Abstract
In Xenopus laevis embryos, the midblastula transition (MBT) at the 12th cell division marks initiation of critical developmental events, including zygotic transcription and the abrupt inclusion of gap phases into the cell cycle. Interestingly, although an ionizing radiation-induced checkpoint response is absent in pre-MBT embryos, introduction of a threshold amount of undamaged plasmid or sperm DNA allows a DNA damage checkpoint response to be activated. We show here that undamaged threshold DNA directly participates in checkpoint signaling, as judged by several dynamic changes, including H2AX phosphorylation, ATM phosphorylation and loading onto chromatin, and Chk1/Chk2 phosphorylation and release from nuclear DNA. These responses on physically separate threshold DNA require γ-H2AX and are triggered by an ATM-dependent soluble signal initiated by damaged DNA. The signal persists in egg extracts even after damaged DNA is removed from the system, indicating that the absence of damaged DNA is not sufficient to end the checkpoint response. The results identify a novel mechanism by which undamaged DNA enhances checkpoint signaling and provide an example of how the transition to cell cycle checkpoint activation during development is accomplished by maternally programmed increases in the DNA-to-cytoplasm ratio.
During embryogenesis in Xenopus laevis, the midblastula transition (MBT) occurs upon completion of the 12th cell division and marks a number of critical changes, including the introduction of gap phases into the cell cycle and the beginning of transcription. Moreover, early embryonic cell cycles lack checkpoints that halt the cell cycle in response to DNA damage, whereas the DNA damage checkpoint functions normally in post-MBT embryos (1, 10). One possible explanation is that one or more of the biochemical components required for DNA damage checkpoint signal transduction are absent in pre-MBT embryos and, thus, zygotic transcription could be necessary to produce elements of the checkpoint pathway. However, the addition of exogenous DNA with free ends mimicking DNA double-strand breaks (DSBs) induces checkpoint activation in pre-MBT embryos or egg extracts, suggesting all components of a functional checkpoint pathway exist before the MBT (6, 11, 15). In the very early embryo, this checkpoint requires both DNA DSBs and a threshold DNA-to-cytoplasm ratio to significantly affect the cell cycle (6). Importantly, even uncut plasmid DNA can facilitate responses to DNA DSBs in pre-MBT embryos (6), suggesting a previously undefined role for undamaged threshold DNA in DNA damage checkpoint signal transduction.
In eukaryotic cells, ataxia-telangiectasia mutated (ATM) and ataxia-telangiectasia and Rad3-related (ATR) kinases are activated by DNA damage and initiate signal transduction pathways that lead to checkpoint activation (28). The mechanisms of DNA damage-induced ATM and ATR activation are still largely unclear. ATM activation has been attributed to DNA damage-induced ATM autophosphorylation at serine 1981 and dissociation of inactive, dimeric ATM into an active, monomeric form (2). Although autophosphorylation of ATM is widely utilized as an indicator of ATM activation, it is dispensable for murine ATM activation (26). Interestingly, although ATM is primarily activated in the vicinity of DNA DSBs, agents altering chromatin structure can activate ATM independently of DNA damage (2). Whereas ATM activation is mainly triggered by DNA DSBs, ATR is activated by single-strand DNA (ssDNA) and stalled replication forks (34). In order to activate ATR, different forms of DNA damage must be processed into a common intermediate structure: ssDNA coated with replication protein A (34). It has also been shown that TopBP1, a BRCT domain protein, is an ATR activator (16). Upon activation, ATM and ATR phosphorylate many cellular substrates critical for controlling checkpoint responses, transcription, or apoptosis, including Chk1, Chk2, H2AX, BRCA1, NBS1, SMC1, and p53 (28). Of these ATM/ATR substrates, Chk1 and Chk2 are two serine/threonine-specific kinases classified as checkpoint kinases owing to their critical roles in regulating cell cycle checkpoints. Activated Chk1/Chk2 phosphorylate and activate the tyrosine kinase Wee1 while inhibiting Cdc25 phosphatase function through phosphorylation-directed proteolysis or 14-3-3 binding (see reference 3 for a review). Lack of Cdc25 blocks the G1/S transition through accumulation of inactive phosphorylated Cdk2, and the activation of Wee1 and inhibition of Cdc25C localization or activity by 14-3-3 binding together control inhibitory phosphorylation of Tyr 15 on Cdc2/cyclin B, a critical regulator of the G2/M transition (24).
The pathway responding to DNA DSBs is highly conserved through evolution. However, ATM and ATR have different roles in yeast and mammals. In Saccharomyces cerevisiae, the ATR homolog (scMec1) plays a central role in DSB-induced checkpoint signaling (22). This contrasts with the situation in mammals, where ATM is the major kinase involved in the response to DSBs and the involvement of ATR is delayed, ATM dependent, and cell cycle regulated (14, 29). A similar DNA damage checkpoint response is also present in Xenopus egg extracts, a cell-free system that has been widely used to elucidate the biochemical basis of cell cycle regulation (20). The addition of exogenous DNA damage activates both ATM and ATR in extracts, eventually resulting in the inhibition of Cdc2 and cell cycle progression (12, 33).
In this study, we show that in both Xenopus embryos and egg extracts, the presence of damage-free DNA, either as uncut plasmid DNA or Xenopus sperm chromatin, greatly increases the sensitivity of DSB-induced checkpoint signaling. We demonstrate this enhancement of DSB-induced ATM-dependent signal transduction, including ATM autophosphorylation and ATM-dependent Chk1 and Chk2 phosphorylation, occurs by recruitment of activated ATM from the soluble fraction to the undamaged threshold DNA in a γ-H2AX-dependent manner.
MATERIALS AND METHODS
Embryos and microinjections.
Embryos were produced and staged as previously described (1, 10). Microinjection was performed at the two-cell stage, approximately 60 min postfertilization with preannealed (dA-dT)70 (6). Embryos subjected to microscopy were collected at the indicated times. For immunoblotting, embryos were collected 3 h postfertilization (2 h following injection), frozen on dry ice, and stored at −80°C until further analysis.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were carried out as previously described (1, 10). Antibodies to human pSer-345 hChk1 (Xenopus Chk1 Ser-342), pThr-387 hChk2, H2AX, and pSer-139 H2AX were obtained from Cell Signaling Technology, Beverly, MA. Antibody to pSer-1981 hATM was obtained from Abcam, Cambridge, MA. Antibody to hMre11 was purchased from Calbiochem, San Diego, CA. Antibodies to Xenopus Chk1, ATM, and Rad1 were kindly provided by Jill Sible (Virginia Polytechnic Institute and State University, Blacksburg, VA), Jean Gautier (Columbia University, New York), and Karlene Cimprich (Stanford University, CA), respectively.
Xenopus egg extract.
Cytostatic factor-mediated metaphase-arrested extracts were freshly prepared as previously described (1). Extracts were stably released into interphase by supplementation with CaCl2 to 0.4 mM and 100 μg/μl cycloheximide and incubated for 30 min at room temperature. Under these conditions, cyclins A and B are absent and the only Cdk2 complex present is cyclin E/Cdk2.
In vitro kinase assay.
