AX (34). As Fig. 6D demonstrates, BACH1 mutant cell lines in the absence of DNA damaging agents displayed increased 53BP1 foci formation and H2AX phosphorylation to similar levels normally encountered with wild-type cells following ionizing radiation. These results indicate that inactivating mutations in BACH1 helicase result in the appearance of DNA strand breaks and the consequent activation of DNA damage checkpoint machinery.
FIG. 6.
Cells expressing mutations in BACH1 helicase display a delay in S-phase progression and increased sensitivity to ionizing radiation. (A) Nuclear extracts from FLAG-WT BACH1, FLAG-K52R (helicase mutant), and FLAG-S990A were immunoprecipitated with anti-FLAG antibody, and the fractions were analyzed by Western blotting with the antibodies shown to the right of the panel. (B) Control 293, FLAG-WT BACH1, FLAG-K52R, and FLAG-S990A cells were synchronized at G1/S with aphidicolin and released into S phase, and the cell cycle progression was monitored at the indicated time points after release by flow cytometric analysis of the total DNA content. The percentage distribution of cells in G1, S, and G2/M phases of the cell cycle as determined by PI staining is shown. (C) Mutation in BACH1 (K52R) result in enhanced sensitivity to ionizing radiation compared to control 293 or WT BACH1 cells. (D) 53BP1 and γ-H2AX foci formation were studied in WT BACH1 and K52R cells. Nonirradiated 293 cells (negative control), irradiated (10 Gy) 293 cells (positive control), and WT BACH1 and K52R cells were stained by immunofluorescence with antibodies against 53BP1 and γ-H2AX as described in Materials and Methods. As a control, cells were stained with DAPI to mark the nuclear domain.
DISCUSSION
The key findings of our results are as follows: first, we have identified a complex containing BRCA1/BRCA2/BARD1 and the DNA-dependent ATPase protein BACH1 that displays increased association with chromatin at S phase. Second, the BACH1 complex is distinct from that of BRCC, as it does not contain either BRCC36 or BRCC45/BRE. Third, BACH1 DNA-dependent ATPase is diminished during the G1 phase of the cell cycle and is reactivated as cells enter S phase. Fourth, depletion of BACH1 or BRCA1 using siRNA results in accumulation of cells in G1 and a delayed entry into the S phase. Fifth, expression of a helicase mutant of BACH1 acts as a dominant negative by disrupting timely progression through S phase. Finally, mutations in BACH1 abrogating its helicase activity also result in enhanced genomic instability and activation of the DNA damage checkpoints. Our experiments are consistent with previous reports (41) indicating that BACH1 predominantly associates with BRCA1 as cells progress through the S phase of the cell cycle. We have extended this observation by showing that this interaction also includes BRCA2 and provided evidence pointing to a role for BACH1 in S-phase progression. Importantly, we show that the helicase activity of BACH1 is required for its role in S-phase progression.
RecQ helicases play an important role in the maintenance of genomic integrity by playing a role in S-phase progression (11). The catalytic activities of a number of RecQ family members are regulated by phosphorylation, leading to changes in the ATPase activity (12, 13, 26). Similar to the RecQ family members, analysis of BACH1 DNA-dependent ATPase activity revealed that it was regulated during cell cycle progression. While BACH1 isolated from cells in S phase exhibited a robust ATPase activity, BACH1 preparations corresponding to the G1 phase of the cell cycle were nearly devoid of any activity. Interestingly, phosphatase treatment of FLAG-BACH1 affinity eluate isolated from cells at the G1 phase of the cell cycle restored the DNA-dependent ATPase activity to levels similar to those observed at S phase. These results indicate that while during the G1 phase of the cell cycle the helicase activity of BACH1 is inhibited via a phosphorylation event, entrance to S phase is concomitant with activation of the helicase by a protein phosphatase which is required for a timely progression through S phase.
