Abstract
Xeroderma pigmentosum group C (XPC) protein plays an essential role in DNA damage recognition in mammalian global genome nucleotide excision repair (NER). Here, we analyze the functional basis of NER inactivation caused by a single amino acid substitution (Trp to Ser at position 690) in XPC, previously identified in the XPC patient XP13PV. The Trp690Ser change dramatically affects the in vivo stability of the XPC protein, thereby causing a significant reduction of its steady-state level in XP13PV fibroblasts. Despite normal heterotrimeric complex formation and physical interactions with other NER factors, the mutant XPC protein lacks binding affinity for both undamaged and damaged DNA. Thus, this single amino acid substitution is sufficient to compromise XPC function through both quantitative and qualitative alterations of the protein. Although the mutant XPC fails to recognize damaged DNA, it is still capable of accumulating in a UV-damaged DNA-binding protein (UV-DDB)-dependent manner to UV-damaged subnuclear domains. However, the NER factors transcription factor IIH and XPA failed to colocalize stably with the mutant XPC. As well as highlighting the importance of UV-DDB in recruiting XPC to UV-damaged sites, these findings demonstrate the role of DNA binding by XPC in the assembly of subsequent NER intermediate complexes.
Nucleotide excision repair (NER) is a major pathway for removing a wide variety of helix-distorting DNA base lesions, including the UV-induced cyclobutane pyrimidine dimer (CPD) and the (6-4) pyrimidine-pyrimidone photoproduct (6-4PP) (11). Hereditary defects in NER are associated with several autosomal recessive disorders, such as xeroderma pigmentosum (XP), which is characterized by cutaneous hypersensitivity to UV exposure and a predisposition to skin cancer. Seven genetic complementation groups have been identified in the NER-defective form of XP (XP group A [XPA] to XPG), and all of the corresponding genes have been cloned (4, 10).
Two subpathways have been discerned in NER: global genome NER (GG-NER), which operates throughout the genome, and transcription-coupled NER, which specifically removes lesions located on the transcribed strand of active genes. In the GG-NER subpathway, XPC plays an essential role in damage recognition and initiation of the repair reaction (30, 36, 42). XPC is present in vivo as a heterotrimeric complex containing one of the two mammalian homologs of Saccharomyces cerevisiae, Rad23p (RAD23A or RAD23B) and centrin 2 (1, 20, 32). We have shown previously that the XPC complex is a structure-specific DNA-binding factor with an affinity for branched structures containing a junction between double- and single-stranded DNA (37, 39). On the basis of such biochemical properties, the XPC complex can recognize and bind not only to certain artificial DNA structures, such as bubbles and loops, but also to sites containing various helix-distorting lesions that do not share any common chemical structure. UV-damaged DNA-binding protein (UV-DDB), which was identified as a heterodimer consisting of DDB1 and DDB2 (XPE) subunits, has been shown to facilitate recruitment of XPC to damaged DNA sites (9, 23, 44), likely through direct physical interactions (38). XPC also interacts with the XPB and p62 subunits of the basal transcription factor IIH (TFIIH) complex, thereby directly recruiting TFIIH to damaged sites (2, 45). The helicase subunits of TFIIH, XPB and XPD, then locally unwind the DNA duplex around the lesion, and the resulting single-stranded DNA region is stabilized by XPA, XPG, and replication protein A (RPA) (7, 8, 24, 43). The open complex formation is a prerequisite for NER dual incision by two structure-specific endonucleases, XPF-ERCC1 and XPG (21, 27, 34). Subsequent gap-filling DNA repair synthesis is performed in a proliferating cell nuclear antigen (PCNA)-dependent manner, thereby allowing DNA ligase to rejoin the DNA strands (3, 33).
The complementation group C of XP is one of the most frequent forms of this disease, and numerous causative mutations in the human XPC gene have been identified. The vast majority of these are nonsense and frameshift mutations which are distributed over the entire gene and exhibit no obvious hot spots (5, 14, 18). Several functional domains have been identified in human XPC (e.g., DNA-, centrin 2-, and TFIIH-binding domains) and have been mapped near the C terminus (26, 29, 41). These domains are overlapped by a region exhibiting significant amino acid sequence homology to the S. cerevisiae XPC ortholog Rad4p. Therefore, mutations causing C-terminal truncations of XPC likely impair normal protein functions. The majority of XPC patients analyzed have significantly reduced XPC mRNA levels, likely due to nonsense-mediated mRNA decay (14-17). Moreover, immunoblot analyses have not detected any truncated versions of the XPC protein. These findings represent a plausible explanation for the relatively homogeneous clinical and cellular phenotypes of XPC patients (i.e., their resemblance to phenotypes of a null mutant).
A few subtle mutations in the XPC gene have been also reported, including two cases with single amino acid substitutions. One patient, XP1MI, was reported to have a missense mutation which resulted in a Pro-to-His substitution at position 334. This mutation reduced the steady-state XPC mRNA level (18). In another patient, designated XP13PV, one XPC allele contained a G-to-C transversion in exon 10, resulting in a Trp-to-Ser substitution at position 690 (W690S) (5). Despite nearly normal XPC mRNA levels and low but detectable expression of the full-length XPC protein, the cellular sensitivity to UV light in patient XP13PV was similar to that observed in XPC patients with mutations resulting in severely truncated XPC products. This finding strongly suggests that this single amino acid substitution is sufficient to compromise XPC function profoundly. Here, we examine how the W690S substitution affects the intracellular behavior and function of XPC. As well as providing novel insights into the structure-function relationship of the XPC protein, the results of this study shed light on the molecular mechanisms underlying the in vivo process of GG-NER.
MATERIALS AND METHODS
Cell culture.
Human skin fibroblasts from a healthy individual (NB1-RGB; obtained from the cell bank at the RIKEN Bioresource Center) and patient XP13PV were cultured at 37°C in Eagle's minimal essential medium (Invitrogen) containing 15% (vol/vol) fetal bovine serum (FBS). Simian virus 40-transformed normal (WI38 VA13) and XPC-deficient (XP4PASV) human fibroblast lines were grown at 37°C in Dulbecco's modified Eagle's medium (Nissui) containing 10% (vol/vol) FBS. HighFive cells were cultured at 27°C in Ex-Cell 405 medium (JRH Bioscience).
Antibodies.