Chk1 was immunoprecipitated from Xenopus egg extracts with antibody against XChk1 and protein A Dynabeads (Dynal Biotech). The Chk1 kinase assay was performed with the immunoprecipitate using 1 μg of a fragment of human glutathione S-transferase (GST)-Cdc25C containing the phosphorylation site for Chk1. Each reaction mixture (20 μl) also contained 50 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM dithiothreitol (DTT), 50 μM cold ATP, and 3 μCi [γ-32P]ATP. The reaction was stopped by addition of sample buffer, and radiolabeled phosphorylated GST-Cdc25C was analyzed by SDS-PAGE and autoradiography.
For the H1 kinase assay, 1 μl of interphase egg extract was diluted in kinase buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM DTT, 50 μM cold ATP, and 3 μCi [γ-32P]ATP) to a final volume of 20 μl. One μg of H1 was added as substrate, and the reaction was performed at 30°C for 20 min before it was stopped by addition of sample buffer. To inhibit Cdk2, Δ34 p27Xic1 was preadded to extracts at 1 μM (32).
Chromatin fractionation.
To reisolate sperm chromatin from egg extracts, extracts were diluted 10-fold with chromatin dilution buffer (50 mM HEPES, pH 7.5, 50 mM KCl, 5 mM MgCl2, 2 mM DTT, 0.5 mM spermine 3 HCl, 0.15 mM spermine 4 HCl, 0.1% Triton X-100). The diluted extract was then layered onto 3 ml of chromatin dilution buffer containing 30% sucrose and centrifuged at 6,000 × g for 15 min. The pellet was resuspended as the chromatin-enriched fraction (7).
Isolation of biotinylated DNA and depletion of ATM from egg extracts.
To isolate biotinylated DNA oligonucleotides, streptavidin-coated magnetic beads (Dynal Biotechnology) were added to Xenopus egg extracts prepared as described above. Beads were prewashed three times in 2× binding/washing buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 2 M NaCl) and then incubated with extracts for 30 min to allow interaction with biotinylated DNA. Beads were then isolated from the extract with a magnet and washed three times with binding/washing buffer. Alternatively, biotinylated oligonucleotides were prebound to streptavidin-coated beads in binding/washing buffer by incubation at room temperature for 30 min. Beads were then washed three times in binding/washing buffer before addition to extracts, with subsequent reisolation with a magnet. For ATM immunodepletion, protein A Dynabeads (Dynal Biotech) were prewashed three times in washing buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM DTT, and 0.5% Tween 20) with 1% bovine serum albumin and then incubated for 1 h at 4°C with antibody against ATM. Beads conjugated with ATM antibody were washed three times and then added to extracts. After incubation for 20 min, the beads were removed with a magnet and the remaining extract was used as immunodepleted extract. The efficiency of ATM depletion was assessed by SDS-PAGE and blotting, using the same ATM antibody.
Slot blotting of DNA onto nitrocellulose membrane using a manifold.
Briefly, samples (egg extracts, chromatin fraction, or bead elutions) were added with 20× SSC (3.0 M NaCl-0.3 M trisodium citrate, pH 7.0) to give a final concentration of 6× SSC. DNA was denatured at 100°C for 10 min. Samples were then transferred onto a nitrocellulose membrane using a Minifold II system (Schleicher & Schuell, Keene, NH). The membrane was then placed on Whatman paper soaked in denaturation buffer (1.5 M NaCl, 0.5 M NaOH) for 10 min and then neutralization buffer (1 M NaCl, 0.5 M Tris-Cl, pH 7.0) for 5 min and dried between two sheets of Whatman paper under vacuum for 1 h at 80°C. Blotting was performed using horseradish peroxidase (HRP)-streptavidin (Vector Labs, Burlingame, CA).
RESULTS
The presence of undamaged threshold DNA enhances DNA damage checkpoint signaling in Xenopus embryos or egg extracts.
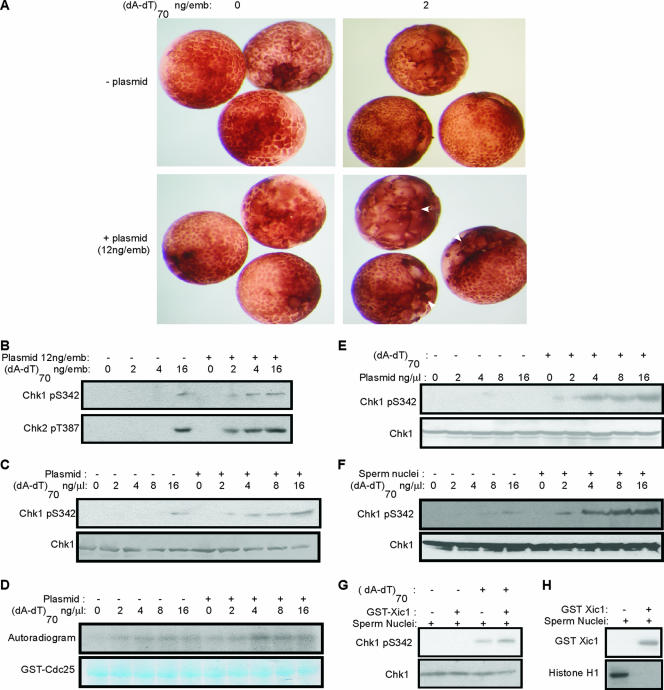
Previously, Conn et al. (6) showed that microinjection of Xenopus embryos with DSB-containing DNA slowed early cleavage cycles through the dose-dependent activation of a caffeine-sensitive checkpoint. A combination of both linearized and uncut plasmids elicited a much stronger checkpoint response than linearized plasmid alone, suggesting an unexpected role for undamaged DNA in checkpoint signaling (6). To systematically analyze the positive effect of undamaged threshold DNA on DNA damage checkpoint activation, embryos were microinjected at the two-cell stage with a DNA double-stranded oligonucleotide, (dA-dT)70, which mimics DNA with DSBs (6, 11, 15), with or without uncut plasmid DNA. In Fig. 1A, one blastomere of each two-cell stage embryo was microinjected and compared over time to the other uninjected blastomere. Cell cycle arrest or delay can be observed morphologically by altered embryonic cleavage divisions, resulting in larger cells compared to controls. A significant delay of cleavage was observed in embryos injected with 12 ng uncut plasmid DNA and 2 ng (dA-dT)70, neither of which alone was able to slow the cell cycle (Fig. 1A). To examine whether DNA damage generates a higher-intensity signal in the presence of a threshold amount of uncut plasmid DNA, we microinjected both blastomeres of two-cell-stage embryos with the indicated amount of (dA-dT)70 and uncut plasmid DNA (Fig. 1B). Injection of large amounts of damaged DNA [16 ng (dA-dT)70/embryo] elicits efficient checkpoint signaling in embryos, as indicated by phosphorylation of Chk1 and Chk2 at sites targeted by ATM/ATR. Interestingly, with 12 ng of uncut plasmid/embryo, the phosphorylation of Chk1 and Chk2 could be initially detected with treatment with as little as 2 ng (dA-dT)70/embryo. Therefore, the presence of the undamaged threshold DNA enhances DNA damage checkpoint signaling by at least four to eight times.
