Abstract
Although abundant in well-differentiated rat thyroid cells, Rap1GAP expression was extinguished in a subset of human thyroid tumor-derived cell lines. Intriguingly, Rap1GAP was downregulated selectively in tumor cell lines that had acquired a mesenchymal morphology. Restoring Rap1GAP expression to these cells inhibited cell migration and invasion, effects that were correlated with the inhibition of Rap1 and Rac1 activity. The reexpression of Rap1GAP also inhibited DNA synthesis and anchorage-independent proliferation. Conversely, eliminating Rap1GAP expression in rat thyroid cells induced a transient increase in cell number. Strikingly, Rap1GAP expression was abolished by Ras transformation. The downregulation of Rap1GAP by Ras required the activation of the Raf/MEK/extracellular signal-regulated kinase cascade and was correlated with the induction of mesenchymal morphology and migratory behavior. Remarkably, the acute expression of oncogenic Ras was sufficient to downregulate Rap1GAP expression in rat thyroid cells, identifying Rap1GAP as a novel target of oncogenic Ras. Collectively, these data implicate Rap1GAP as a putative tumor/invasion suppressor in the thyroid. In support of that notion, Rap1GAP was highly expressed in normal human thyroid cells and downregulated in primary thyroid tumors.
Rap1GAP (30, 33) is a member of a family of GTPase-activating proteins (GAPs) for Rap1/2 GTPases that includes the splice variant Rap1GAPII, SPA-1, and E6TP1. Rap1GAP shares structural similarities with the RhebGAP tuberin. Tuberin is subject to mutational inactivation and loss in tuberous sclerosis, a disease syndrome associated with the formation of multiple benign tumors (15, 19, 22, 37). The downregulation of E6TP1 by human papillomavirus E6 protein is believed to contribute to cervical cancer (10, 11), and an SPA-1 deficiency in mice results in a spectrum of myelodysplastic disorders similar to chronic myelogenous leukemia (13). The rap1GAP gene has been mapped to 1p35-36, a chromosomal region subject to deletion in a variety of human tumors including breast (28) and endocrine (41) neoplasia. Recently, decreased expression and loss of heterozygosity for Rap1GAP were reported for human oropharyngeal squamous cell (43) and pancreatic (21, 42) carcinomas.
Rap1GAP is abundant in rat thyroid epithelial cells, where thyroid-stimulating hormone (TSH) regulates Rap1GAP protein stability. The stable overexpression of Rap1GAP in thyroid cells impaired DNA synthesis and the growth rate, and based on this, we suggested that Rap1GAP might function as a tumor suppressor (34). We now provide further support for this idea. Eliminating Rap1GAP expression in differentiated rat thyroid cells induced a transient increase in cell proliferation. Moreover, while highly expressed in normal thyroid follicular cells, Rap1GAP expression was downregulated in primary thyroid tumors and in thyroid carcinoma cell lines. In vitro, decreased expression of Rap1GAP was observed selectively in thyroid carcinoma cell lines that exhibited migratory and invasive properties. Restoring Rap1GAP expression in these cells inhibited not only cell proliferation but also tumor cell migration and invasion. Remarkably, acute or chronic expression of activated Ras in rat thyroid cells abolished Rap1GAP expression. These findings identify Rap1GAP as being a putative tumor/invasion suppressor in thyroid cells and a novel target of oncogenic Ras.
MATERIALS AND METHODS
Reagents.
Rap1GAP antibodies generously provided by Michiyuki Matsuda (Osaka University) and purchased from Santa Cruz (Santa Cruz, CA) were used. Hemagglutinin (HA) antibody was a kind gift from Jeffrey Field (University of Pennsylvania). Rap1, extracellular signal-regulated kinase 2 (ERK2), and actin antibodies were purchased from Santa Cruz (Santa Cruz, CA). Vimentin antibody was purchased from Sigma (Saint Louis, MO), E-cadherin antibody was purchased from R&D Systems (Minneapolis, MN), Rac1 antibody was purchased from Upstate (Lake Placid, NY), and phospho-ERK and AKT antibodies were purchased from Cell Signaling (Beverly, MA). The β2-chimerin antibody was described previously (38).
Cell culture.
Wistar rat thyroid (WRT) cells were propagated in Coon's modified Ham's F-12 medium containing TSH, insulin, transferrin, and 5% calf serum (referred to as 3H medium). Cells were starved in growth factor- and serum-free Coon's modified Ham's F-12 or basal medium. NPA and KAT10 papillary thyroid carcinoma lines, FTC133 and WRO follicular thyroid carcinoma lines, and ARO, KAT4B, and SW1736 anaplastic thyroid carcinoma lines were studied. FTC-133 cells (12) were obtained from O. Clark (University of California, San Francisco). KAT10 and KAT4B cells were a generous gift from K. Ain (University of Kentucky), and SW1736 cells were obtained from M. S. Brose's laboratory. ARO, NPA, and WRO cells (29) were kindly provided by M. Ringel (The Ohio State University College of Medicine). FTC-133 cells were grown in 3H medium and starved in basal medium. ARO, NPA, and WRO cells were propagated in RPMI-10% fetal bovine serum. KAT10, KAT4B, and SW1736 cells were grown in phenol red-free RPMI supplemented with 10% fetal bovine serum, nonessential amino acids, and sodium pyruvate. Starvation was performed in serum-free RPMI medium. RasV12-transformed WRT cells (7, 26) were propagated in 3H medium and starved in basal medium.
Transient transfection and anchorage-independent proliferation.
Transfection was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Cells were plated in six-well plates 18 h prior to transfection, transferred into Opti-MEM I (Invitrogen), and exposed to 4 μg of pShuttle or pShuttle-HA-Rap1GAP DNA and 8 μl of Lipofectamine 2000. Twenty-four hours posttransfection, cells (103) were resuspended in 1.5 ml 0.33% agar in 3H medium and plated onto a bottom layer of 0.5% agar. Cells were refed every 4 to 5 days for 3 to 4 weeks. Colonies were visualized by staining with 2 mg/ml MTT (methylthiazolyldiphenyl-tetrazolium bromide) for 16 h at 37°C.