We can envision a scenario by which the BACH1/BRCA1/BARD1/BRCA2 complex assists in resolving difficult structural motifs encountered by the replication forks during DNA replication. Defects in helicase activity of BACH1 may then result in stalled replication forks, which may signal for a delay in S-phase progression. Such a role for the BRCA2 protein had been previously envisioned based on observations in BRCA2 mutant murine embryonic fibroblasts (17). We have extended this contention to implicate a role for BACH1 and BRCA1 in promoting S-phase progression through assisting stalled replication forks and consequently preventing DNA breakage and chromosomal instability. Such a contention is also consistent with recent reports delineating a role for BACH1 (also known as BRIP1) in Fanconi anemia (FA) (3, 15, 16), a syndrome associated with increased genomic instability. Importantly, we found BACH1 in complex with BRCA2, whose mutations were also recently shown to confer FA-like phenotypes (42), suggesting a role for the BACH1/BRCA2 complex in the FA pathway.
Acknowledgments
We thank T. Beer of the Wistar Proteomics Facility for expertise in the microcapillary HPLC/mass spectrometry.
R.S. was supported by a grant from NIH (CA 90758).
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Alberg, A. J., A. P. Lam, and K. J. Helzlsouer. 1999. Epidemiology, prevention, and early detection of breast cancer. Curr. Opin. Oncol. 11:435-441. [DOI] [PubMed] [Google Scholar]
- 2.Bork, P., K. Hofmann, P. Bucher, A. F. Neuwald, S. F. Altschul, and E. V. Koonin. 1997. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 11:68-76. [PubMed] [Google Scholar]
- 3.Bridge, W. L., C. J. Vandenberg, R. J. Franklin, and K. Hiom. 2005. The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat. Genet. 37:953-957. [DOI] [PubMed] [Google Scholar]
- 4.Callebaut, I., and J. P. Mornon. 1997. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 400:25-30. [DOI] [PubMed] [Google Scholar]
- 5.Cantor, S. B., D. W. Bell, S. Ganesan, E. M. Kass, R. Drapkin, S. Grossman, D. C. Wahrer, D. C. Sgroi, W. S. Lane, D. A. Haber, and D. M. Livingston. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105:149-160. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., C. F. Chen, D. J. Riley, D. C. Allred, P. L. Chen, D. Von Hoff, C. K. Osborne, and W. H. Lee. 1995. Aberrant subcellular localization of BRCA1 in breast cancer. Science 270:789-791. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, I., M. Schertzer, A. Rose, and P. M. Lansdorp. 2002. Disruption of dog-1 in Caenorhabditis elegans triggers deletions upstream of guanine-rich DNA. Nat. Genet. 31:405-409. [DOI] [PubMed] [Google Scholar]
- 8.Dong, Y., M. A. Hakimi, X. Chen, E. Kumaraswamy, N. S. Cooch, A. K. Godwin, and R. Shiekhattar. 2003. Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol. Cell 12:1087-1099. [DOI] [PubMed] [Google Scholar]
- 9.Futreal, P. A., Q. Liu, D. Shattuck-Eidens, C. Cochran, K. Harshman, S. Tavtigian, L. M. Bennett, A. Haugen-Strano, J. Swensen, Y. Miki, et al. 1994. BRCA1 mutations in primary breast and ovarian carcinomas. Science 266:120-122. [DOI] [PubMed] [Google Scholar]
- 10.Hand, R., and J. German. 1975. A retarded rate of DNA chain growth in Bloom's syndrome. Proc. Natl. Acad. Sci. USA 72:758-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickson, I. D. 2003. RecQ helicases: caretakers of the genome. Nat. Rev. Cancer 3:169-178. [DOI] [PubMed] [Google Scholar]