FIG. 1.
The presence of threshold DNA increases the DNA damage checkpoint response in pre-MBT Xenopus embryos and egg extracts. (A) One blastomere of a two-cell Xenopus embryo was microinjected with double-stranded oligonucleotides (dA-dT)70 and supercoiled pGEX plasmid DNA, as indicated, and visualized by microscopy 3.5 h after injection. White arrows indicate the injected blastomeres that displayed delayed cleavage divisions due to cell cycle arrest. (B) Both blastomeres of two-cell embryos were microinjected with the indicated concentrations of (dA-dT)70 and plasmid DNA. Embryos were then collected 2 h postinjection and lysed, and phospho-Chk1 and phospho-Chk2 were detected by immunoblotting as described in Materials and Methods. (C) Xenopus interphase egg extracts were supplemented with plasmid DNA and (dA-dT)70 as indicated and analyzed for Chk1 and phospho-Chk1 by immunoblotting. (D) Xenopus interphase egg extracts were treated with plasmid DNA and (dA-dT)70 as indicated. Immunoprecipitation was then performed using Chk1 antibody, and Chk1 kinase activity in each immunoprecipitate was measured using a fragment of GST-CDC25C as substrate, as described in Materials and Methods. An autoradiogram is shown in the upper panel, and equal loading was confirmed by Coomassie staining of the gel in the bottom panel. (E) Xenopus interphase egg extracts were supplemented with plasmid DNA as indicated, with or without 4 ng/μl of (dA-dT)70, and analyzed for Chk1 and phospho-Chk1 by immunoblotting. (F) Xenopus interphase egg extracts were treated with sperm nuclei at 1,000/μl and (dA-dT)70 as indicated and analyzed by immunoblotting for Chk1 and phospho-Chk1. (G) Xenopus interphase egg extracts were treated with sperm nuclei at 1,000/μl, with or without Δ34 GST-Xic1 and 4 ng/μl of (dA-dT)70. Chk1 and phospho-Chk1 were measured by immunoblotting as described in Materials and Methods. (H) Xenopus interphase egg extracts were treated with sperm nuclei and Δ34 GST-Xic1 as indicated. The addition of Δ34 GST-Xic1 was confirmed by immunoblotting for GST. The histone H1 kinase assay reflecting cyclin E/Cdk2 activity was performed as described in Materials and Methods, and an autoradiogram is shown.
Xenopus interphase egg extracts have been widely adopted as an in vitro system to study the DNA damage response. In these extracts, the addition of damaged DNA elicits an ATM/ATR-dependent DNA damage checkpoint response to activate Chk1, inhibit Cdk activities, and delay cell cycle progression (15). We therefore asked if threshold DNA in Xenopus egg extracts enhances DNA damage checkpoint signaling in a manner similar to that observed in early embryos. In extracts, a minimum of 16 ng (dA-dT)70/μl (roughly comparable to 16 ng in one embryo before the MBT) was required to induce detectable Chk1 phosphorylation (Fig. 1C). In contrast, 2 ng (dA-dT)70/μl was sufficient to produce Chk1 phosphorylation in extracts supplemented with a threshold amount of circular plasmid DNA (Fig. 1C). Consistent with the level of Chk1 phosphorylation, DNA damage increased Chk1 kinase activity towards Cdc25C more efficiently in the presence of plasmid (Fig. 1D). The supercoiled plasmid DNA is damage free and by itself at 16 ng/μl did not activate the checkpoint. In contrast, as little as 4 ng/μl of this supercoiled plasmid DNA was sufficient to promote the DNA damage checkpoint induced by (dA-dT)70 (Fig. 1E). The effect of threshold DNA on checkpoint signaling was evident with several different DNA plasmids without apparent sequence specificity (data not shown). Importantly, Xenopus genomic chromatin from demembranated sperm nuclei was also capable of serving as threshold DNA and promoting DNA damage-induced Chk1 phosphorylation (Fig. 1F).
Both sperm chromatin and plasmid DNA are capable of replication in interphase egg extracts, although several hours are required for detectable replication of plasmid DNA (9, 21). As we discussed before (6), however, the ability of either type of DNA to enhance DNA damage checkpoint activation is unlikely to be replication dependent. Here we further address this point by inhibiting DNA replication with the CDK2 inhibitor p27Xic1 (32). Interestingly, in replication-incompetent extracts, DNA damage checkpoint signaling promoted by threshold DNA was not impaired (Fig. 1G and H).
ATM recruitment and ATM-dependent phosphorylation occur on undamaged threshold DNA.
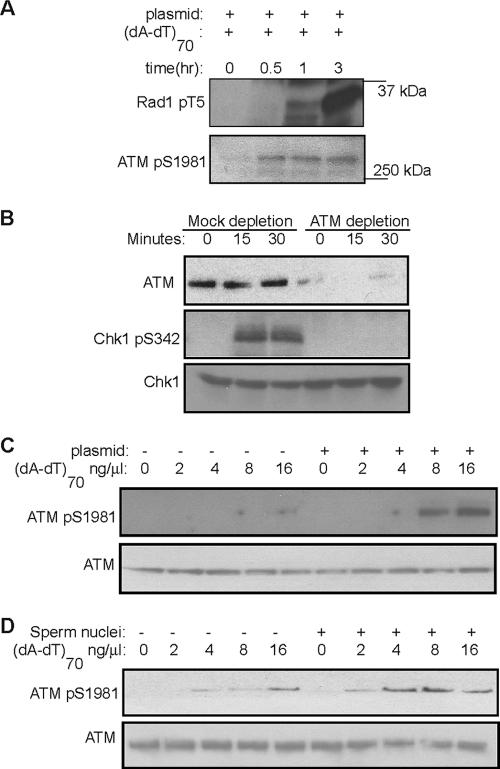
The phosphatidyl inositol (PI) 3-kinase-related kinases ATM and ATR, the upstream kinases that phosphorylate and activate Chk1/Chk2 (28), are both activated in Xenopus egg extracts by DNA DSBs (Fig. 2A), as judged by ATM autophosphorylation at Ser 1981 and ATR-dependent phosphorylation at Thr 5 of Rad1, a specific indicator of ATR kinase activity (19), although ATM autophosphorylation occurs much faster than Rad1 phosphorylation. ATR is the primary kinase that phosphorylates Chk1 in response to hydroxyurea and UV, whereas DSB-induced Chk1 and Chk2 phosphorylations are both dependent on ATM (23). Consistently, DSBs failed to induce Chk1 phosphorylation in extracts from which ATM had been depleted (Fig. 2B) or in which ATM was inhibited by caffeine (Fig. 3E). Since undamaged threshold DNA promotes DSB-induced Chk1 and Chk2 phosphorylation, it may also enhance the activation of ATM, the upstream activator of Chk1 and Chk2. Indeed, ATM activity assayed by its autophosphorylation at Ser 1981 is clearly increased in the presence of threshold DNA, added as either plasmid (Fig. 2C) or sperm nuclei (Fig. 2D).
FIG. 2.