Anti-full-length XPC [XPC (FL)], anti-human RAD23B, anti-C-terminal XPC, anti-6-4PP (64 M-2), and anti-CPD (TDM-2) antibodies were prepared as described previously (22, 28, 35). The anti-DDB2 monoclonal antibody was established by the MAB Institute Co., Ltd. (Yokosuka, Japan). Anti-lamin B (C-20), anti-XPB (S-19), and anti-XPA (FL-273) antibodies were purchased from Santa Cruz Biotechnology. The anti-FLAG (M5) antibody was purchased from Sigma.
Isolation of stably transfected cell lines.
The bicistronic mammalian expression vector pIREShyg (Clontech) was used for stable expression of wild-type and mutant XPC (fused to the N-terminal FLAG tag). The expression constructs were linearized and introduced into XP4PASV cells by electroporation using a gene pulser II (Bio-Rad). Stable transfectants were selected initially by culture in the presence of 200 μg/ml hygromycin B1 (Invitrogen). After colonies were isolated, the concentration of hygromycin B1 was reduced gradually, and clones expressing appropriate levels of XPC were then selected from the results of immunoblot analyses.
Preparation of cell extracts.
Cell monolayers cultured in 60-mm dishes were washed twice with phosphate-buffered saline (PBS) and lysed with 0.5 ml of ice-cold NP lysis buffer (25 mM Tris-HCl, pH 8.0, 1 mM EDTA, 10% [vol/vol] glycerol, 1% [vol/vol] Nonidet P-40, 1 mM dithiothreitol, 0.25 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail [Complete; Roche Diagnostics]) containing 0.3 M NaCl. After 1 h of incubation on ice, the lysates were scraped into microfuge tubes and centrifuged at 20,000 × g for 10 min. The clarified soluble cell extracts were used for immunoblot and immunoprecipitation analyses. The whole-cell extracts used for experiments with results shown in Fig. 3A were prepared using a sodium dodecyl sulfate (SDS) denaturing method described previously (28).
FIG. 3.
The W690S mutation causes destabilization and functional defects of XPC in vivo. (A) Establishment of stably transfected cell lines expressing FLAG-tagged XPC (wild type [wt] or W690S mutant). Whole-cell extracts prepared from the transfectants, as well as from the parental XP4PASV cells and the simian virus 40-transformed normal human fibroblast line WI38 VA13, were subjected to immunoblot analyses using the antibodies indicated (α-XPC, anti-XPC). The XPC band intensity was quantified, normalized to lamin B1 for each lane, and then expressed as a relative value compared to its expression in WI38 VA13 cells. (B and C) The experiments were performed as described in the legend for Fig. 2, using the XP4PASV transformed cells expressing wt or mutant FLAG-XPC. Arrows indicate the position of full-length XPC. The ∼80-kDa band that appeared during the simultaneous treatment (+) with cycloheximide (CHX) and MG132 is shown by an arrowhead. Asterisks, nonspecific bands. (D) Global genome repair kinetics of UV-induced 6-4PP. The cell lines indicated were treated with 6 mM thymidine for 2 h to block DNA replication, irradiated with a UVC dose of 10 J/m2, and further incubated in the presence of 6 mM thymidine for the times indicated. The amount of 6-4PP remaining in genomic DNA at each time point was measured by an enzyme-linked immunosorbent assay using a lesion-specific monoclonal antibody.
Preparation of recombinant proteins.
Bacterial expression and purification of hexahistidine-tagged human RAD23B (RAD23B-His) and centrin 2 (nontagged and glutathione S-transferase [GST] tagged) were performed as described previously (1, 19, 26). Recombinant baculoviruses were constructed using a Bac-to-Bac system (Invitrogen). Wild-type FLAG-XPC was expressed in HighFive insect cells, purified, and used to form in vitro complexes with RAD23B-His, as described previously (37). Since FLAG-XPC (W690S) was somewhat unstable during purification, we added an excess of RAD23B-His to the insect cell extract, enabling heterodimer formation. The two initial column chromatography steps (using heparin and anti-FLAG) were carried out as described previously (37). The FLAG peptide-containing eluate was loaded onto a HiTrap chelating HP column (1 ml; GE Healthcare Bioscience), which had been bound with Ni2+ ions and equilibrated with buffer A (20 mM sodium phosphate, pH 7.8, 10% [vol/vol] glycerol, 0.01% [vol/vol] Triton X-100, 1 mM 2-mercaptoethanol, and 0.25 mM phenylmethylsulfonyl fluoride) containing 0.3 M NaCl. After extensive washing with buffer A containing 1 M NaCl, bound proteins were eluted stepwise with the same buffer containing 20, 100, and 250 mM imidazole-HCl (pH 7.8). The 100 mM imidazole fractions containing XPC complexes were adjusted to 1.5 M NaCl and loaded onto a HiTrap butyl FF column (1 ml; GE Healthcare Bioscience) equilibrated with buffer A containing 1.5 M NaCl. The protein was eluted stepwise with buffer A containing 1.5, 0.5, and 0.2 M NaCl, and the XPC complex was recovered in the 0.5 M NaCl eluate. This fraction was diluted in buffer A to 0.3 M NaCl and loaded onto a Mono S PC 1.6/5 column that had been connected to a SMART system (GE Healthcare Bioscience) and equilibrated with buffer A containing 0.3 M NaCl. Bound proteins were eluted in buffer A containing 0.7 M NaCl.
The GST-tagged XPB and p62 proteins were expressed in HighFive cells (seven 150-mm culture dishes each) and solubilized as described for XPC. The cell lysates were dialyzed against buffer A containing 0.1 M NaCl and loaded onto a GSTrap FF column (1 ml; GE Healthcare Bioscience) equilibrated in the same buffer. After extensive washing, bound proteins were eluted in buffer A containing 0.1 M NaCl and 10 mM glutathione (Sigma). In order to concentrate GST-XPB and GST-p62, eluates were loaded onto mini Q PC 3.2/3 and mini S PC 3.2/3 columns (GE Healthcare Bioscience), respectively. Both columns were connected to a SMART system, preequilibrated with buffer A containing 0.1 M NaCl, and developed with a 3-ml gradient of 0.1 to 1 M NaCl in buffer A.