- 12.Ishimi, Y., Y. Komamura-Kohno, Z. You, A. Omori, and M. Kitagawa. 2000. Inhibition of Mcm4, 6, 7 helicase activity by phosphorylation with cyclin A/Cdk2. J. Biol. Chem. 275:16235-16241. [DOI] [PubMed] [Google Scholar]
- 13.Karmakar, P., J. Piotrowski, R. M. Brosh, Jr., J. A. Sommers, S. P. Miller, W. H. Cheng, C. M. Snowden, D. A. Ramsden, and V. A. Bohr. 2002. Werner protein is a target of DNA-dependent protein kinase in vivo and in vitro, and its catalytic activities are regulated by phosphorylation. J. Biol. Chem. 277:18291-18302. [DOI] [PubMed] [Google Scholar]
- 14.Koonin, E. V., S. F. Altschul, and P. Bork. 1996. BRCA1 protein products. Functional motifs. Nat. Genet. 13:266-268. [DOI] [PubMed] [Google Scholar]
- 15.Levitus, M., Q. Waisfisz, B. C. Godthelp, Y. Vries, S. Hussain, W. W. Wiegant, E. Elghalbzouri-Maghrani, J. Steltenpool, M. A. Rooimans, G. Pals, F. Arwert, C. G. Mathew, M. Z. Zdzienicka, K. Hiom, J. P. De Winter, and H. Joenje. 2005. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat. Genet. 37:934-935. [DOI] [PubMed] [Google Scholar]
- 16.Levran, O., C. Attwooll, R. T. Henry, K. L. Milton, K. Neveling, P. Rio, S. D. Batish, R. Kalb, E. Velleuer, S. Barral, J. Ott, J. Petrini, D. Schindler, H. Hanenberg, and A. D. Auerbach. 2005. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat. Genet. 37:931-933. [DOI] [PubMed] [Google Scholar]
- 17.Lomonosov, M., S. Anand, M. Sangrithi, R. Davies, and A. R. Venkitaraman. 2003. Stabilization of stalled DNA replication forks by the BRCA2 breast cancer susceptibility protein. Genes Dev. 17:3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonn, U., S. Lonn, U. Nylen, G. Winblad, and J. German. 1990. An abnormal profile of DNA replication intermediates in Bloom's syndrome. Cancer Res 50:3141-3145. [PubMed] [Google Scholar]
- 19.Ludwig, T., P. Fisher, S. Ganesan, and A. Efstratiadis. 2001. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 15:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmorstein, L. Y., A. V. Kinev, G. K. Chan, D. A. Bochar, H. Beniya, J. A. Epstein, T. J. Yen, and R. Shiekhattar. 2001. A human BRCA2 complex containing a structural DNA binding component influences cell cycle progression. Cell 104:247-257. [DOI] [PubMed] [Google Scholar]
- 21.Mendez, J., and B. Stillman. 2000. Chromatin association of human origin recognition complex, Cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20:8602-8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menichini, P., and M. Linial. 2001. SUVi and BACH1: a new subfamily of mammalian helicases? Mutat. Res. 487:67-71. [DOI] [PubMed] [Google Scholar]
- 23.Miki, Y., J. Swensen, D. Shattuck-Eidens, P. A. Futreal, K. Harshman, S. Tavtigian, Q. Liu, C. Cochran, L. M. Bennett, W. Ding, et al. 1994. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66-71. [DOI] [PubMed] [Google Scholar]
- 24.Moynahan, M. E., J. W. Chiu, B. H. Koller, and M. Jasin. 1999. Brca1 controls homology-directed DNA repair. Mol. Cell 4:511-518. [DOI] [PubMed] [Google Scholar]
- 25.Nathanson, K. L., R. Wooster, B. L. Weber, and K. N. Nathanson. 2001. Breast cancer genetics: what we know and what we need. Nat. Med. 7:552-556. [DOI] [PubMed] [Google Scholar]