Threshold DNA promotes ATM-dependent checkpoint responses. (A) Xenopus interphase egg extracts treated with 12 ng of plasmid DNA/μl and 8 ng of (dA-dT)70/μl were incubated at room temperature for the indicated times. ATR-dependent phosphorylation at Thr 5 of Rad1 and ATM autophosphorylation were assayed by immunoblotting with phospho-specific antibodies as described in Materials and Methods. (B) Xenopus interphase egg extracts were mock treated or immunodepleted for ATM, then treated with 12 ng of plasmid DNA/μl and 8 ng of (dA-dT)70/μl, and incubated at room temperature for the indicated times. ATM, Chk1, and phospho-Chk1 were analyzed by immunoblotting as described in Materials and Methods. (C) Xenopus interphase egg extracts were treated with 12 ng of plasmid DNA/μl and (dA-dT)70/μl at the indicated concentrations. After 30 min, phosphorylation of ATM at Ser 1981 was measured by immunoblotting as described in Materials and Methods. (D) Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70 as indicated. After 30 min, extracts were then measured for ATM phosphorylation at Ser 1981 as for panel C.
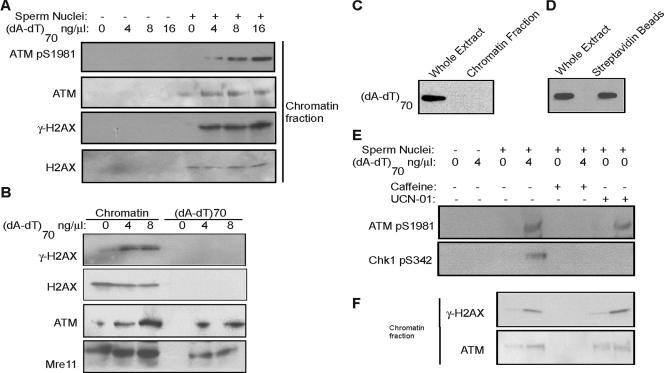
FIG. 3.
DNA damage induces H2AX phosphorylation (γ-H2AX) and ATM association on undamaged threshold DNA. (A) Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70 as indicated. The chromatin fraction was then isolated and analyzed by immunoblotting for phospho-ATM, ATM, γ-H2AX, and H2AX. (B) Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70 as indicated and then both (dA-dT)70 and chromatin fractions were isolated as described in Materials and Methods. γ-H2AX, H2AX, ATM, and Mre11 were measured by immunoblotting with specific antibodies. (C) As for the experiment in panel B, Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70, and then total chromatin was isolated by centrifugation as described in Materials and Methods. Using HRP-streptavidin blotting, the presence of (dA-dT)70 was assessed in the whole egg extract and the slot-blotted chromatin fraction. (D) Xenopus interphase egg extracts were treated with sperm nuclei and biotinylated (dA-dT)70. Oligonucleotides were then recovered with streptavidin beads as described in Materials and Methods. The presence of (dA-dT)70 in the whole egg extract and on the isolated beads was measured by HRP-streptavidin blotting. (E) Xenopus interphase egg extracts were treated with sperm nuclei, (dA-dT)70, caffeine (10 mM), and UCN-01 (0.1 μM) as indicated. Immunoblotting was then performed for phosphorylated ATM and phospho-Chk1. (F) Xenopus interphase egg extracts were treated with sperm nuclei, (dA-dT)70, caffeine, and UCN-01 as for panel E and then chromatin fractions were isolated and subjected to immunoblotting for γ-H2AX and ATM.
To elucidate how undamaged threshold DNA promotes an ATM-dependent checkpoint, we reisolated threshold sperm chromatin from extracts and analyzed associated proteins. A small amount of ATM is associated with chromatin even in the absence of damaged DNA, whereas this association is greatly increased after DNA damage (Fig. 3A). Consistent with the chromatin association of ATM, more ATM autophosphorylation and ATM-dependent H2AX phosphorylation occur on chromatin in the presence of DNA damage (Fig. 3A). ATM recruitment and ATM-dependent H2AX phosphorylation are typical DNA damage response events that have been shown to occur at chromatin domains adjacent to DSBs (25), and so it is striking that these events also took place on undamaged, physically separate threshold DNA. The chromatin fractionation is specific, because without sperm nuclei, no positive signal is evident (Fig. 3A). Furthermore, it is also unlikely that these events reflect the presence of (dA-dT)70 incorporated into and recovered together with chromatin DNA, because γ-H2AX is absent in isolated (dA-dT)70 (Fig. 3B), and only DNA fragments of 1 kb or longer can bind H2AX (8). Therefore, threshold DNA directly recruits ATM and promotes ATM-dependent phosphorylation in response to physically separate damaged DNA. However, (dA-dT)70 also associates with ATM and Mre11 in an H2AX-independent manner (Fig. 3B), perhaps related to the recognition of DSBs and initiation of signal transduction. The separation of (dA-dT)70 from chromatin is complete, as shown in Fig. 3C by slot blotting of the total chromatin fraction, and the streptavidin beads recover most of the biotin-labeled oligonucleotides from extracts (Fig. 3D). Previously, Conn et al. (6) demonstrated that the DNA damage checkpoint in early Xenopus embryos is sensitive to caffeine, an inhibitor of ATM/ATR kinases. Similarly, caffeine treatment in Xenopus egg extracts abrogates DNA damage-induced ATM and Chk1 phosphorylation (Fig. 3E). Interestingly, only caffeine, not UCN-01, a potent Chk1 inhibitor, disrupts ATM loading and ATM-dependent H2AX phosphorylation on threshold DNA (Fig. 3F). Thus, DNA damage-induced ATM loading and phosphorylation on threshold chromatin require ATM kinase activity.
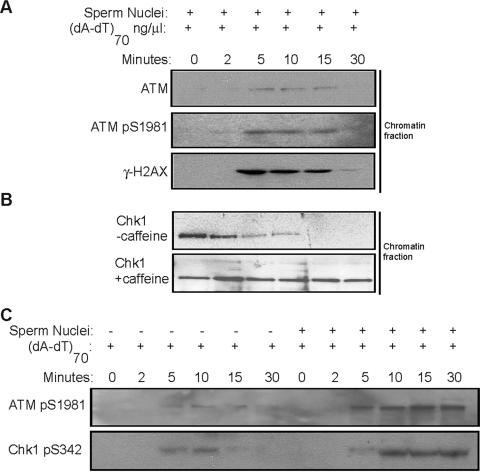
A kinetic analysis showed that DNA damage-induced ATM loading on sperm chromatin threshold DNA is a dynamic process. Chromatin association of ATM increases rapidly, within 5 min after the introduction of damaged DNA, and it then diminishes gradually. ATM autophosphorylation and H2AX phosphorylation on threshold DNA exhibit similar kinetics (Fig. 4A). In the absence of DNA damage, it has been reported that a fraction of Chk1 and Chk2 are localized on chromatin during all phases of the cell cycle (17, 30). In response to DNA damage, the chromatin-bound Chk1 and Chk2 are phosphorylated and dissociate from chromatin, perhaps as part of a mechanism to transmit the DNA damage signal (17, 30). We observed that on sperm chromatin threshold DNA, Chk1 dissociated from chromatin within 5 min after addition of damaged DNA, consistent with the kinetics of ATM loading and phosphorylation (Fig. 4B). Inhibition of ATM kinase activity with caffeine blocks both ATM loading on threshold DNA and Chk1 dissociation from chromatin (Fig. 4B). In accordance with the kinetics of these dynamic events on chromatin, including ATM loading, H2AX phosphorylation, and Chk1 dissociation, the phosphorylation levels of ATM and Chk1 continue to increase even after 5 min in extracts containing threshold DNA (Fig. 4C).