To prepare the UV-DDB complex, HighFive cells (10 150-mm culture dishes) were coinfected with recombinant baculoviruses expressing His-DDB1 and GST-DDB2. Crude cell extracts were prepared and dialyzed as described previously (38) and then loaded onto a HiPrep heparin 16/10 column (GE Healthcare Bioscience) equilibrated with buffer A containing 0.1 M NaCl. Following stepwise elution with buffer A containing 0.1, 0.2, and 1 M NaCl, the 1 M NaCl fraction was loaded onto a HiTrap chelating HP column (1 ml) bound with Ni2+ ions, which had been preequilibrated with buffer A containing 1 M NaCl. Bound proteins were eluted stepwise in the same buffer containing 20, 100, and 250 mM imidazole-HCl (pH 7.8). The purity of the 250 mM imidazole fraction containing UV-DDB heterodimers was determined by SDS-polyacrylamide gel electrophoresis, after which the fraction was loaded onto a GSTrap FF column (1 ml) equilibrated with buffer A containing 1 M NaCl. Proteins were eluted in the same buffer containing 10 mM glutathione and dialyzed against buffer A containing 0.1 M NaCl. The protein was then loaded onto a mini Q PC 3.2/3 column connected to a SMART system and eluted stepwise with buffer A containing 0.2, 0.5, and 1 M NaCl. The UV-DDB heterodimer was recovered in the 0.2 and 0.5 M NaCl fractions.
GST pull-down assays.
Ten microliters of glutathione-Sepharose FF beads (GE Healthcare Bioscience) was washed with buffer B (25 mM Tris-HCl, pH 7.5, 0.3 M NaCl, 1 mM EDTA, 10% [vol/vol] glycerol, 0.5% [vol/vol] Triton X-100, 1 mM dithiothreitol) before being mixed with 500 μl of buffer B containing 10 μg/ml bovine serum albumin (BSA). The purified GST fusion proteins (5 pmol) were added to the beads and incubated at 4°C for 2 h with gentle rotation. The beads were then washed four times with 500 μl buffer B and resuspended in 50 μl buffer B containing 10 μg/ml BSA and 1 pmol of the FLAG-XPC/RAD23B-His complex containing either wild-type or W690S mutant XPC. After incubation at 4°C with gentle agitation, the beads were washed eight times with 500 μl buffer B, and the bound proteins were eluted in 40 μl buffer B containing 10 mM glutathione. The eluate was recovered by centrifugation, and the precipitation of the XPC complex was examined by immunoblotting with anti-XPC (FL) antibody.
Local UV irradiation and immunofluorescence analyses.
Cells were cultured in poly-d-lysine-coated, 35-mm glass-bottomed dishes (MatTek). Local UV irradiation was performed using isopore membrane filters (Millipore TMTP; 5 μm pore size) as described previously (13). The cells were washed twice in ice-cold PBS and fixed at 4°C for 15 min with 1.6% (wt/vol) formaldehyde (Wako Pure Chemicals). After additional washes with ice-cold PBS, the cells were treated for 10 min with ice-cold PBS containing 0.5% (vol/vol) Triton X-100 and then incubated at room temperature for 30 min in PBS containing 3% (vol/vol) FBS to block nonspecific antibody adsorption. During the following procedure, the dishes were washed five times with PBS after each incubation. Samples were incubated for 2 h at room temperature with the appropriate primary antibody and then for 1 h at room temperature with a corresponding Alexa Fluor 488-labeled secondary antibody (1:500 dilution; Molecular Probes). All antibodies were diluted with PBS containing 0.05% (vol/vol) Tween 20 and 0.5% (vol/vol) FBS. For visualizing UV-induced photolesions, the samples were further treated successively for 10 min at 37°C with 1.4% (wt/vol) formaldehyde, for 10 min at 37°C with 2 M HCl (to denature DNA), for 30 min at 37°C with lesion-specific monoclonal antibody (64 M-2 or TDM-2), and then for 30 min at 37°C with an Alexa Fluor 594-labeled, anti-mouse immunoglobulin G antibody (1:1,000 dilution; Molecular Probes). In addition, nuclear DNA was counterstained for 5 min at room temperature with 0.05 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) or 10 μg/ml Hoechst 33342 in PBS. Dishes were mounted in drops of Vectashield (Vector Laboratories). Fluorescence microscopy was performed using an Olympus IX70 instrument and IP Lab software (Solution Systems).
siRNA knockdown.
The small interfering RNA (siRNA) for human DDB2 was purchased from QIAGEN (Hs-DDB2-1 HP). The siRNA was transfected into cells using Lipofectamine 2000 reagent and Opti-MEM medium (both from Invitrogen) according to the manufacturer's instructions. The transfected cells were incubated at 37°C for 72 h before being used in immunoblotting or local UV irradiation experiments.
Other materials and methods.
The amount of UV-induced 6-4PP in genomic DNA was measured by an enzyme-linked immunosorbent assay using a lesion-specific monoclonal antibody as described previously (22). Genomic DNA was purified using a QIAamp DNA blood mini kit (QIAGEN). Electrophoretic mobility shift assays and cell-free NER incision assays were performed as described previously (37), except for the omission of formaldehyde fixation in the former assay. Cycloheximide (Sigma) and MG132 (Calbiochem) were purchased. Protein concentrations of cell extracts were determined according to the method of Schaffner and Weissmann (31), while purified proteins were quantified using a Bio-Rad protein assay kit. For both assays, BSA was used as the standard.
RESULTS
The W690S substitution destabilizes XPC in vivo.
The position of the missense mutation identified in patient XP13PV is located within the C-terminal domain, which shares significant sequence homology with S. cerevisiae Rad4p (Fig. 1). Moreover, the mutated Trp is strongly conserved throughout evolution (Fig. 1B). Since low levels of the XPC protein were reported for XP13PV cell lysates (5), we examined the in vivo stability of XPC in XP13PV primary fibroblasts. Previously, we reported that endogenously expressed XPC is quite stable and that its turnover is slow in wild-type mouse embryonic fibroblasts (28). We confirmed these findings by showing that there was little change in XPC protein levels in normal human fibroblasts treated with cycloheximide, an inhibitor of de novo protein synthesis, over a 12-h period (Fig. 2A, lanes 1 to 6). In striking contrast, similar treatment of XP13PV primary fibroblasts resulted in a rapid reduction of XPC levels (Fig. 2B, lanes 1 to 6). To examine whether the 26S proteasome was involved in XPC degradation, the cells were treated simultaneously with a proteasome inhibitor (MG132) and cycloheximide. We confirmed that MG132 blocked proteolysis by observing the accumulation of the p21 tumor suppressor protein (data not shown). Although we found that the addition of MG132 failed to stabilize full-length XPC, this treatment did result in the accumulation of an ∼80-kDa band, which reacted with the anti-XPC antibody (Fig. 2B, lanes 8 to 11).
FIG. 1.