DNA synthesis.
FTC-133 cells plated onto glass coverslips were transfected with 8 μg of pShuttle or pShuttle-HA-Rap1GAP DNA and 10 μl of Lipofectamine 2000 in 1 ml of Opti-MEM I. After 5 h, cells were transferred into 3H medium. After 24 and 48 h, cells were pulse-labeled with bromodeoxyuridine (BrdU) for 4 h, fixed, and stained for HA-Rap1GAP and BrdU as previously described (34).
Viral infection.
Cells were infected overnight in basal or serum-free medium and transferred into growth medium for 24 h before they were used in experiments. HA-Rap1GAP and HA-Rap1bN17 adenoviruses were constructed using the AdEasy vector system (Q-Biogene, Carlsbad, CA). HA-pShuttle was kindly provided by M. Kazanietz (University of Pennsylvania). Rap1GAP cDNA was obtained by PCR from pCMV2-FLAG-Rap1GAP, kindly provided by Lawrence Quilliam (Indiana University). Rap1b cDNA was obtained by reverse transcription (RT)-PCR from WRT cell RNA. The PCR fragments were cloned into EcoRV and XhoI sites of HA-pShuttle. Site-directed mutagenesis of Rap1b to Rap1bN17 was performed as described previously (34). The resulting constructs were used for recombination with adenoviral DNA in BJ5183 competent cells. QBI-293A cells were transfected with recombinants and analyzed for adenovirus production by plaque assay. Adenoviruses were propagated according to the manufacturer's recommendations, and titers were determined by using the Adeno-X rapid titer kit (Clontech, Mountain View, CA). Control virus (LacZ) was used at an equal multiplicity of infection (MOI) (infectious units/cell for banded viruses and particles/cell for unbanded viruses) as test viruses.
HA-RasV12 adenovirus was constructed as described previously (8). Adenoviruses expressing Ras effector domain mutants were generated in a similar fashion. A virus expressing activated MEK1 was a kind gift from Zohre German (UT Southwestern). WRT cells were infected with viruses overnight in basal medium, washed, and transferred into fresh 3H medium for the indicated times. β2-Chimerin adenovirus was described elsewhere previously (6).
Wound assay.
Confluent monolayers of control and infected cells (at 24 to 48 h postinfection) were wounded with a pipette tip, refed to remove floating cells, and imaged immediately and at various times thereafter using a Nikon Eclipse TE2000 microscope.
Transwell migration assay.
Cells were trypsinized and counted, and 105 cells (in 100 μl) were plated into the upper chamber of Transwell 24-well plates (Fisher, Pittsburgh, PA) containing 8-μm-pore-size filters. FTC-133 and RasV12S35 cells were plated in 3H medium, and the lower chamber was supplemented with the same medium. NPA cells were plated in serum-free RPMI medium and exposed to RPMI-10% fetal calf serum in the lower chamber. Migration was analyzed after 24 h. Triplicate samples were analyzed for total and migrated cells. For migrated cells, cells on the top of the filters were removed using a cotton swab. Cells were fixed in 3.7% formaldehyde-phosphate-buffered saline (PBS) for 15 min, stained with 0.1% crystal violet for 15 min, and solubilized in 1% sodium deoxycholate, and the absorbance was read at 450 nm. The percent migrated cells was calculated against total cells, which was set as 100%. To assess invasion, FTC-133 and RasV12S35 cells were plated in basal medium containing 1% fetal calf serum in the upper chamber of BioCoat Matrigel Invasion chambers (BD Biosciences, Bedford, MA) and exposed to epidermal growth factor (10 ng/ml)-supplemented 3H medium in the lower chamber. Percent invasion was analyzed in triplicate samples after 72 h.
RT-PCR.
RNA was isolated using TRIzol (Invitrogen). RT-PCR was performed using the AccessQuick RT-PCR system (Promega, Madison, WI) according to the manufacturer's protocol. Briefly, 3 μg RNA was mixed with 24 μl master mix containing Rap1GAP or β-actin primers, heated at 45°C for 1 h, and followed with PCR cycling as specified by manufacturer. Reaction products were analyzed on 1.5% agarose gels and imaged using GelDoc XR and Quantity One 4.5.2 software (Bio-Rad, Hercules, CA).
Rap1 and Rac1 activity.
Rap1 activation was analyzed as described previously (35), and Rac1 activation was analyzed as described previously (38). Rac1 activity was assessed in growing RasV12S35 cells and 4 h after replating FTC-133 cells. Cells were lysed in 600 μl Rac lysis buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 50 μg glutathione S-transferase-Pak-CRIB). Precleared lysate (450 μl) was incubated with 50 μl glutathione-Sepharose 4B, with rotating for 1 h at 4°C. Beads were washed three times with lysis buffer, resuspended in 2× sample buffer, boiled, and analyzed on 12% gels. Total cell extracts (30 μl) were run in parallel as a loading control.
Adhesion assay.
FTC-133 cells were trypsinized, counted, and plated on laminin-treated coverslips. After various times, cells were fixed, stained for HA-Rap1GAP and actin using rhodamine-conjugated phalloidin, and analyzed using a Zeiss axiophot fluorescence microscope.
siRNA transfection and cell proliferation assays.
Small interfering RNA (siRNA) duplexes (100 nM) were introduced into WRT cells (1.3 × 106 to 1.5 × 106 cells) using the Amaxa Nucleofector (Germany). Scrambled siRNA (catalog no. 1027280) and Rap1GAP siRNA duplexes 1 (catalog no. SI01737043) and 2 (catalog no. SI01737050) were purchased from QIAGEN (Valencia, CA). siRNA-transfected cells were plated in 96-well plates (105 cells/well), and cell proliferation was analyzed using the Rapid cell proliferation kit (Calbiochem, San Diego, CA) on days 1 and 3 posttransfection. In brief, 10 μl of WST-1 reagent was added to each well and incubated at 37°C for 1 h, and absorbance was read at 450 nm. All samples were performed in triplicate. Alternatively, transfected cells (5 × 105) were plated in 60-mm dishes in triplicate, trypsinized, and counted on days 2 to 3 posttransfection using a Z1 Coulter particle counter (Beckman Coulter, Fullerton, CA).