FIG. 4.
DNA damage induces dynamic events on threshold DNA. (A) Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70, followed by incubation at room temperature as indicated. Chromatin fractions were then isolated and analyzed by immunoblotting for ATM, phospho-ATM, and γ-H2AX. (B) Xenopus interphase egg extracts of panel A were treated with or without caffeine. Chromatin factions were then isolated, and the level of chromatin-bound Chk1 was analyzed by immunoblotting. (C) Xenopus interphase egg extracts were treated with 16 ng of (dA-dT)70/μl and sperm nuclei. After incubation at room temperature for the indicated times, ATM and Chk1 phosphorylation levels were then assayed by immunoblotting.
γ-H2AX is required for DNA damage checkpoint activation promoted by undamaged threshold DNA.
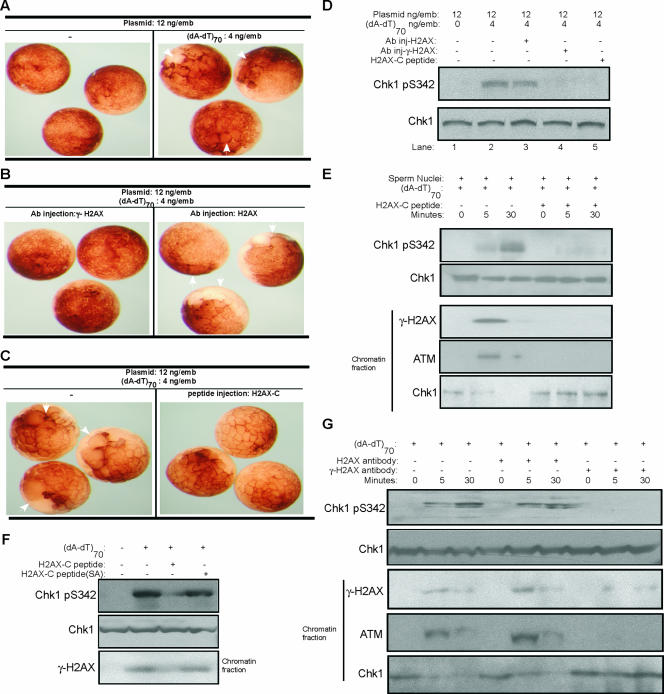
H2AX is a major variant form of the core histone H2A that accounts for approximately 2 to 25% of the whole H2A pool (27). Distinct from other H2A variants, human H2AX contains a C-terminal tail with a serine residue (Ser 139 in human) that is phosphorylated quickly by ATM upon exposure to DNA damage (4). Ser 139 and its surrounding sequence are highly conserved in Xenopus H2AX. Phosphorylated H2AX (γ-H2AX) is found particularly in the chromatin regions surrounding DNA DSBs, regions also defined as nuclear foci. γ-H2AX recruits and retains DNA damage factors, thus playing a crucial role in both checkpoint activation and DNA repair (5). It is striking that the addition of damaged DNA induces accumulation of γ-H2AX on threshold DNA, which itself doesn't contain any DNA damage lesions. We asked whether γ-H2AX on threshold DNA has a required role in checkpoint activation. In each Xenopus embryo, 4 ng (dA-dT)70 and 12 ng plasmid DNA microinjected together activated the checkpoint to slow cell division (Fig. 5A). This checkpoint effect is clearly attenuated by the simultaneous injection of γ-H2AX antibody, which recognizes and masks the epitope created by phosphorylation of H2AX at Ser 139 (Fig. 5B). As a control, the injection of H2AX antibody not directed against the Ser 139 phosphorylation site did not affect DNA damage checkpoint activation (Fig. 5B). In addition, the injection of a peptide corresponding to the COOH terminus of H2AX also abrogated the DNA damage-induced checkpoint activation in Xenopus embryos (Fig. 5C), most likely by competing for phosphorylation of full-length H2AX. Consistent with the results on cell cycle arrest, Chk1 phosphorylation induced by DNA damage is reduced by either antibody against human γ-H2AX or by a peptide derived from the Xenopus H2AX COOH terminus (Fig. 5D). Further studies in extracts confirmed that addition of the H2AX COOH terminus peptide disrupted H2AX phosphorylation, ATM loading, and Chk1 release on threshold DNA (Fig. 5E). Importantly, a mutant H2AX COOH terminus peptide lacking the conserved Ser 139 phosphorylation site did not inhibit checkpoint activation (Fig. 5F). Moreover, the addition of a neutralizing antibody against γ-H2AX, but not the control antibody against H2AX, displayed effects similar to those induced by the H2AX COOH terminus peptide, namely, inhibition of DNA damage-induced Chk1 phosphorylation and events on threshold chromatin (Fig. 5G).
FIG. 5.
Phosphorylation of H2AX is required for threshold DNA to promote DNA damage-induced checkpoint activation. (A) One blastomere of each two-cell Xenopus embryo was microinjected with 12 ng of plasmid DNA/μl with or without (dA-dT)70 and then visualized by microscopy. White arrows indicate the injected blastomeres that display delayed cleavage divisions due to cell cycle arrest, compared to the control uninjected sites. (B) One blastomere of each two-cell Xenopus embryo was microinjected with 12 ng of plasmid DNA/μl, 4 ng of (dA-dT)70/μl, and antibody against γ-H2AX or H2AX. Embryos were then visualized by microscopy. White arrows indicate the injected blastomeres that displayed delayed cleavage divisions due to cell cycle arrest. (C) One blastomere of each two-cell Xenopus embryo was microinjected with 12 ng of plasmid DNA/μl and 4 ng of (dA-dT)70/μl, with or without peptide encoding the Xenopus COOH terminus of H2AX (CKKSSQQSQEY). Embryos were then visualized by microscopy. White arrows indicate the injected blastomeres that displayed delayed cleavage divisions due to cell cycle arrest. (D) Both blastomeres of two-cell Xenopus embryos were microinjected with plasmid DNA, (dA-dT)70, and antibody against human γ-H2AX, human H2AX, or a Xenopus H2AX COOH-terminal peptide, as indicated. Embryos were then lysed and subjected to immunoblotting for phospho-Chk1 and Chk1. (E) Xenopus egg extracts supplemented with buffer or a Xenopus H2AX COOH-terminal peptide were treated with (dA-dT)70 and sperm chromatin as indicated. Chk1 and phospho-Chk1 were measured by immunoblotting. Chromatin fractions were also isolated from the extracts and assayed by immunoblotting for γ-H2AX, ATM, and Chk1. (F) Xenopus egg extracts were treated with (dA-dT)70, a Xenopus H2AX COOH-terminal peptide, or a mutant peptide (CKKSSQQAQEY), in which the conserved Ser 139 phosphorylation site was changed to Ala. Chk1 and phospho-Chk1 were measured by immunoblotting. Chromatin fractions were also isolated from extracts and assayed by immunoblotting for γ-H2AX. (G) Xenopus egg extracts with (dA-dT)70 (4 ng/μl) and sperm chromatin (1,000/μl) were added with buffer, H2AX antibody, or γ-H2AX antibody. Samples were prepared at 0, 5, or 30 min after (dA-dT)70 addition, and Chk1 and phospho-Chk1 were measured by immunoblotting. Chromatin fractions were also isolated from extracts and assayed by immunoblotting for ATM, γ-H2AX, and Chk1.
