Abstract
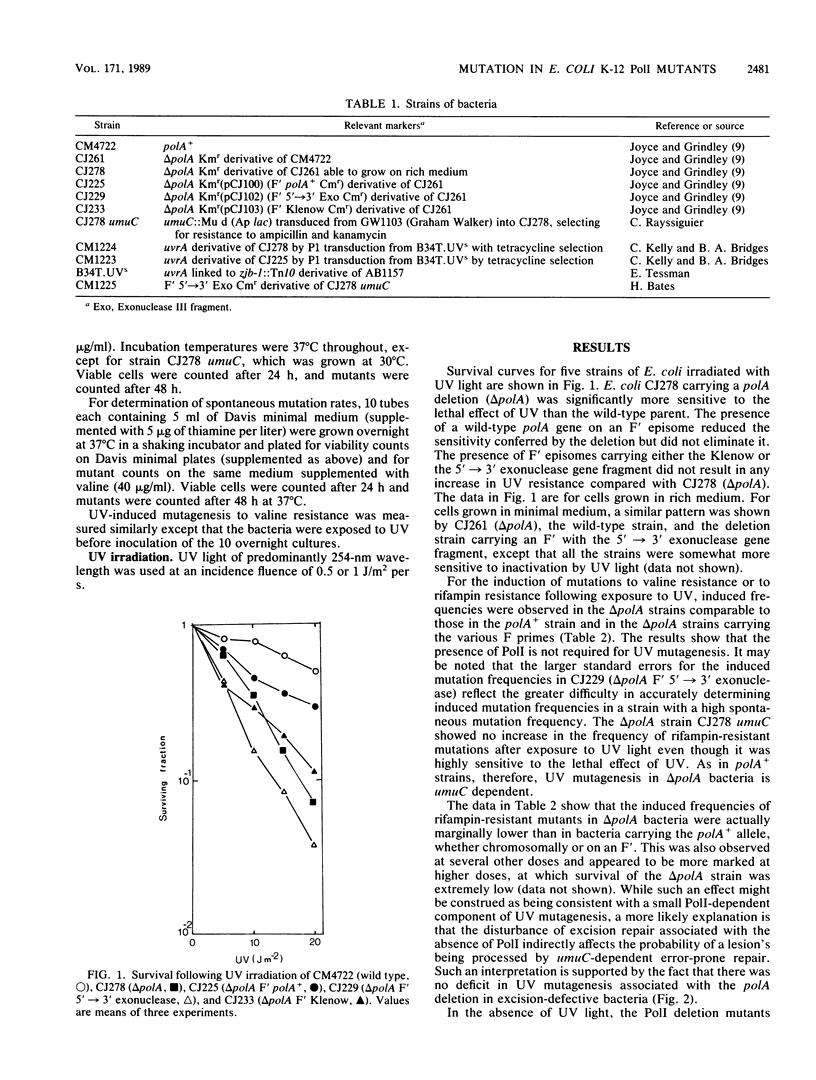
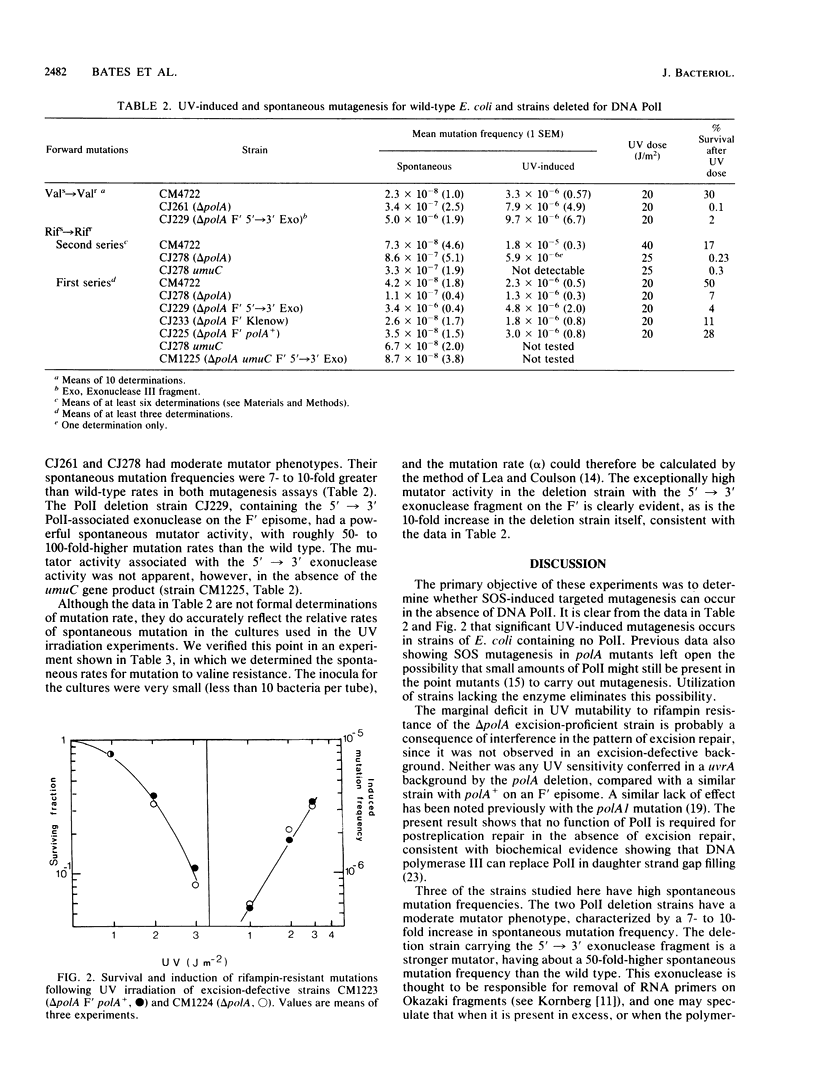
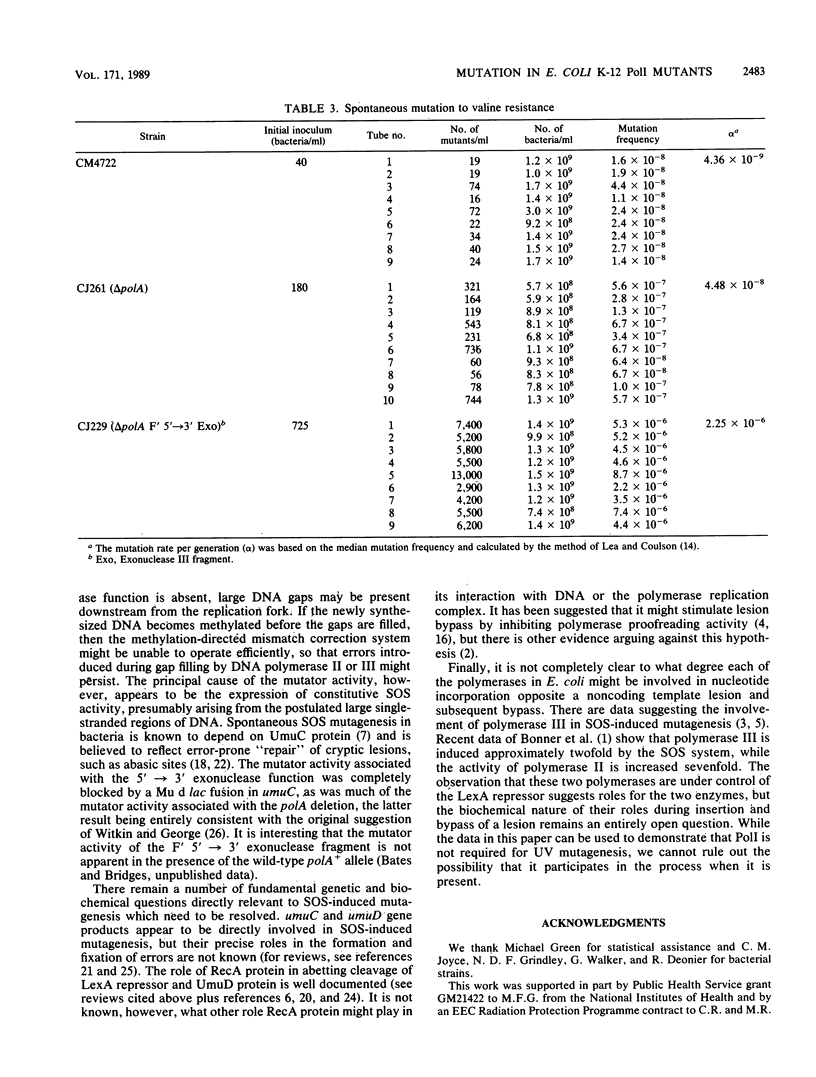
The induction of mutations to valine resistance and to rifampin resistance occurs after UV irradiation in bacteria carrying a deletion through the polA gene (delta polA), showing that DNA polymerase I (PolI) is not an essential enzyme for this process. The PolI deletion strain showed a 7- to 10-fold-higher spontaneous mutation frequency than the wild type. The presence in the deletion strain of the 5'----3' exonuclease fragment on an F' episome caused an additional 10-fold increase in spontaneous mutation frequency, resulting in mutation frequencies on the order of 50- to 100-fold greater than wild type. The mutator effect associated with the 5'----3' exonuclease gene fragment together with much of the effect attributable to the polA deletion was blocked in bacteria carrying a umuC mutation. The mutator activity therefore appears to reflect constitutive SOS induction. Excision-proficient polA deletion strains exhibited increased sensitivity to the lethal effect of UV light which was only partially ameliorated by the presence of polA+ on an F' episome. The UV-induced mutation rate to rifampin resistance was marginally lower in delta polA bacteria than in bacteria carrying the polA+ allele. This effect is unlikely to be caused by the existence of a PolI-dependent mutagenic pathway and is probably an indirect effect caused by an alteration in the pattern of excision repair, since it did not occur in excision-deficient (uvrA) bacteria. An excision-deficient polA deletion strain possessed UV sensitivity similar to that of an isogenic strain carrying polA+ on an F' episome, showing