Western blotting.
Western blotting was performed essentially as described previously (34). Proteins were detected and analyzed using the FUJI LAS-3000 system and Multi Gauge 3.0 software (Fuji, Japan).
Immunohistochemistry.
Tissue blocks from five patients diagnosed with classic papillary thyroid carcinoma were obtained from Yu Lv, Professor of Pathology, Beijing Chaoyang Hospital, Capital University of Medical Sciences, Beijing, People's Republic of China. Fresh hematoxylin and eosin sections were made and reviewed by trained pathologists from the Hospital of the University of Pennsylvania. Sections (5 μm) were freshly cut for immunohistochemical staining. Sections were incubated at 58°C for 20 min, deparaffinized in xylene twice for 15 min, and rehydrated. Sections were incubated in antigen-unmasking solution (Vector Laboratories, CA) at 95 to 98°C for 20 min and cooled to room temperature, and endogenous peroxidase activity was blocked by incubation in 3% H2O2 at room temperature for 15 min. Sections were washed in PBS-Tween, incubated in blocking buffer (10% normal goat serum-1% bovine serum albumin in PBS-Tween), and then incubated at 4°C overnight with primary antibody (Rap1GAP, 1:500; Santa Cruz). Following washing, sections were stained with biotinylated goat anti-rabbit IgG (1:200) for 30 min at room temperature, washed, and incubated in ABC complex (Vector Vectastain Elite ABC kit) at room temperature for 30 min. Bound antibody was visualized by DAB (Vector Laboratories, CA). Sections were analyzed by two pathologists, and staining intensity was scored as 1 to 5.
Graphic and statistical analyses.
Statistical analyses were performed using GraphPad Prism 3.0 software. Data are presented as means ± standard deviations, and significance was assessed by t test. A P value of <0.05 was considered to be statistically significant.
RESULTS
Rap1GAP expression is abolished in human thyroid tumor cell lines.
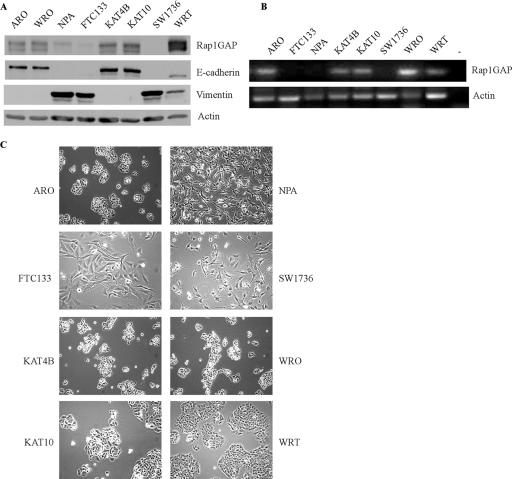
We previously reported that Rap1GAP is abundant in differentiated rat thyroid cells and suggested a role for Rap1GAP as a tumor suppressor (34). Two recent reports provided support for this hypothesis and showed that Rap1GAP expression was decreased in oropharyngeal squamous cell (43) and pancreatic (42) carcinomas. Here, we analyzed Rap1GAP expression in cell lines isolated from human follicular, papillary, and anaplastic thyroid carcinomas. Western blotting revealed that Rap1GAP protein levels were markedly decreased in NPA, FTC-133, and SW1736 cells compared to the other lines examined (Fig. 1A). As NPA, FTC-133, and SW1736 cells were isolated from papillary, follicular, and anaplastic carcinomas, respectively, the downregulation of Rap1GAP was not correlated with a specific tumor subtype. To assess whether the loss of the Rap1GAP protein reflected transcriptional effects, Rap1GAP message levels were examined. Rap1GAP mRNA was absent from FTC-133, NPA, and SW1736 cells and present in ARO, KAT-4B, KAT-10, and WRO cells (Fig. 1B), consistent with the notion that the rap1GAP gene is silenced or lost during thyroid carcinogenesis.
FIG. 1.
Downregulation of Rap1GAP in human thyroid cancer cell lines. (A) Rap1GAP expression was analyzed by Western blotting in a panel of human thyroid carcinoma cell lines (see Materials and Methods) and WRT cells. The same filter was probed for E-cadherin and vimentin. Equal protein loading was confirmed by blotting for actin. More than three experiments were performed, with similar results. (B) Rap1GAP mRNA was analyzed by RT-PCR. No DNA was added to the negative control (−), and WRT cells provided a positive control. Four experiments were performed, with similar results. (C) Phase-contrast images of human thyroid carcinoma and WRT cells.
We noted a striking correlation between the loss of expression of Rap1GAP and transformed morphology (Fig. 1C). Tumor cell lines that expressed Rap1GAP (ARO, KAT-4B, KAT-10, and WRO) exhibited an epithelial morphology and formed well-defined colonies with extensive cell-cell contacts, similar to WRT cells. In contrast, the tumor cell lines that lacked Rap1GAP (FTC-133, NPA, and SW1736) were fibroblast-like and exhibited extensive cell scattering. Notably, the downregulation of Rap1GAP was correlated with the loss of E-cadherin and the acquisition of vimentin expression (Fig. 1A). Hence, these data revealed a striking correlation between the loss of Rap1GAP and alterations in cell morphology and gene expression characteristic of an epithelial-to-mesenchymal transition.
Rap1GAP inhibits cell spreading, migration, and invasion.