- 26.Nuesch, J. P., S. Dettwiler, R. Corbau, and J. Rommelaere. 1998. Replicative functions of minute virus of mice NS1 protein are regulated in vitro by phosphorylation through protein kinase C. J. Virol. 72:9966-9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poot, M., H. Hoehn, T. M. Runger, and G. M. Martin. 1992. Impaired S-phase transit of Werner syndrome cells expressed in lymphoblastoid cell lines. Exp. Cell. Res. 202:267-273. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez, J. A., W. W. Au, and B. R. Henderson. 2004. Cytoplasmic mislocalization of BRCA1 caused by cancer-associated mutations in the BRCT domain. Exp. Cell. Res. 293:14-21. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez, M., X. Yu, J. Chen, and Z. Songyang. 2003. Phosphopeptide binding specificities of BRCA1 COOH-terminal (BRCT) domains. J. Biol. Chem. 278:52914-52918. [DOI] [PubMed] [Google Scholar]
- 30.Ruffner, H., and I. M. Verma. 1997. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc. Natl. Acad. Sci. USA 94:7138-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz, L. B., N. H. Chehab, A. Malikzay, and T. D. Halazonetis. 2000. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 151:1381-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scully, R., J. Chen, R. L. Ochs, K. Keegan, M. Hoekstra, J. Feunteun, and D. M. Livingston. 1997. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90:425-435. [DOI] [PubMed] [Google Scholar]
- 33.Scully, R., S. Ganesan, K. Vlasakova, J. Chen, M. Socolovsky, and D. M. Livingston. 1999. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol. Cell 4:1093-1099. [DOI] [PubMed] [Google Scholar]
- 34.Sedelnikova, O. A., D. R. Pilch, C. Redon, and W. M. Bonner. 2003. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2:233-235. [DOI] [PubMed] [Google Scholar]
- 35.Skibbens, R. V. 2004. Chl1p, a DNA helicase-like protein in budding yeast, functions in sister-chromatid cohesion. Genetics 166:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobhian, B., G. Shao, D. R. Lilli, A. C. Culhane, L. A. Moreau, B. Xia, D. M. Livingston, and R. A. Greenberg. 2007. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316:1198-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavtigian, S. V., J. Simard, J. Rommens, F. Couch, D. Shattuck-Eidens, S. Neuhausen, S. Merajver, S. Thorlacius, K. Offit, D. Stoppa-Lyonnet, C. Belanger, R. Bell, S. Berry, R. Bogden, Q. Chen, T. Davis, M. Dumont, C. Frye, T. Hattier, S. Jammulapati, T. Janecki, P. Jiang, R. Kehrer, J. F. Leblanc, D. E. Goldgar, et al. 1996. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat. Genet. 12:333-337. [DOI] [PubMed] [Google Scholar]
- 38.Weber, C. A., E. P. Salazar, S. A. Stewart, and L. H. Thompson. 1990. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 9:1437-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, R. S., D. I. Chasman, D. D. Hau, B. Hui, A. Y. Lau, and J. N. Glover. 2003. Detection of protein folding defects caused by BRCA1-BRCT truncation and missense mutations. J. Biol. Chem. 278:53007-53016. [DOI] [PubMed] [Google Scholar]
- 40.Williams, R. S., R. Green, and J. N. Glover. 2001. Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1. Nat. Struct. Biol. 8:838-842. [DOI] [PubMed] [Google Scholar]
- 41.Yu, X., C. C. Chini, M. He, G. Mer, and J. Chen. 2003. The BRCT domain is a phospho-protein binding domain. Science 302:639-642. [DOI] [PubMed] [Google Scholar]
- 42.Zdzienicka, M. Z., and F. Arwert. 2002. Breast cancer and Fanconi anemia: what are the connections? Trends Mol. Med. 8:458-460. [DOI] [PubMed] [Google Scholar]
- 43.Zgheib, O., Y. Huyen, R. A. Ditullio, Jr., A. Snyder, M. Venere, E. S. Stavridi, and T. D. Halazonetis. 2005. ATM signaling and 53BP1. Radiother Oncol. 76:119-122. [DOI] [PubMed] [Google Scholar]
- 44.Zhong, Q., C. F. Chen, S. Li, Y. Chen, C. C. Wang, J. Xiao, P. L. Chen, Z. D. Sharp, and W. H. Lee. 1999. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 285:747-750. [DOI] [PubMed] [Google Scholar]