DNA damage produces an ATM-dependent soluble signal that can be transmitted by undamaged threshold DNA.
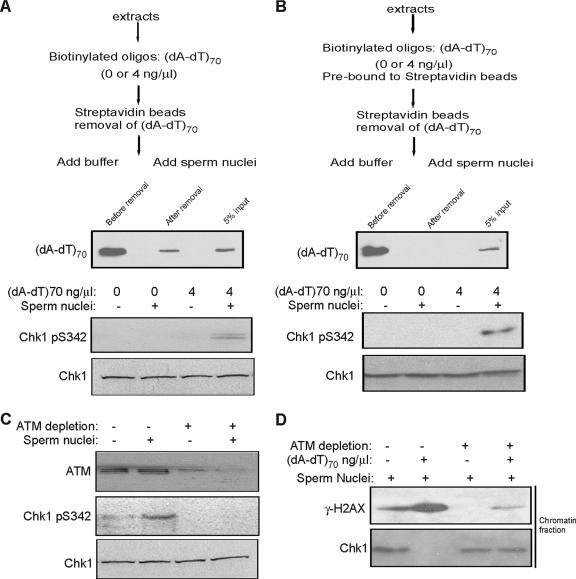
These results show that damaged DNA in Xenopus egg extracts induces dynamic ATM loading and phosphorylation on undamaged, physically separate threshold chromatin, but it is not clear how these dynamic changes on threshold chromatin are achieved. Since (dA-dT)70 itself associates with ATM and Mre11 (Fig. 3B), one possibility is that damaged DNA assembles a signaling complex, which is released into the soluble fraction and transmitted to the threshold DNA. To test this hypothesis, Xenopus egg extracts were incubated with a biotinylated form of (dA-dT)70, which was then removed with streptavidin beads (Fig. 6A). Strikingly, even after removal of the damaged DNA, the extract still elicited Chk1 phosphorylation after the addition of sperm chromatin (Fig. 6A). The oligonucleotide remaining after depletion is less than 5% of the initial input, far below the threshold needed to activate the checkpoint and affect the cell cycle (Fig. 1F). An alternative approach in which oligonucleotides were preconjugated with streptavidin beads before incubation with and removal from extracts achieved a quantitatively complete depletion and showed similar results (Fig. 6B). We propose that DNA damage produces a soluble signal which is insufficient to trigger Chk1 phosphorylation but which can be transmitted and enhanced by threshold DNA.
FIG. 6.
DNA damage produces a soluble signal that can be transmitted by threshold DNA. (A) Extracts previously supplemented with biotinylated oligonucleotides (dA-dT)70 were then incubated with streptavidin beads to bind the oligonucleotides. Oligonucleotides were then removed by a magnet. The level of (dA-dT)70 in the extract is shown before and after the removal by HRP-streptavidin blotting. The extracts were then supplemented with sperm nuclei as indicated, and immunoblotting was performed using antibodies against Chk1 and phospho-Chk1. (B) As an alternative approach to the procedure of panel A, biotinylated oligonucleotides were first bound to streptavidin beads and then added to extracts, the beads were removed by a magnet, and sperm nuclei were added. The presence of (dA-dT)70 was measured before and after removal by HRP-streptavidin blotting, and the levels of Chk1 and phospho-Chk1 were determined by immunoblotting. (C) Extracts were treated as for panel A, with or without an additional step to deplete ATM. Immunoblotting was performed using antibodies against ATM, Chk1, and phospho-Chk1. (D) Extracts were mock treated or immunodepleted for ATM as for panel C and then supplemented with sperm nuclei and (dA-dT)70 as indicated. Chromatin fractions were reisolated from extracts and immunoblotted for γ-H2AX and Chk1.
The nature of the soluble signal transmitted to threshold DNA is not known. It is not damaged DNA itself, because all damaged DNA can be removed without blocking the signal. One possibility is that ATM kinase itself serves as a signal, for the following reasons: (i) ATM kinase activity is required to trigger a DNA damage response on threshold DNA (Fig. 3E); (ii) in response to DNA damage, the amount of ATM localized on threshold DNA increases, especially the active, autophosphorylated form (Fig. 3A); (iii) activated ATM is not exclusively localized on damaged chromatin (2), and a soluble fraction of Xenopus egg extracts pretreated with damaged DNA has been reported to phosphorylate a peptide corresponding to the COOH-terminal sequence of H2AX (8). Therefore, to test this possibility, ATM was completely immunodepleted from Xenopus egg extracts pretreated with damaged DNA before the addition of sperm nuclei. Unlike control extracts, ATM-depleted extracts were unable to elicit Chk1 phosphorylation after addition of sperm chromatin (Fig. 6C). Moreover, no H2AX phosphorylation or Chk1 release was evident on threshold chromatin in ATM-depleted extracts (Fig. 6D). These effects of ATM depletion are similar to those resulting from ATM kinase inhibition by caffeine. We thus propose a model in which DNA damage initiates and releases activated ATM, which is then translocated to the threshold DNA, leading to checkpoint activation (Fig. 7).
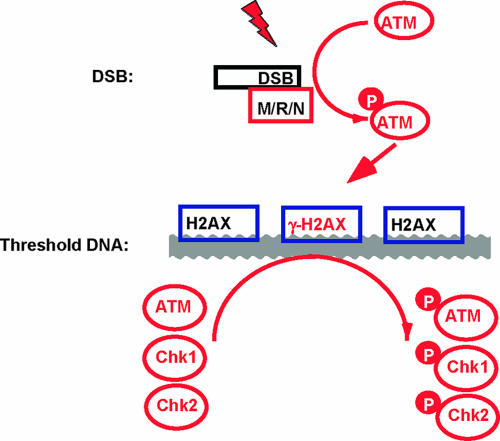
FIG. 7.
Model for the role of threshold DNA in DNA damage checkpoint signaling. DNA damage-induced ATM activation is initiated at a DSB, which triggers a set of dynamic events on threshold DNA within 5 min, including increased ATM loading and H2AX phosphorylation. We propose that threshold DNA functions as a platform to promote ATM, Chk1, and Chk2 phosphorylation and trigger Chk1 and Chk2 release and thus enhance DNA damage checkpoint regulation at a level sufficient for cell cycle arrest.