that none of the functions of PolI are needed for postreplication repair in the absence of excision repair. Our data provide no evidence for a pathway of UV mutagenesis dependent on PolI, although it remains an open question whether PolI is able to participate when it is present.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner C. A., Randall S. K., Rayssiguier C., Radman M., Eritja R., Kaplan B. E., McEntee K., Goodman M. F. Purification and characterization of an inducible Escherichia coli DNA polymerase capable of insertion and bypass at abasic lesions in DNA. J Biol Chem. 1988 Dec 15;263(35):18946–18952. [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcorne-Lannoye A., Maenhaut-Michel G., Radman M. Involvement of DNA polymerase III in UV-induced mutagenesis of bacteriophage lambda. Mol Gen Genet. 1985;199(1):64–69. doi: 10.1007/BF00327511. [DOI] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieśla Z. Plasmid pKM101-mediated mutagenesis in Escherichia coli is inducible. Mol Gen Genet. 1982;186(2):298–300. doi: 10.1007/BF00331866. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Kondo S. Spontaneous and radiation-induced deletion mutations in Escherichia coli strains with different DNA repair capacities. Mutat Res. 1972 Sep;16(7):13–25. doi: 10.1016/0027-5107(72)90059-0. [DOI] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey D., Krauss S. W., Linn S. Characterization of DNA polymerase I*, a form of DNA polymerase I found in Escherichia coli expressing SOS functions. J Biol Chem. 1985 Mar 10;260(5):3178–3184. [PubMed] [Google Scholar]