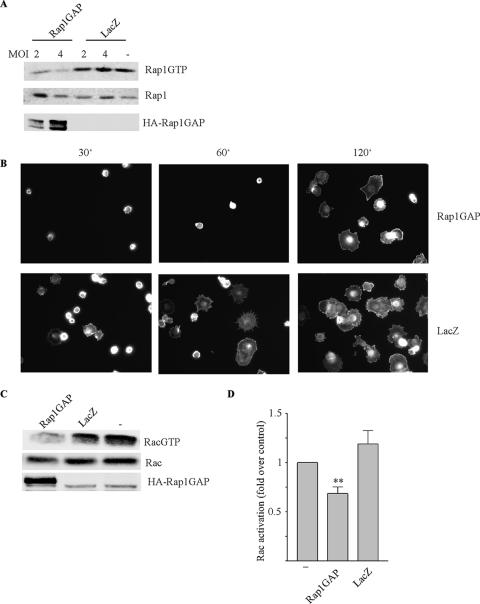
To assess if there were functional consequences associated with Rap1GAP depletion, HA-Rap1GAP was expressed in FTC-133 cells using an adenovirus. Preliminary experiments were conducted to determine the lowest dose of virus that resulted in sufficient Rap1GAP expression to inhibit endogenous Rap1 activity (Fig. 2A). Infection with the Rap1GAP virus dose-dependently impaired Rap1 activity, with maximal inhibition observed at an MOI of 4. Under these conditions, Rap1GAP-expressing cells were impaired in their ability to adhere to and spread on laminin-treated coverslips (Fig. 2B), consistent with the previously established roles of Rap in the regulation of cell adhesion (reviewed in references 5 and 18). Rap1 has been shown to facilitate Rac activation by recruiting a subset of Rac guanine nucleotide exchange factors to the plasma membrane (3). Rac1 activity in FTC-133 cells increased over time following replating, and this was impaired by the expression of Rap1GAP (Fig. 2C and D). The inhibition of Rac1 activity and cell spreading were transient (data not shown), suggesting that Rap1GAP selectively inhibits early events associated with cell attachment and spreading.
FIG. 2.
Rap1GAP blocks Rac activation and cell spreading. (A) FTC-133 cells were infected with Rap1GAP or LacZ virus (at the MOIs indicated), and Rap1 activity was assessed at 24 h postinfection. Two dose-response experiments were performed, with similar results. (B) Rap1GAP and LacZ virus-infected (MOI of 4) FTC-133 cells were plated onto laminin-coated coverslips for the times indicated (minutes), fixed, and stained with rhodamine-conjugated phalloidin. Two time course experiments were performed with similar results. More than 90% of Rap1GAP-infected cells were HA positive (data not shown). (C and D) Rac1 activity was examined in Rap1GAP and LacZ (MOI of 4) virus-infected FTC-133 cells 4 h after plating. Total cell extracts were analyzed for Rac1 and HA-Rap1GAP expression. Representative data and a summary from four experiments are presented. Rac1 activation in mock-infected cells (−) was set as 1. Rap1GAP significantly reduced Rac1 activity (**, P < 0.005).
Expression of Rap1GAP in FTC-133 cells delayed wound closure (Fig. 3A) and impaired actin polymerization at the leading edge of migrating cells (Fig. 3B). To fortify these findings, cell migration was analyzed in transwell assays. Compared to LacZ virus-infected cells, the expression of Rap1GAP significantly reduced cell migration (Fig. 3C) as well as epidermal growth factor-induced invasion through a basement membrane (Matrigel) (Fig. 3D). To assess whether Rap1GAP elicited similar effects on other migratory thyroid tumor cell lines, Rap1GAP was expressed in NPA cells. When expressed at levels sufficient to block Rap1 activity (MOI of 8) (Fig. 3E), Rap1GAP inhibited the migration of NPA cells (Fig. 3F).
FIG. 3.
Rap1GAP inhibits the migration and invasion of human thyroid carcinoma cells. (A) Rap1GAP and LacZ virus-infected FTC-133 cells (at the MOIs indicated) were plated to yield a confluent monolayer at 24 h postinfection. Cells were wounded, and images were acquired immediately and 24 h later. Three experiments were performed, with similar results. (B) Immunostaining for HA-Rap1GAP and actin at 6 h after wounding is shown. The extension of actin-rich cell protrusions was markedly impaired in Rap1GAP-expressing cells (arrow). Two experiments were performed, with similar results. (C) At 24 h postinfection, FTC-133 cells were plated in transwell chambers, and cell migration was analyzed (see Materials and Methods). Results from three independent experiments performed in triplicate are summarized. Rap1GAP significantly inhibited cell migration (*, P < 0.05). (D) Rap1GAP impaired the ability of FTC-133 cells to invade through Matrigel. Cells were infected as described above (C) and plated in transwell chambers containing Matrigel-coated filters (see Materials and Methods). Results from two independent experiments (triplicate samples) are shown. Rap1GAP significantly reduced invasion (*, P < 0.05). (E) Rap1GAP dose-dependently inhibited Rap1 activity in NPA cells. Two dose-response experiments performed at the MOIs indicated yielded similar results. (F) Rap1GAP and LacZ virus-infected (MOI of 8) NPA cells were plated in transwell chambers, and cell migration was analyzed. Results from two experiments (triplicate samples) are shown. Rap1GAP significantly decreased cell migration in NPA cells (*, P < 0.05).
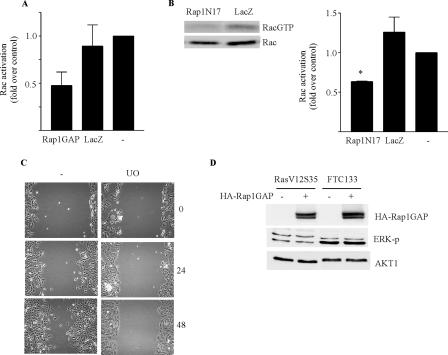
To assess whether the inhibition of Rap activity was sufficient to impair cell migration, dominant negative Rap1 (Rap1N17) was expressed in FTC-133 cells using an adenovirus. The expression of Rap1N17 inhibited cell migration in transwell (Fig. 4A) and wound closure (data not shown) assays. As for Rap1GAP, the dose response over which Rap1N17 inhibited Rap1 activity was similar to that over which it impaired migration (data not shown).