A single amino acid substitution in XPC identified from patient XP13PV. (A) Position of the mutation site with respect to known domains of human XPC. The domain sharing amino acid (aa) sequence homology to S. cerevisiae Rad4p is shown by a shaded box, and several previously determined interacting domains are indicated by solid lines. (B) Evolutionary conservation of the Trp residue (indicated by an arrow). An alignment of corresponding amino acid sequences from XPC orthologs is shown. Amino acids that are identical or similar among three or more species are shaded. H. sapiens, Homo sapiens; M. musculus, Mus musculus; D. melanogaster, Drosophila melanogaster; C. elegans, Caenorhabditis elegans.
FIG. 2.
Instability of XPC expressed in XP13PV primary fibroblasts. Human skin fibroblasts from a healthy individual (NB1-RGB) (A) or from patient XP13PV (B) were incubated, for the time periods indicated, in the presence (+) or absence (−) of 0.1 mM cycloheximide (CHX) and/or 10 μM MG132. Soluble cell extracts (5 μg protein per lane) were subjected to immunoblot analyses using the antibodies indicated (α-XPC, anti-XPC). Arrows indicate the position of full-length XPC. The ∼80-kDa band observed during simultaneous treatment with CHX and MG132 is shown by an arrowhead. Asterisk, nonspecific band.
To examine whether the single amino acid substitution is sufficient to induce destabilization of XPC, stably transformed clones expressing near-physiological levels of wild-type or W690S mutant FLAG-tagged XPC were isolated from the XP4PASV cell line, which is defective in endogenous XPC (Fig. 3A). When the transformed cells were treated with cycloheximide as described for Fig. 2, the ectopically expressed W690S mutant protein was less stable than its wild-type counterpart (Fig. 3B and C, compare lanes 1 to 6). However, unlike results for the endogenous protein in XP13PV primary fibroblasts, the addition of MG132 appeared to stabilize the full-length mutant protein and resulted in the accumulation of the ∼80-kDa band (Fig. 3C, lanes 8 to 11). We found that the ∼80-kDa band also reacted with an anti-FLAG antibody (data not shown), indicating that it was a cleavage product of FLAG-XPC (W690S) containing the N terminus (see Discussion).
The W690S substitution abolishes the GG-NER function of XPC in vivo.
To examine whether W690S mutant XPC can function in GG-NER, the removal of 6-4PP was analyzed. The XP4PASV stably transformed cells expressing FLAG-XPC (wild type or W690S mutant) were UV irradiated at 10 J/m2 and were harvested at various time points thereafter. After purification of genomic DNA, the remaining 6-4PPs were measured using a lesion-specific monoclonal antibody. Control cells expressing wild-type FLAG-XPC showed repair kinetics similar to those of the normal human fibroblast line WI38 VA13 (Fig. 3D). In contrast, the stably transformed cells expressing the mutant protein had removed little 6-4PP from the genomic DNA, as was observed for the parental XP4PASV cells. Thus, the mutant XPC did not contribute significantly to GG-NER, despite its presence at relatively high steady-state levels.
Functional defects of the XPC (W690S) mutant protein.
To further explore which XPC functions are affected by the amino acid substitution, we first investigated the ability of the XPC (W690S) mutant protein to form heterotrimeric complexes in vivo. Wild-type or mutant FLAG-XPC was immunoprecipitated from extracts of XP4PASV stably transformed cells. Both RAD23B and centrin 2 bound to XPC, and there was no significant difference between wild-type and mutant XPC in the stoichiometry of the three subunits (Fig. 4A). The XPB subunit of TFIIH also coimmunoprecipitated equally well with wild-type and mutant XPC (Fig. 4A). We also purified recombinant protein complexes containing wild-type or W690S mutant FLAG-XPC and RAD23B-His (Fig. 4B) and investigated their interactions with several GST-tagged NER proteins that had previously been shown to interact directly with XPC (Fig. 4C). Both wild-type and mutant XPC were capable of direct physical interaction with the two TFIIH subunits, XPB and p62, and with UV-DDB (Fig. 4C).
FIG. 4.
Mutant XPC retains normal physical interactions with other NER factors. (A) FLAG-XPC (wild type [wt] or W690S mutant) was immunoprecipitated with an anti (α)-FLAG antibody from soluble extracts of XP4PASV transfected cells. Coimmunoprecipitation of each subunit of the XPC complex as well as the XPB subunit of TFIIH was examined by immunoblotting. Three different amounts (2.5, 5, and 10%) of the immunoprecipitates were loaded in parallel. (B) Purified recombinant FLAG-XPC/RAD23B-His complexes (containing wt or mutant XPC) were subjected to SDS-polyacrylamide gel electrophoresis and visualized by silver staining. (C) GST-tagged NER proteins were immobilized on glutathione-Sepharose beads and incubated with purified FLAG-XPC/RAD23B-His complexes (wt or W690S mutant). FLAG-XPC coimmunoprecipitated with each GST fusion protein as well as 0.2% of the input XPC complex.
Next, we examined the DNA-binding activity of the mutant XPC complex. Electrophoretic mobility shift assays using radiolabeled DNA fragments that either contained or were lacking single 6-4PP lesions were performed (Fig. 5A). The wild-type XPC complex bound specifically to DNA fragments with 6-4PP lesions. In contrast, mutant XPC did not bind to undamaged or damaged DNA at the same concentrations of protein (Fig. 5A). Furthermore, the mutant failed to support cell-free NER dual incision in an XPC-deficient cell extract (Fig. 5B). Immunoblot analyses confirmed that this inability was not due to degradation of the W690S XPC protein during incubation with the crude cell extract (see Fig. S1 in the supplemental material). These results indicate that the W690S XPC mutant protein is defective in its ability to bind to DNA.
FIG. 5.
The XPC (W690S) mutant lacks damage recognition activity. (A) Electrophoretic mobility shift assays using a 32P-labeled DNA fragment (∼180 bp) with or without a single 6-4PP (ND, no damage). Purified wild-type (wt) or W690S mutant FLAG-XPC/RAD23B-His complexes were included in binding reactions (10 μl) at 0 nM (lanes 1, 5, 9, and 13), 0.66 nM (lanes 2, 6, 10, and 14), 1.32 nM (lanes 3, 7, 11, and 15), and 2.65 nM (lanes 4, 8, 12, and 16), together with a 6.7-fold molar excess of purified centrin 2 (i.e., equivalent amounts in weight) and 0.35 nM DNA probe. Following incubation for 30 min at 30°C, protein-DNA complexes were separated by nondenaturing polyacrylamide gel electrophoresis (PAGE) and detected by autoradiography. (B) Cell-free NER dual incision assay. The double-stranded circular DNA substrate containing an internal 32P label (∼2.5 × 105 cpm) near a site-specific 6-4PP was incubated in a 25-μl mixture containing XP3BE (XPC-deficient) cell extract (100 μg protein) and purified FLAG-XPC/RAD23B-His complexes, containing either wt XPC or W690S mutant XPC. The concentrations of XPC complexes included in the reactions were 0 pM (lane 1), 13 pM (lanes 2 and 6), 66 pM (lanes 3 and 7), 330 pM (lanes 4 and 8), and 530 pM (lanes 5 and 9). Following incubation for 1 h at 30°C, DNA samples were purified and subjected to denaturing PAGE, followed by autoradiography. Part of the autoradiogram is presented, showing damage-containing oligonucleotides excised by NER.