DISCUSSION
It has been shown that irradiation of Xenopus embryos prior to the MBT does not activate DNA damage checkpoints, whereas the same treatment in post-MBT embryos does activate the checkpoint and delays cell cycle progression (1, 10). However, in both pre-MBT embryos and egg extracts, the addition of adequate DSB-containing DNA oligonucleotides efficiently induces checkpoint activation, indicating that pre-MBT embryos are capable of DNA damage checkpoint activation (6, 11, 15). One of the reasons possibly contributing to the difference in checkpoint behavior of Xenopus embryos before and after the MBT is the DNA-to-cytoplasm ratio (6). During early embryonic cycles, the DNA content is low and doubles after each round of replication and cleavage. Importantly, supplementation of uncut supercoiled plasmid DNA or undamaged sperm chromatin in pre-MBT Xenopus embryos or egg extracts greatly enhances the checkpoint response to DNA damage, indicating a novel role of the undamaged threshold DNA in checkpoint signaling (6). One may argue that undamaged threshold DNA, provided by either plasmid DNA or sperm chromatin, may undergo replication and, thus, induce activation of the replication checkpoint. As we have discussed before (6), while sperm chromatin can be replicated in egg extracts, plasmid DNA is not replicated in extracts under our conditions (9, 21). This makes it unlikely that replication is responsible for the enhanced signaling in the presence of threshold DNA. Here we show new evidence that inhibiting replication with the CDK2 inhibitor p27Xic1 does not reduce checkpoint signaling. This notion is also consistent with the molecular analysis: ATR-dependent Rad1 phosphorylation at Thr 5, a marker of ATR activation (19), was not evident in the time frame of these experiments (Fig. 2A). Moreover, our data show that Chk1 activation promoted by threshold DNA requires ATM, whereas Chk1 phosphorylation in response to replication stress or UV is ATM independent (23).
Our results indicate that in response to DNA damage, threshold DNA recruits activated ATM kinase and facilitates H2AX, Chk1, and Chk2 phosphorylation, and thus threshold DNA may serve as a platform to transmit and spread the DNA damage signal (Fig. 7). Consistent with this hypothesis, our kinetic analysis also suggests that DNA damage-induced dynamic changes in threshold chromatin may sustain and amplify ATM and Chk1 phosphorylation. More importantly, reagents disrupting these DNA damage-induced changes on undamaged DNA are also found to impair checkpoint signaling. These results identify a novel mechanism of checkpoint regulation by the DNA-to-cytoplasm ratio: in late embryonic and somatic cell cycles, the high content of DNA transmits and enhances the DNA damage checkpoint signal to a level sufficient for cell cycle arrest, whereas the low DNA concentration in early embryonic cell cycles is insufficient for checkpoint activation at a level that affects the cell cycle, thus ensuring rapid and synchronous cell division. The initial DNA-to-cytoplasm ratio achieved at the MBT provides an example in development how maternally programmed rapid cell divisions can lead to abrupt appearance of cell cycle checkpoints at a specific time.
A potential caveat in this study is that the isolated chromatin fraction might contain a substantial amount of the double-stranded oligonucleotide through contamination or recombination. In this case, the DNA damage-induced events observed on threshold DNA could be in fact due to the incorporation of the damaged DNA into chromatin. However, several lines of evidence rule out this possibility: (i) the double-stranded oligonucleotide we used does not bind H2AX and therefore cannot contribute to the increased level of γ-H2AX on threshold DNA; (ii) we showed directly that no biotinylated oligonucleotide is found in slot blotted chromatin whereas streptavidin beads recover most (if not all) of the input oligonucleotide, confirming a complete separation of the oligonucleotide from chromatin; (iii) in Fig. 6B, we completely depleted the oligonucleotide prebound to streptavidin beads before chromatin was added and still observed checkpoint responses on threshold DNA. These results indicate that the signal was transmitted by a soluble signal, not the oligonucleotide itself.
In eukaryotic cells, DSBs induce H2AX phosphorylation at large, flanking chromatin areas; γ-H2AX then recruits ATM and other DNA damage response factors to form nuclear foci, the putative centers of DNA damage responses (25, 31). Our study presents novel evidence that DSBs also induce dynamic ATM loading and H2AX phosphorylation on physically separate threshold DNA. Therefore, DNA damage induces similar events on chromatin flanking DSBs and undamaged threshold DNA, both of which promote checkpoint signaling. However, there are clear differences between the events on the flanking chromatin and threshold DNA: (i) DNA damage directly induces ATM activation and H2AX phosphorylation of the flanking chromatin, while those events on threshold DNA are transmitted through a soluble signal; (ii) the formation of DNA damage foci at the flanking areas is much more intense than at other remote chromatin areas; and (iii) H2AX phosphorylation and ATM loading last significantly longer at flanking areas than at remote sites (25). Importantly, our work shows that γ-H2AX is required for threshold DNA to promote the DNA damage checkpoint, as injection of phospho-specific γ-H2AX antibody or a peptide corresponding to the COOH-terminal sequence of H2AX disrupted ATM accumulation and Chk1 release on chromatin and strongly interfered with checkpoint activation. We therefore hypothesize that, like its well-established role in recruiting DNA damage foci, γ-H2AX on threshold DNA also facilitates checkpoint activation by concentrating and retaining DNA damage response factors. Our experimental system is unique in that DSBs were present as 70-bp DNA oligonucleotides that do not have areas of flanking chromatin. However, the role of threshold DNA may be also conserved in other systems, as irradiation of mammalian cells also induces γ-H2AX foci in nuclear areas other than DSBs (13).
By transiently exposing egg extracts to DNA DSBs, we showed that DNA damage checkpoint signaling initiated by DNA DSBs may involve a subsequent release of signal into the soluble fraction. In our experiments, low levels of DNA ends, e.g., 2.8 × 1010 or 5.6 × 1010/μl, did not directly induce Chk1 phosphorylation. However, a signal was clearly sent, although unheard, so that after removal of the damaged DNA, the addition of threshold sperm chromatin was able to transmit and amplify signaling and Chk1 phosphorylation. One plausible theory is that ATM may serve as such a soluble signal: ATM activated on DNA with DSBs may be released into the soluble fraction, where it is recruited by threshold DNA to phosphorylate its substrates (Fig. 7). Consistent with this hypothesis, depletion of ATM from the soluble fraction disrupted H2AX phosphorylation and Chk1 release on threshold chromatin. Interestingly, an independent study showed increased kinase activity towards H2AX in soluble factions isolated from Xenopus egg extracts treated with DNA DSBs (1.2 × 1011 or 3.6 × 1011/μl) (8). In general, compared to signaling by DSBs, less is known about how checkpoint responses are reversed when DNA damage is repaired. One might have expected that removal of damaged DNA would be a permissive condition for reversal or decay of the checkpoint response. However, we observed that the soluble signal for checkpoint activation was evident for a prolonged period (30 min) after DSB removal (Fig. 6C).
In mammalian cells, a small pool of Chk2 is associated with undamaged chromatin, and ionizing radiation induces a rapid (<10-min) dissociation of Chk2 from chromatin in an ATM-dependent manner (17). Those authors thus proposed that a PI-3 kinase-related kinase is recruited onto chromatin after DNA damage, where it phosphorylates and releases the small pool of Chk2 that was associated with chromatin prior to DNA damage. Similarly, chromatin association and dissociation also appear to be important for proper Chk1 regulation (30). Interestingly, immobilization of Chk1 and Chk2 to chromatin by H2B fusion attenuated their downstream activation and cell cycle arrest (18, 30). Our study presents evidence that ATM is recruited onto chromatin and thus phosphorylates chromatin-bound substrates, such as H2AX, Chk1, and Chk2, leading to dissociation of Chk1 and Chk2 from chromatin. Using damage-free chromatin, we demonstrated that this process is not limited to the previously proposed flanking areas of DSBs. We hypothesize that this DNA damage-induced dissociation of chromatin-bound Chk1 and Chk2 facilitates the transmission of DNA damage signals to downstream targets and perhaps also the activation of soluble pools of Chk1 and Chk2.