FIG. 4.
Rap1 and Rac1 activity are required for the migration of human thyroid carcinoma cells. (A) Rap1N17 and LacZ virus-infected FTC-133 cells were plated in transwell chambers, and cell migration was analyzed. The remaining cells were analyzed for HA-Rap1N17 expression. Results from three experiments are summarized. Rap1N17 significantly reduced cell migration (**, P < 0.005). (B) Infection with β2-chimerin but not LacZ virus dose-dependently impaired Rac1 activity in FTC-133 cells. Results from three independent experiments are summarized. Rac1 activity in growing FTC-133 cells was set as 1. (C) β2-Chimerin dose-dependently impaired migration in FTC-133 cells. Cells were infected as described above. Results from three independent experiments are summarized.
We confirmed that the migration of FTC-133 cells required Rac1 activity. The expression of β2-chimerin, a highly selective RacGAP (38), inhibited both Rac1 activation (Fig. 4B) and cell migration (Fig. 4C) with a similar dose response. Collectively, these findings indicate that Rap1GAP impairs the migration of human thyroid tumor cells, possibly through the inhibition of Rac1 activity.
Rap1GAP inhibits cell proliferation.
If Rap1GAP functions as a tumor suppressor, manipulating its expression should elicit effects on cell proliferation. We previously reported that Rap1GAP inhibits cell proliferation when overexpressed in rat thyroid cells (34). To assess whether decreased Rap1GAP expression contributed to the proliferation of human thyroid tumor cells, the effects of restoring Rap1GAP expression on DNA synthesis and anchorage-independent growth were investigated. DNA synthesis was significantly reduced in Rap1GAP- versus vector-transfected FTC-133 cells (Fig. 5A). Similarly, the transient expression of Rap1GAP reduced colony formation in agar (Fig. 5B). To examine whether Rap1GAP negatively regulates cell proliferation in differentiated rat thyroid cells, cell proliferation was analyzed in WRT cells in which Rap1GAP expression was transiently reduced using RNA interference. The elimination of Rap1GAP expression induced a significant increase in cell proliferation, as assessed by increased mitochondrial activity (Fig. 5C) and cell number (Fig. 5D). Hence, the proliferation of differentiated rat thyroid cells and human thyroid carcinoma cells is sensitive to Rap1GAP expression levels.
FIG. 5.
Rap1GAP regulates cell proliferation. (A) DNA synthesis was examined in Rap1GAP- versus vector (pShuttle)-transfected FTC-133 cells. Cells were pulse-labeled with BrdU for 4 h on day 1 posttransfection. Percent BrdU-positive versus total DAPI (4′,6′-diamidino-2-phenylindole)-positive nuclei is shown for pShuttle, and percent BrdU-positive, HA-positive cells is shown for Rap1GAP. Results shown are representative of three experiments (*, P < 0.05). (B) pShuttle HA-Rap1GAP and pShuttle-transfected FTC-133 cells were plated in soft agar at day 1 posttransfection, and the number of colonies formed was assessed after 3 weeks. Data shown are representative of three independent experiments (***, P < 0.001). (C) WRT cells were transfected with two different sets of Rap1GAP siRNAs (siGAP) (1 and 2) versus scrambled controls (scr) and plated overnight, and cell proliferation was analyzed by WST-1 assay at days 1 (open bars) and 3 (black bars) posttransfection. The optical density at 450 nm at day 1 was set as 1 for each sample. Parallel samples were analyzed for Rap1GAP expression. Results shown are summarized from two experiments (*, P < 0.05). (D) WRT cells were transfected as described above (C) and plated overnight, and the cell number was analyzed at days 2 (open bars) and 3 (black bars) posttransfection. The cell number at day 2 was set as 1 for each sample. Parallel samples were analyzed for Rap1GAP expression. Results shown are summarized from three independent experiments (*, P < 0.05; **, P < 0.005).
Rap1GAP expression is downregulated by Ras transformation.
To further assess the significance of Rap1GAP downregulation to thyroid cell transformation, we examined whether the downregulation of Rap1GAP could be induced by a thyroid oncogene. Mutations in H-Ras, K-Ras, and N-Ras are frequent in human thyroid tumors, where Ras contributes to tumor initiation and progression (reviewed in references 25 and 36). Strikingly, Rap1GAP protein (Fig. 6A) and message (data not shown) levels were abolished in human H-Ras (RasV12)-transformed thyroid cells (7, 26). The downregulation of Rap1GAP was restricted to Ras proteins capable of signaling to the Raf/MEK/ERK cascade, as Rap1GAP expression was abolished by RasV12S35, a mutant that activates Raf (40), and retained in cells transformed with RasV12G37 and RasV12C40, mutants that signal preferentially to RalGDS and phosphatidylinositol 3-kinase, respectively. The downregulation of Rap1GAP was correlated with increases in both Rap1 activity and expression (Fig. 6B). Following normalization for differences in expression, Rap1 activity was increased in RasV12 and RasV12S35 cells compared to cells expressing the other Ras effector domain mutants (Fig. 6C).
FIG. 6.
Rap1GAP is downregulated in Ras-transformed cells. (A) Rap1GAP expression was analyzed in WRT cells transformed with human HA-RasV12 and the indicated Ras effector domain mutants (7, 26). Expression of HA-Ras is shown; Western blotting for actin confirmed equal protein loading. (B and C) Rap1 activity was analyzed in parental and Ras-transformed cells. Normalization for Rap1 expression confirmed that Rap1 activity was increased in RasV12 and RasV12S35 cells compared to the other lines. Rap1 activity in WRT cells was set as 1. Results shown are summarized from two independent experiments. (D) Confluent RasV12-transformed cells were wounded, imaged immediately, and imaged again after 24 h. Three experiments were performed, with similar results. (E) Rap1GAP and LacZ virus-infected (MOI of 6) RasV12S35 cells were wounded and imaged immediately and after 48 h. Two experiments were performed, with similar results. (F) Rap1GAP and LacZ virus-infected RasV12S35 cells were plated in transwell chambers, and cell migration was analyzed. Results from three experiments (triplicate samples) are summarized.