The W690S XPC mutant can be recruited to sites of damage in a UV-DDB-dependent manner.
The finding that the mutant XPC protein lacks damage recognition activity prompted us to investigate the localization of the mutant protein in locally UV-damaged cells. We locally UV irradiated XP4PASV transformed cells expressing FLAG-XPC and performed immunofluorescence analyses using an anti-XPC antibody. Within 30 min, wild-type FLAG-XPC accumulated in damaged areas, as identified by counterstaining with an anti-6-4PP lesion-specific antibody (Fig. 6A, left) or anti-CPD antibody (data not shown). At 3 h postirradiation, most 6-4PP appeared to be repaired, and the FLAG-XPC had dispersed throughout the nucleus. Although most CPDs still remain at this time point (data not shown), the amount of XPC engaged in CPD repair may be too small to form discernible foci under these experimental conditions. Surprisingly, despite defective damage-specific DNA-binding activity, significant accumulation of the mutant XPC protein in UV-damaged areas could also be observed within 30 min postirradiation (Fig. 6A, right; see also quantitative data in Fig. 6D). As predicted from the data in Fig. 3D, very little (if any) 6-4PP repair could be observed at 3 h following irradiation, and the local accumulation of mutant XPC still persisted at that time point.
FIG. 6.
UV-DDB-dependent recruitment of the XPC (W690S) mutant to subnuclear UV-damaged areas. (A) XP4PASV transfected cell lines expressing FLAG-XPC (wild type [wt]or mutant) were irradiated with UV (100 J/m2) through isopore membrane filters. After 0.5 or 3 h at 37°C, the localization of XPC (green) and 6-4PP (red) was visualized by immunofluorescence. Nuclear DNA was counterstained with DAPI. ND, nondamaged control cells. (B) siRNA knockdown of endogenous DDB2. The XP4PASV transfected cell lines were transfected with siRNA for DDB2 (+) or mock treated (−). After incubation at 37°C for 72 h, soluble cell extracts (each 10 μg protein) were subjected to immunoblot analyses using the indicated antibodies (α-DDB2, anti-DDB2). (C) The XP4PASV stably transformed cells expressing FLAG-XPC (wt or W690S mutant) were transfected with siRNA for DDB2 and incubated for 72 h. After local UV irradiation followed by 0.5 h of incubation at 37°C, the localization of XPC (green) and CPD (red) was visualized by immunofluorescence analyses. Nuclear DNA was counterstained with Hoechst 33342. (D) From the immunofluorescence data, the numbers of cells containing visible XPC foci 30 min after UV irradiation were counted and expressed as percentages of the total numbers of cells that had acquired local UV damage (i.e., cells containing CPD foci). The mean values and standard errors were calculated from two independent experiments, each of which contained 100 CPD-positive cells.
A possible explanation for the results discussed above is that the mutant XPC was recruited to sites of damage through its interaction with UV-DDB. To investigate this possibility, we knocked down the expression levels of DDB2 by using siRNA and determined whether XPC localization was subsequently affected. As shown in Fig. 6B, treatment with DDB2 siRNA suppressed the expression of the endogenous DDB2 protein within the XP4PASV stably transformed cells expressing either wild-type or mutant XPC. Under these conditions, accumulation of wild-type XPC was still observed for a substantial proportion of the cells that acquired local UV damage (Fig. 6C and D). In contrast, the percentage of cells containing visible foci of mutant XPC dramatically decreased after the knockdown of DDB2. This was not due to a delay of XPC accumulation, since the number of cells containing XPC foci did not increase up to 3 h later (data not shown). Although recruitment of wild-type XPC to UV damage may be facilitated by UV-DDB, as reported previously (6, 9, 23, 44), our results indicate that accumulation of mutant XPC depends heavily on the presence of functional UV-DDB.
Behavior of other NER factors in the presence of mutant XPC.
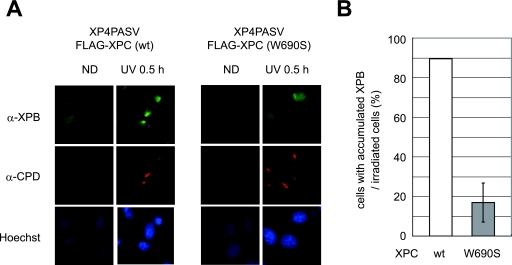
Although the mutant XPC protein appeared to localize to damaged sites in a UV-DDB-dependent manner, the NER process must be impaired at some stage. Therefore, we observed the accumulation of XPB and XPA, both of which are known to be involved in later stages of GG-NER than XPC, following local UV irradiation. In XP4PASV transformed cells expressing wild-type FLAG-XPC, XPB accumulated at locally damaged sites within 30 min postirradiation (Fig. 7A, left). At this time point, the percentage of cells that accumulated XPB was comparable to the percentage of cells containing XPC foci (approximately 90%) (compare Fig. 6D and 7B). Three hours after irradiation, XPB dispersed throughout the nucleus similarly to XPC (data not shown). In contrast, in the presence of mutant XPC, only ∼20% of locally irradiated cells accumulated XPB within 30 min (Fig. 7B), while accumulation of mutant XPC could be observed in as many as ∼70% of the irradiated cells. Furthermore, in the cells expressing wild-type XPC, XPA behaved similarly to XPB and XPC (accumulating at 30 min and dispersing by 3 h), whereas XPA did not accumulate at any time point in cells expressing only mutant XPC (data not shown). These results strongly suggest that XPC must bind properly to DNA for the assembly of stable NER complexes involving TFIIH and XPA.
FIG. 7.