Acknowledgments
We are grateful to Jill Sible (Virginia Polytechnic Institute and State University, Blacksburg, VA), Jean Gautier (Columbia University, New York) and Karlene Cimprich (Stanford University, CA) for reagents. We thank David Bentley for providing access to his DNA slot blot apparatus. We also thank members of this lab for technical assistance and stimulating discussions and Bryn Grimison for proofreading the manuscript.
A.P. is an Associate and J.L.M. an Investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Anderson, J. A., A. L. Lewellyn, and J. L. Maller. 1997. Ionizing radiation induces apoptosis and elevates cyclin A1 Cdk2 activity before but not after the midblastula transition in Xenopus. Mol. Biol. Cell 8:1195-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. [DOI] [PubMed] [Google Scholar]
- 3.Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. [DOI] [PubMed] [Google Scholar]
- 4.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 5.Celeste, A., S. Petersen, P. J. Romanienko, O. Fernandez-Capetillo, H. T. Chen, O. A. Sedelnikova, B. Reina-San-Martin, V. Coppola, E. Meffre, M. J. Difilippantonio, C. Redon, D. R. Pilch, A. Olaru, M. Eckhaus, R. D. Camerini-Otero, L. Tessarollo, F. Livak, K. Manova, W. M. Bonner, M. C. Nussenzweig, and A. Nussenzweig. 2002. Genomic instability in mice lacking histone H2AX. Science 296:922-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conn, C. W., A. L. Lewellyn, and J. L. Maller. 2004. The DNA damage checkpoint in embryonic cell cycles is dependent on the DNA-to-cytoplasmic ratio. Dev. Cell 7:275-281. [DOI] [PubMed] [Google Scholar]
- 7.Costanzo, V., K. Robertson, and J. Gautier. 2004. Xenopus cell-free extracts to study the DNA damage response. Methods Mol. Biol 280:213-227. [DOI] [PubMed] [Google Scholar]
- 8.Dupre, A., L. Boyer-Chatenet, and J. Gautier. 2006. Two-step activation of ATM by DNA and the Mre11-Rad50-Nbs1 complex. Nat. Struct. Mol. Biol. 13:451-457. [DOI] [PubMed] [Google Scholar]
- 9.Endean, D. J., and O. Smithies. 1989. Replication of plasmid DNA in fertilized Xenopus eggs is sensitive to both the topology and size of the injected template. Chromosoma 97:307-314. [DOI] [PubMed] [Google Scholar]
- 10.Finkielstein, C. V., A. L. Lewellyn, and J. L. Maller. 2001. The midblastula transition in Xenopus embryos activates multiple pathways to prevent apoptosis in response to DNA damage. Proc. Natl. Acad. Sci. USA 98:1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, Z. J., and W. G. Dunphy. 2000. Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol. Biol. Cell 11:1535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo, Z. J., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, J. X., M. J. Hendzel, and J. Allalunis-Turner. 2006. Quantitative analysis reveals asynchronous and more than DSB-associated histone H2AX phosphorylation after exposure to ionizing radiation. Radiat. Res. 165:283-292. [DOI] [PubMed] [Google Scholar]
- 14.Jazayeri, A., J. Falck, C. Lukas, J. Bartek, G. C. M. Smith, J. Lukas, and S. P. Jackson. 2006. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8:37-45. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai, A., Z. J. Guo, K. H. Emami, S. X. Wang, and W. G. Dunphy. 1998. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 142:1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai, A., J. Lee, H. Y. Yoo, and W. G. Dunphy. 2006. TopBP1 activates the ATR-ATRIP complex. Cell 124:943-955. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., and D. F. Stern. 2005. DNA damage regulates Chk2 association with chromatin. J. Biol. Chem. 280:37948-37956. [DOI] [PubMed] [Google Scholar]
- 18.Lukas, C., J. Falck, J. Bartkova, J. Bartek, and J. Lukas. 2003. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5:255-260. [DOI] [PubMed] [Google Scholar]
- 19.Lupardus, P. J., and K. A. Cimprich. 2006. Phosphorylation of Xenopus Rad1 and Hus1 defines a readout for ATR activation that is independent of claspin and the Rad9 carboxy terminus. Mol. Biol. Cell 17:1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maller, J. L. 1990. Xenopus oocytes and the biochemistry of cell-division. Biochemistry 29:3157-3166. [DOI] [PubMed] [Google Scholar]
- 21.Marini, N. J., L. D. Etkin, and R. M. Benbow. 1988. Persistence and replication of plasmid DNA microinjected into early embryos of Xenopus laevis. Dev. Biol. 127:421-434. [DOI] [PubMed] [Google Scholar]
- 22.Melo, J., and D. Toczyski. 2002. A unified view of the DNA-damage checkpoint. Curr. Opin. Cell Biol. 14:237-245. [DOI] [PubMed] [Google Scholar]
- 23.Myers, J. S., and D. Cortez. 2006. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J. Biol. Chem. 281:9346-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohi, R., and K. L. Gould. 1999. Regulating the onset of mitosis. Curr. Opin. Cell Biol. 11:267-273. [DOI] [PubMed] [Google Scholar]
- 25.Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert, and W. M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886-895. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini, M., A. Celeste, S. Difilippantonio, R. Guo, W. D. Wang, L. Feigenbaum, and A. Nussenzweig. 2006. Autophosphorylation at serine 1987 is dispensable for murine ATM activation in vivo. Nature 443:222-225. [DOI] [PubMed] [Google Scholar]
- 27.Rogakou, E. P., D. R. Pilch, A. H. Orr, V. S. Ivanova, and W. M. Bonner. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273:5858-5868. [DOI] [PubMed] [Google Scholar]
- 28.Shiloh, Y. 2001. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11:71-77. [DOI] [PubMed] [Google Scholar]
- 29.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3:155-168. [DOI] [PubMed] [Google Scholar]
- 30.Smits, V. A. J., P. M. Reaper, and S. P. Jackson. 2006. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr. Biol. 16:150-159. [DOI] [PubMed] [Google Scholar]
- 31.Stucki, M., and S. P. Jackson. 2006. Gamma H2AX and MDC1: anchoring the DNA damage response machinery to broken chromosomes. DNA Repair 5:534-543. [DOI] [PubMed] [Google Scholar]
- 32.Su, J., R. E. Remple, E. Erikson, and J. L. Maller. 1995. Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor p27Xic1. Proc. Natl. Acad. Sci. USA 92:10187-10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You, Z. S., C. Chahwan, J. Bailis, T. Hunter, and P. Russell. 2005. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol. Cell. Biol. 25:5363-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of replication protein A (RPA)-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]