The selective downregulation of Rap1GAP in RasV12- and RasV12S35-expressing cells was striking in that only thyroid cells transformed by these Ras proteins exhibited morphological transformation (7, 26). Wound assays revealed that only RasV12- and RasV12S35-expressing cells were migratory (Fig. 6D). As predicted, restoring Rap1GAP expression to RasV12S35 cells impaired wound closure (Fig. 6E) and transwell migration (Fig. 6F). Similar effects were observed with Rap1N17 (data not shown). Rap1GAP expression also blocked the invasion of RasV12S35 cells through Matrigel (data not shown).
Interference with Rap1 activity through the expression of either Rap1GAP (Fig. 7A) or dominant negative Rap1 (Fig. 7B) reduced Rac1 activity in RasV12S35 cells as in human thyroid carcinoma cells (Fig. 2). Although the migration of RasV12S35 cells required MEK1 activity (Fig. 7C), the expression of Rap1GAP in these cells or in FTC-133 cells did not decrease ERK activity (Fig. 7D). Similar results were reported previously for pancreatic carcinoma cell lines, where the stable expression of Rap1GAP impaired cell motility and invasion but not ERK phosphorylation (42). Rac1 activity in RasV12S35 cells was insensitive to the MEK1 inhibitor (data not shown). These data suggest that multiple pathways contribute to the migration of RasV12S35 cells, only some of which are impaired by interference with Rap activity. However, they do not exclude the inhibition of localized pools of ERK by Rap1GAP, as Rap1 has been shown to selectively activate plasma membrane-localized ERK (39). Thus, the downregulation of Rap1GAP appears to contribute to the migratory behavior of Ras-transformed rat thyroid cells as well as human thyroid tumor cell lines.
FIG. 7.
Interference with Rap1 activity impairs Rac1 but not ERK activity in Ras-transformed cells. (A) Rac1 activity was assessed in Rap1GAP and LacZ virus-infected RasV12S35 cells. Rac1 activity in mock-infected cells (−) was set as 1. Results from three experiments are shown. (B) Rac1 activity was examined in Rap1N17 and LacZ virus-infected (MOI of 152) RasV12S35 cells. Rac1 activity in mock-infected cells was set as 1. Results from two experiments performed in triplicate (*, P < 0.05) are summarized. (C) RasV12S35 cells were pretreated with 10 μM UO126 (UO) for 30 min prior to wounding. Cells were imaged immediately and after 24 and 48 h. Three experiments were performed, with similar results. (D) Activating phosphorylation of ERK2 was compared in LacZ versus Rap1GAP virus-infected RasV12S35 (MOI of 6) and FTC-133 (MOI of 4) cells at 24 h postinfection. Equal protein loading was confirmed by Western blotting for AKT1. Three experiments were performed with RasV12S35 cells, and four were performed with FTC-133 cells, with similar results.
Acute expression of oncogenic Ras downregulates Rap1GAP expression.
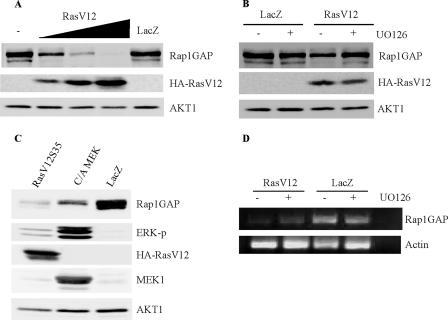
To determine whether the downregulation of Rap1GAP was an early event in Ras transformation, we analyzed the acute effects of oncogenic Ras on Rap1GAP. WRT cells were infected overnight with an adenovirus expressing activated H-Ras (RasV12) (8), and Rap1GAP protein expression was analyzed after 48 h. Rap1GAP expression was extinguished in a dose-dependent manner following infection with human H-RasV12 but not LacZ-expressing adenovirus (Fig. 8A). Similar effects were observed when Rap1GAP was analyzed after 24 h (data not shown). The decrease in Rap1GAP required activated and membrane-localized Ras, as the expression of cellular Ras (Ras G12) or of an activated mutant impaired in membrane localization (RasV12C186A) (8) did not affect Rap1GAP protein levels (data not shown). Rap1GAP protein levels were partially restored by treatment with the MEK1 inhibitor UO126 (Fig. 8B). In addition, acute expression of RasV12S35 or constitutively active MEK1 was sufficient to decrease Rap1GAP protein levels (Fig. 8C). The expression of RasV12 also decreased Rap1GAP message levels (Fig. 8D). However, compared to LacZ virus-infected cells, treatment with UO126 had little effect on Rap1GAP message levels in RasV12-expressing cells. These results suggest that RasV12 elicits potentially independent effects on Rap1GAP protein and message levels. The acute downregulation of Rap1GAP by Ras reveals the potential for collaboration between Ras and Rap1 in thyroid cell transformation.
FIG. 8.