The XPC W690S mutant fails to induce the stable accumulation of XPB following local UV irradiation. (A) XP4PASV transfected cell lines stably expressing FLAG-XPC (wild type [wt] or W690S mutant) were locally UV irradiated through isopore membrane filters. Following 0.5 h of incubation at 37°C, the distribution of XPB was visualized by immunostaining with an anti-XPB (α-XPB) antibody (green). Counterstaining with anti-CPD antibody (red) and Hoechst 33342 (blue) was also performed. ND, nondamaged control cells. (B) The percentages of cells containing detectable XPB foci were calculated for each cell line. The mean values and standard errors were calculated from two independent experiments, each of which contained 100 CPD-positive cells.
DISCUSSION
Instability of the XPC (W690S) mutant protein.
We have demonstrated that the single amino acid substitution (Ser for Trp at position 690) significantly destabilizes human XPC in vivo. Although we have shown previously that the mammalian Rad23p homolog stabilizes XPC (25, 28), we have found here that the interaction between W690S mutant XPC and RAD23B is apparently normal (Fig. 4A and B). It is possible that the mutant protein may have a slightly lower affinity than wild-type XPC for RAD23, and this may contribute, at least in part, to its instability. However, unlike XPC in the RAD23-deficient cells, the mutant XPC protein was not stabilized by UV irradiation (see Fig. S2 in the supplemental material), suggesting an alternative destabilization mechanism. Although the proteasome inhibitor MG132 failed to block degradation of the mutant XPC protein in XP13PV primary fibroblasts, this treatment resulted in accumulation of an ∼80-kDa band (Fig. 2B), which probably corresponds to an N-terminal fragment of the XPC (W690S) mutant. Notably, the apparent size of the fragment largely coincides with the estimated molecular weight of the N-terminal XPC sequence upstream of this mutation site. This may indicate that the amino acid substitution induces a change in the local protein structure, resulting in cleavage near the mutation site by an endolytic protease, followed by degradation via the ubiquitin-proteasome system. Unfortunately, however, no protease has been predicted from the amino acid sequence around the mutation site.
The FLAG-XPC (W690S) protein that was ectopically expressed in XP4PASV cells may be degraded by a different mechanism than the endogenously expressed XPC from patient XP13PV. When the transfected cells were treated with MG132 and cycloheximide, both the ∼80-kDa fragment and the full-length FLAG-XPC (W690S) protein were stabilized (Fig. 3C). Thus, at least part of FLAG-XPC (W690S) may be degraded directly by the 26S proteasome without the involvement of an endolytic protease. Although the mutant protein appears to be less stable than wild-type XPC, we analyzed a stably transformed cell line that expressed near-physiological steady-state levels of FLAG-XPC (W690S) protein. Therefore, a possible explanation for apparent differences in the mutants’ responses to MG132 may be that XPC expressed in these transformed cells is metabolized more rapidly than the endogenously expressed mutant protein. However, we cannot exclude the possibility that the addition of a FLAG tag may affect the structure of the protein and its susceptibility to the protease cleavage.
The W690S substitution abolishes the DNA-binding activity of XPC.
Although the instability and reduced steady-state levels of mutant XPC may account for the GG-NER deficiency in patient XP13PV, the ability of XPC to recognize DNA damage is also profoundly affected by the mutation. This is not surprising considering that the DNA-binding domain in XPC (41) contains the Trp residue that is mutated in patient XP13PV (Fig. 1A). Although the structural basis underlying the XPC-DNA interaction remains to be elucidated, the Trp residue may be important for DNA binding specifically or for the maintenance of the local protein domain structure through hydrophobic interactions. Notably, the XPC (W690S) mutant appears to retain many physical interactions with other NER proteins (Fig. 4), indicating that any structural perturbation of XPC caused by the amino acid substitution is probably restricted to a very specific, highly localized region. Nevertheless, our results indicate that the W690S mutation causes both quantitative and functional defects in XPC, which explains why fibroblasts from patient XP13PV exhibit levels of UV sensitivity and UV-induced unscheduled DNA synthesis similar to those of XPC-null cells, despite the detectable expression of the full-length XPC (5).
Roles for the XPC DNA-binding activity in GG-NER.
The XPC (W690S) mutant protein lacks damage recognition capability but still accumulates to subnuclear UV-damaged areas in the presence of functional UV-DDB (Fig. 6). Similar nonfunctional recruitment of mutant XPG proteins has also been reported previously (40). Our results strongly support the model that UV-DDB promotes recruitment of XPC to sites of UV damage, likely through direct physical interactions. However, the presence of mutant XPC at a damage site was not sufficient to induce the repair process (Fig. 3D), indicating that the DNA-binding activity of XPC is essential for GG-NER. Since TFIIH interacts directly with both wild-type and W690S mutant XPC (Fig. 4), it is possible that TFIIH may be recruited transiently to a lesion site by mutant XPC bound to UV-DDB. Even in the presence of mutant XPC, a small number of cells still accumulated XPB (Fig. 7B), which could reflect the inefficient recruitment of TFIIH. In the absence of DNA binding by XPC, however, neither TFIIH nor XPA appears to be stably assembled into the NER complex. Scanning force microscopic analyses revealed that when the XPC complex is bound to a site of DNA damage, an ∼40° bending of the DNA duplex is induced (12). Thus, it is very likely that such a conformational change in the DNA is important for the later steps of NER, such as entry of the TFIIH helicases.
In conclusion, we have shown that a single amino acid substitution in XPC can reduce its stability in vivo and abolish its damage recognition capability. Although the XPC mutant can localize to sites of DNA damage in vivo (likely through its interaction with UV-DDB), its defective DNA-binding activity severely compromises the recruitment and assembly of NER factors. Further analyses of other subtle XPC mutations will improve our understanding of the structure-function relationship of XPC as well as the molecular mechanisms underlying GG-NER.
ADDENDUM IN PROOF
During review of this paper, two articles concerning roles of the XPC Trp690 residue in DNA damage recognition were published (C. G. Bunick, M. R. Miller, B. E. Fuller, E. Fanning, and W. J. Chazin, Biochemistry 45:14965-14979, 2006; O. Maillard, S. Solyom, and H. Naegeli, PLoS Biol. 5:e79, 2007).