- Lackey D., Krauss S. W., Linn S. Isolation of an altered form of DNA polymerase I from Escherichia coli cells induced for recA/lexA functions. Proc Natl Acad Sci U S A. 1982 Jan;79(2):330–334. doi: 10.1073/pnas.79.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman I. R., Chien J. R. Persistence of deoxyribonucleic acid polymerase I and its 5'--3' exonuclease activity in PolA mutants of Escherichia coli K12. J Biol Chem. 1973 Nov 25;248(22):7717–7723. [PubMed] [Google Scholar]
- Lu C., Scheuermann R. H., Echols H. Capacity of RecA protein to bind preferentially to UV lesions and inhibit the editing subunit (epsilon) of DNA polymerase III: a possible mechanism for SOS-induced targeted mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):619–623. doi: 10.1073/pnas.83.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Low K. B. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell. 1984 Jun;37(2):675–682. doi: 10.1016/0092-8674(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Ossanna N., Thliveris A. T., Ennis D. G., Mount D. W. Derepression of specific genes promotes DNA repair and mutagenesis in Escherichia coli. J Bacteriol. 1988 Jan;170(1):1–4. doi: 10.1128/jb.170.1.1-4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Glickman B. W., Loeb L. A. Mutagenesis resulting from depurination is an SOS process. Mutat Res. 1982 Nov;106(1):1–9. doi: 10.1016/0027-5107(82)90186-5. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Requirement for either DNA polymerase I or DNA polymerase 3 in post-replication repair in excision-proficient Escherichia coli. Nature. 1974 May 24;249(455):348–349. doi: 10.1038/249348a0. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis in strains of E. coli deficient in DNA polymerase. Nat New Biol. 1971 Jan 20;229(3):81–82. doi: 10.1038/newbio229081a0. [DOI] [PubMed] [Google Scholar]