RasV12 acutely downregulates Rap1GAP protein and message levels. (A) WRT cells were infected with RasV12 (300, 1,000, and 2,500 particles/cell) or LacZ (1,000 particles/cell) virus overnight, transferred into growth medium, and harvested after 48 h. Total cell lysates were analyzed for endogenous Rap1GAP and HA-Ras expression. Western blotting for AKT1 confirmed equal protein loading. Three experiments were performed, with similar results. (B) WRT cells were infected overnight with RasV12 or LacZ virus (300 particles/cell), transferred into growth medium in the presence and absence of 10 μM UO126, and harvested 48 h later. Rap1GAP expression and HA-Ras expression were analyzed. Western blotting for AKT1 confirmed protein loading. Three experiments were performed, with similar results. (C) WRT cells were infected with viruses expressing RasV12S35, activated MEK1 (C/A MEK), and β-galactosidase (LacZ) (2,500 particles/cell), and Rap1GAP expression was analyzed at 48 h. Expression of HA-Ras and MEK1 and ERK activity (ERK-p) are shown. Equal protein loading was confirmed by analyzing AKT1 expression. Three experiments were performed, with similar results. (D) WRT cells were infected with RasV12 and LacZ (1,000 particles/cell) viruses overnight and then transferred into growth medium in the absence (−) and presence (+) of 10 μM UO126 for 48 h. Cells were harvested, total RNA was isolated, and Rap1GAP message levels were analyzed as described in the legend of Fig. 1B. Five experiments revealed that RasV12 decreased Rap1GAP mRNA. Two experiments revealed little or no effect of UO126 on Rap1GAP message levels.
Downregulation of Rap1GAP in primary thyroid tumors.
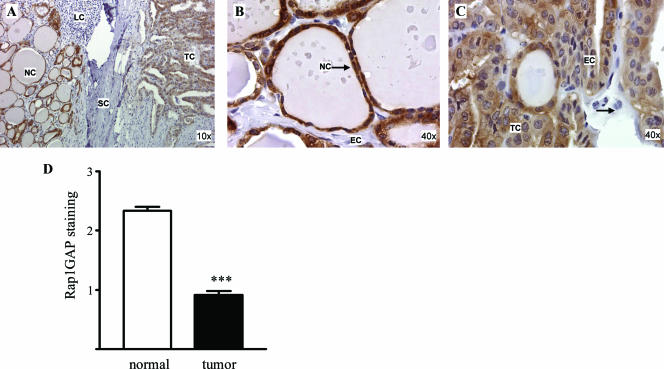
The loss of Rap1GAP from the thyroid tumor cell lines provided a compelling rationale for examining Rap1GAP expression in primary thyroid tumors. Five classic human papillary thyroid carcinomas were selected for analysis, as this is the most frequent type of thyroid cancer. We restricted our analysis to tissue blocks that contained both normal tissue and tumor tissue in the same sample. Rap1GAP expression was examined by immunohistochemistry and scored independently by two pathologists. As we predicted, Rap1GAP was highly expressed in normal follicular epithelial cells (Fig. 9A and B). Stromal cells, endothelial cells, and lymphocytes did not express Rap1GAP and served as internal negative controls for Rap1GAP staining. Although Rap1GAP was detected in tumor cells, there was a marked decrease in staining intensity in these cells compared to normal thyroid tissue in the same sample (Fig. 9A and C). Similar results were observed for all five samples analyzed (Fig. 9D). These data strongly suggest that Rap1GAP is downregulated in human thyroid tumors in vivo.
FIG. 9.
Rap1GAP protein expression is decreased in primary thyroid tumors. Five classic papillary thyroid carcinomas were stained for Rap1GAP (see Materials and Methods). (A) Low-power fields showing that Rap1GAP is expressed in follicular epithelial cells (normal cells [NC]) and tumor cells (TC) but not in stromal cells (SC), endothelial cells (EC) (see B), or lymphocytes (LC). (B and C) High-power fields of normal thyroid tissue (B) versus tumor tissue from the same section (C). Rap1GAP expression was consistently decreased in tumor cells versus adjacent normal cells in the same sections. The scoring results from five samples are summarized in panel D.
DISCUSSION
One of the most critical steps in the progression to malignancy is the acquisition of the ability to migrate and metastasize by tumor cells. As the most frequent endocrine malignancy, and one whose incidence is increasing, thyroid cancer poses a significant clinical challenge. There are no curative therapies for patients with progressive or recurrent thyroid cancer. An elucidation of the molecular events that contribute to the metastatic progression of thyroid tumors is desperately needed in order to devise more effective therapies.
Rap1GAP is abundant in differentiated rat thyroid cells, cells in which Rap1GAP protein stability is regulated by TSH (34). As the expression of the TSH receptor is extinguished in thyroid tumors, Rap1GAP expression was analyzed in human follicular, papillary, and anaplastic thyroid tumor cell lines. Rap1GAP expression was selectively abolished in lines that were highly migratory. Decreased expression of Rap1GAP was strictly correlated with the loss of E-cadherin and acquisition of vimentin expression and mesenchymal morphology. Importantly, transient expression of Rap1GAP in the Rap1GAP-deficient tumor cell lines inhibited cell migration and invasion.
Based on in vitro data, we hypothesized that Rap1GAP expression would be decreased in invasive thyroid cancers compared to normal thyroid tissue. To assess this, five classic papillary thyroid cancers were analyzed for Rap1GAP protein expression by immunohistochemistry. Rap1GAP staining was decreased in all five tumors compared to adjacent normal tissue. Although the tumor cells were not as mesenchymal in morphology as the thyroid cancer cell lines, they were morphologically dissimilar from normal thyroid follicular cells and invasive clinically. Whether further reductions in Rap1GAP expression would lead to more complete morphological transformation remains to be determined. These results fortify our in vitro data and implicate a role for Rap1GAP in thyroid cancer. We are actively pursuing the significance of these findings in a larger set of human thyroid cancers.