Supplementary Material
Acknowledgments
We thank Hirotaka Kobayashi for technical assistance and other members of the Cellular Physiology Laboratory of RIKEN for helpful discussions and encouragement. We also thank Yasue Ichikawa and Rie Nakazawa (Bioarchitect Research Group, RIKEN) for DNA sequencing.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Human Frontier Science Program, and by Solution Oriented Research for Science and Technology (SORST) from the Japan Science and Technology Agency. G.Y. and R.N. were supported by a fellowship from the Center of Excellence (COE) Program of the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 6 August 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Araki, M., C. Masutani, M. Takemura, A. Uchida, K. Sugasawa, J. Kondoh, Y. Ohkuma, and F. Hanaoka. 2001. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 276:18665-18672. [DOI] [PubMed] [Google Scholar]
- 2.Araújo, S. J., E. A. Nigg, and R. D. Wood. 2001. Strong functional interactions of TFIIH with XPC and XPG in human DNA nucleotide excision repair, without a preassembled repairosome. Mol. Cell. Biol. 21:2281-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araújo, S. J., F. Tirode, F. Coin, H. Pospiech, J. E. Syväoja, M. Stucki, U. Hübscher, J.-M. Egly, and R. D. Wood. 2000. Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev. 14:349-359. [PMC free article] [PubMed] [Google Scholar]
- 4.Bootsma, D., K. H. Kraemer, J. E. Cleaver, and J. H. J. Hoeijmakers. 2001. Nucleotide excision repair syndromes: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy, p. 677-703. In C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle (ed.), The metabolic and molecular basis of inherited disease, vol. 1. McGraw-Hill Book Co., New York, NY. [Google Scholar]
- 5.Chavanne, F., B. C. Broughton, D. Pietra, T. Nardo, A. Browitt, A. R. Lehmann, and M. Stefanini. 2000. Mutations in the XPC gene in families with xeroderma pigmentosum and consequences at the cell, protein, and transcript levels. Cancer Res. 60:1974-1982. [PubMed] [Google Scholar]
- 6.El-Mahdy, M. A., Q. Zhu, Q. E. Wang, G. Wani, M. Praetorius-Ibba, and A. A. Wani. 2006. Cullin 4A-mediated proteolysis of DDB2 protein at DNA damage sites regulates in vivo lesion recognition by XPC. J. Biol. Chem. 281:13404-13411. [DOI] [PubMed] [Google Scholar]
- 7.Evans, E., J. Fellows, A. Coffer, and R. D. Wood. 1997. Open complex formation around a lesion during nucleotide excision repair provides a structure for cleavage by human XPG protein. EMBO J. 16:625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, E., J. G. Moggs, J. R. Hwang, J.-M. Egly, and R. D. Wood. 1997. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 16:6559-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitch, M. E., S. Nakajima, A. Yasui, and J. M. Ford. 2003. In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J. Biol. Chem. 278:46906-46910. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg, E. C. 2001. How nucleotide excision repair protects against cancer. Nat. Rev. Cancer 1:22-33. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. Ellenberger. 2006. DNA repair and mutagenesis, 2nd ed. ASM Press, Washington, DC.
- 12.Janicijevic, A., K. Sugasawa, Y. Shimizu, F. Hanaoka, N. Wijgers, M. Djurica, J. H. Hoeijmakers, and C. Wyman. 2003. DNA bending by the human damage recognition complex XPC-HR23B. DNA Repair 2:325-336. [DOI] [PubMed] [Google Scholar]
- 13.Katsumi, S., N. Kobayashi, K. Imoto, A. Nakagawa, Y. Yamashina, T. Muramatsu, T. Shirai, S. Miyagawa, S. Sugiura, F. Hanaoka, T. Matsunaga, O. Nikaido, and T. Mori. 2001. In situ visualization of ultraviolet-light-induced DNA damage repair in locally irradiated human fibroblasts. J. Investig. Dermatol. 117:1156-1161. [DOI] [PubMed] [Google Scholar]
- 14.Khan, S. G., H. L. Levy, R. Legerski, E. Quackenbush, J. T. Reardon, S. Emmert, A. Sancar, L. Li, T. D. Schneider, J. E. Cleaver, and K. H. Kraemer. 1998. Xeroderma pigmentosum group C splice mutation associated with autism and hypoglycinemia. J. Investig. Dermatol. 111:791-796. [DOI] [PubMed] [Google Scholar]
- 15.Khan, S. G., A. Metin, E. Gozukara, H. Inui, T. Shahlavi, V. Muniz-Medina, C. C. Baker, T. Ueda, J. R. Aiken, T. D. Schneider, and K. H. Kraemer. 2004. Two essential splice lariat branchpoint sequences in one intron in a xeroderma pigmentosum DNA repair gene: mutations result in reduced XPC mRNA levels that correlate with cancer risk. Hum. Mol. Genet. 13:343-352. [DOI] [PubMed] [Google Scholar]
- 16.Khan, S. G., K. S. Oh, T. Shahlavi, T. Ueda, D. B. Busch, H. Inui, S. Emmert, K. Imoto, V. Muniz-Medina, C. C. Baker, J. J. DiGiovanna, D. Schmidt, A. Khadavi, A. Metin, E. Gozukara, H. Slor, A. Sarasin, and K. H. Kraemer. 2006. Reduced XPC DNA repair gene mRNA levels in clinically normal parents of xeroderma pigmentosum patients. Carcinogenesis 27:84-94. [DOI] [PubMed] [Google Scholar]
- 17.Legerski, R., and C. Peterson. 1992. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature 359:70-73. [DOI] [PubMed] [Google Scholar]
- 18.Li, L., E. S. Bales, C. A. Peterson, and R. J. Legerski. 1993. Characterization of molecular defects in xeroderma pigmentosum group C. Nat. Genet. 5:413-417. [DOI] [PubMed] [Google Scholar]
- 19.Masutani, C., M. Araki, K. Sugasawa, P. J. van der Spek, A. Yamada, A. Uchida, T. Maekawa, D. Bootsma, J. H. Hoeijmakers, and F. Hanaoka. 1997. Identification and characterization of XPC-binding domain of hHR23B. Mol. Cell. Biol. 17:6915-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masutani, C., K. Sugasawa, J. Yanagisawa, T. Sonoyama, M. Ui, T. Enomoto, K. Takio, K. Tanaka, P. J. van der Spek, D. Bootsma, J. H. J. Hoeijmakers, and F. Hanaoka. 1994. Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homolog of yeast RAD23. EMBO J. 13:1831-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsunaga, T., D. Mu, C. H. Park, J. T. Reardon, and A. Sancar. 1995. Human DNA repair excision nuclease. Analysis of the roles of the subunits involved in dual incisions by using anti-XPG and anti-ERCC1 antibodies. J. Biol. Chem. 270:20862-20869. [DOI] [PubMed] [Google Scholar]
- 22.Mori, T., M. Nakane, T. Hattori, T. Matsunaga, M. Ihara, and O. Nikaido. 1991. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem. Photobiol. 54:225-232. [DOI] [PubMed] [Google Scholar]
- 23.Moser, J., M. Volker, H. Kool, S. Alekseev, H. Vrieling, A. Yasui, A. A. van Zeeland, and L. H. F. Mullenders. 2005. The UV-damaged DNA binding protein mediates efficient targeting of the nucleotide excision repair complex to UV-induced photo lesions. DNA Repair 4:571-582. [DOI] [PubMed] [Google Scholar]
- 24.Mu, D., M. Wakasugi, D. S. Hsu, and A. Sancar. 1997. Characterization of reaction intermediates of human excision repair nuclease. J. Biol. Chem. 272:28971-28979. [DOI] [PubMed] [Google Scholar]
- 25.Ng, J. M. Y., W. Vermeulen, G. T. J. van der Horst, S. Bergink, K. Sugasawa, H. Vrieling, and J. H. J. Hoeijmakers. 2003. A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 17:1630-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishi, R., Y. Okuda, E. Watanabe, T. Mori, S. Iwai, C. Masutani, K. Sugasawa, and F. Hanaoka. 2005. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol. Cell. Biol. 25:5664-5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donovan, A., A. A. Davies, J. G. Moggs, S. C. West, and R. D. Wood. 1994. XPG endonuclease makes the 3′ incision in human DNA nucleotide excision repair. Nature 371:432-435. [DOI] [PubMed] [Google Scholar]
- 28.Okuda, Y., R. Nishi, J. M. Y. Ng, W. Vermeulen, G. T. J. van der Horst, T. Mori, J. H. J. Hoeijmakers, F. Hanaoka, and K. Sugasawa. 2004. Relative levels of the two mammalian Rad23 homologs determine composition and stability of the xeroderma pigmentosum group C protein complex. DNA Repair 3:1285-1295. [DOI] [PubMed] [Google Scholar]
- 29.Popescu, A., S. Miron, Y. Blouquit, P. Duchambon, P. Christova, and C. T. Craescu. 2003. Xeroderma pigmentosum group C protein possesses a high affinity binding site to human centrin 2 and calmodulin. J. Biol. Chem. 278:40252-40261. [DOI] [PubMed] [Google Scholar]
- 30.Riedl, T., F. Hanaoka, and J.-M. Egly. 2003. The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 22:5293-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaffner, W., and C. Weissmann. 1973. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56:502-514. [DOI] [PubMed] [Google Scholar]
- 32.Shivji, M. K. K., A. P. M. Eker, and R. D. Wood. 1994. DNA repair defect in xeroderma pigmentosum group C and complementing factor from HeLa cells. J. Biol. Chem. 269:22749-22757. [PubMed] [Google Scholar]
- 33.Shivji, M. K. K., V. N. Podust, U. Hübscher, and R. D. Wood. 1995. Nucleotide excision repair DNA synthesis by DNA polymerase e in the presence of PCNA, RFC, and RPA. Biochemistry 34:5011-5017. [DOI] [PubMed] [Google Scholar]
- 34.Sijbers, A. M., W. L. de Laat, R. R. Ariza, M. Biggerstaff, Y.-F. Wei, J. G. Moggs, K. C. Carter, B. K. Shell, E. Evans, M. C. de Jong, S. Rademakers, J. de Rooij, N. G. J. Jaspers, J. H. J. Hoeijmakers, and R. D. Wood. 1996. Xeroderma pigmentosum group F caused by a defect of in structure-specific DNA repair endonuclease. Cell 86:811-822. [DOI] [PubMed] [Google Scholar]
- 35.Sugasawa, K., C. Masutani, A. Uchida, T. Maekawa, P. J. van der Spek, D. Bootsma, J. H. Hoeijmakers, and F. Hanaoka. 1996. HHR23B, a human Rad23 homolog, stimulates XPC protein in nucleotide excision repair in vitro. Mol. Cell. Biol. 16:4852-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugasawa, K., J. M. Y. Ng, C. Masutani, S. Iwai, P. J. van der Spek, A. P. M. Eker, F. Hanaoka, D. Bootsma, and J. H. J. Hoeijmakers. 1998. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell 2:223-232. [DOI] [PubMed] [Google Scholar]
- 37.Sugasawa, K., T. Okamoto, Y. Shimizu, C. Masutani, S. Iwai, and F. Hanaoka. 2001. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev. 15:507-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugasawa, K., Y. Okuda, M. Saijo, R. Nishi, N. Matsuda, G. Chu, T. Mori, S. Iwai, K. Tanaka, K. Tanaka, and F. Hanaoka. 2005. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell 121:387-400. [DOI] [PubMed] [Google Scholar]
- 39.Sugasawa, K., Y. Shimizu, S. Iwai, and F. Hanaoka. 2002. A molecular mechanism for DNA damage recognition by the xeroderma pigmentosum group C protein complex. DNA Repair 1:95-107. [DOI] [PubMed] [Google Scholar]
- 40.Thorel, F., A. Constantinou, I. Dunand-Sauthier, T. Nouspikel, P. Lalle, A. Raams, N. G. Jaspers, W. Vermeulen, M. K. Shivji, R. D. Wood, and S. G. Clarkson. 2004. Definition of a short region of XPG necessary for TFIIH interaction and stable recruitment to sites of UV damage. Mol. Cell. Biol. 24:10670-10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida, A., K. Sugasawa, C. Masutani, N. Dohmae, M. Araki, M. Yokoi, Y. Ohkuma, and F. Hanaoka. 2002. The carboxy-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH. DNA Repair 1:449-461. [DOI] [PubMed] [Google Scholar]
- 42.Volker, M., M. J. Moné, P. Karmakar, A. van Hoffen, W. Schul, W. Vermeulen, J. H. J. Hoeijmakers, R. van Driel, A. A. van Zeeland, and L. H. F. Mullenders. 2001. Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell 8:213-224. [DOI] [PubMed] [Google Scholar]
- 43.Wakasugi, M., J. Reardon, and A. Sancar. 1997. The non-catalytic function of XPG protein during dual incision in human nucleotide excision repair. J. Biol. Chem. 272:16030-16034. [DOI] [PubMed] [Google Scholar]
- 44.Wang, Q. E., Q. Zhu, G. Wani, J. Chen, and A. A. Wani. 2004. UV radiation-induced XPC translocation within chromatin is mediated by damaged-DNA binding protein, DDB2. Carcinogenesis 25:1033-1043. [DOI] [PubMed] [Google Scholar]
- 45.Yokoi, M., C. Masutani, T. Maekawa, K. Sugasawa, Y. Ohkuma, and F. Hanaoka. 2000. The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J. Biol. Chem. 275:9870-9875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.