The mechanism through which Rap1GAP inhibits cell motility was explored in the FTC-133 follicular thyroid carcinoma cell line (12). The results strongly suggest that Rap1GAP impairs cell migration through the inhibition of Rap1 and Rac1 activity. However, while the expression of dominant negative Rap1 inhibited cell migration, the depletion of Rap1A and Rap1B expression failed to do so (data not shown). These results suggest that low levels or specific pools of Rap1 or additional targets of Rap1GAP, for example, Rap2 (24), contribute to migratory behavior. Rap1GAP delayed the transient increase in Rac1 activity induced by plating, consistent with recent findings that Rap1 recruits Rac guanine nucleotide exchange factors to the plasma membrane (3). Similar to our findings, the overexpression of Rap1GAP inhibited Rac1 activity, membrane protrusion, and cell spreading in HeLa cells (3); Rac1 activation by serotonin in COS cells expressing the 5-hydroxytryptamine receptor (23); and cell migration in human umbilical vein endothelial cells (9). Although Rac activity was required for the migration of thyroid cancer cells, RacV12 failed to restore migration to Rap1GAP-expressing cells and impaired migration when expressed alone (data not shown). Thus, although it seems likely that Rap1GAP impairs migration through the inhibition of Rac1, sustained Rac1 activity is not sufficient to restore cell migration. These results imply that the cycling of Rac between GDP- and GTP-bound forms and/or additional factors targeted by Rap1GAP contribute to cell migration in these cells.
Rap1GAP expression has been found to be decreased in other tumor types, suggesting that it may be a common event in cell transformation. Rap1GAP expression was decreased in invasive pancreatic carcinomas compared to benign lesions (42) and in a mouse model of glioblastoma multiform (14). Similar to the results reported here, the stable expression of Rap1GAP in pancreatic carcinoma cell lines inhibited cell motility (42). Our findings that the transient expression of Rap1GAP is sufficient to inhibit migration argue that impaired motility is a primary effect of Rap1GAP rather than a consequence of secondary changes associated with the isolation of stable cell lines. Rap1GAP decreased growth rate and tumor formation when stably expressed in pancreatic carcinoma cells (42) and slowed cell cycle progression in squamous cell carcinomas, although these effects were modest and seen only in synchronized cells (43). We demonstrate that the transient expression of Rap1GAP impairs tumor cell proliferation and, importantly, that the elimination of Rap1GAP expression induces a transient increase in cell proliferation. These findings clearly indicate that Rap1GAP expression is a determinant of proliferative capacity in some cells.
Activating mutations in H-ras, K-ras, and N-ras are prevalent in follicular thyroid carcinomas (25, 36). Our findings identify Rap1GAP as being a target for Ras and suggest that the depletion of Rap1GAP contributes to at least some aspects of Ras transformation. Rap1GAP protein and message levels were decreased by transformation with RasV12 and RasV12S35 but not in response to Ras mutants impaired in activating the Raf/MEK/ERK cascade. Only RasV12 and RasV12S35 induced features of an epithelial-to-mesenchymal transition in thyroid cells, including the downregulation of E-cadherin and upregulation of vimentin expression. As predicted, RasV12- and RasV12S35-transformed thyroid cells were migratory, and the expression of Rap1GAP in these cells inhibited cell migration and invasion.
The transient expression of RasV12 in rat thyroid cells downregulated Rap1GAP at the protein and message levels. Acute downregulation of Rap1GAP protein levels by Ras required MEK1 activity, and transient expression of activated MEK1 was sufficient to decrease Rap1GAP protein expression. Acutely, RasV12 also decreased Rap1GAP message levels; however, this decrease was largely insensitive to MEK1 inhibition, suggesting that Ras elicits multiple, independent effects on Rap1GAP expression. Together with our previous report that TSH regulates Rap1GAP protein stability, these data indicate that Rap1GAP expression is subject to multiple levels of regulation and raise the interesting question as to whether Rap1GAP is a marker of thyroid differentiation that can be extinguished by Ras. The mechanism through which Ras decreases Rap1GAP message levels is under investigation. Ras has been shown to upregulate the expression of DNA methyltransferases in epithelial cells (31). Although a CpG-rich island has been identified upstream from the rap1gap transcriptional start site (42), the demethylating agent 5-aza-cytidine failed to restore Rap1GAP expression in Ras-transformed thyroid cells (our unpublished results). The acute expression of Ras induces chromosomal instability in rat thyroid cells (1, 17). A loss of heterozygosity for rap1gap has been reported for pancreatic carcinomas (42), tumors where Ras mutations are frequent. Whether Ras induces losses or rearrangements that affect the chromosomal region containing rap1gap (1p35-36) remains to be determined.
Although first identified as being a suppressor of Ras transformation (16), it is now clear that Rap1 functions independently from Ras in most instances. Recent genetic evidence from Drosophila melanogaster revealed a collaboration between Ras and Rap1 in the regulation of ERK activity (27). Other recent findings place Rap1 functionally downstream from Ras. The Rap1 guanine nucleotide exchange factor Epac2 contains cAMP and Ras association domains (reviewed in reference 4). Elevations in cAMP induce a conformational change in Epac2, resulting in the activation of Rap1 on endomembranes. When coexpressed with activated Ras, Epac2 localized to the plasma membrane, where it activated a pool of membrane-localized Rap1 (20). Our data reveal a novel mechanism through which Ras might regulate Rap1 via the downregulation of Rap1GAP. The significance of this regulation remains to be explored; however, the ability of Rap1GAP to impair the migratory and invasive properties of Ras-transformed cells raises the potential for collaborative effects of Ras and Rap1 in the regulation of thyroid cell motility and perhaps other aspects of cell transformation.
In sum, we provide evidence that Rap1GAP expression is downregulated in primary thyroid tumors. This is particularly noteworthy given the proposed roles for Rap in thyroid cell proliferation (2) and transformation (32) together with the regulation of Rap1GAP by TSH (34). Although widely used as a tool to impair Rap activity, it is surprising how little is known about the regulation and function of cellular Rap1GAP. Studies to assess whether Rap1GAP is a marker of a favorable prognosis in thyroid cancer would reveal whether strategies to express Rap1GAP in thyroid tumors should be exploited.
Acknowledgments
This work was supported by Public Health Service grant DK55757 awarded to J.L.M. M.S.B. is a Damon Runyon-Seimens Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (CI-25-05).
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Abulaiti, A., A. J. Fikaris, O. M. Tsygankova, and J. L. Meinkoth. 2006. Ras induces chromosome instability and abrogation of the DNA damage response. Cancer Res. 66:10505-10512. [DOI] [PubMed] [Google Scholar]
- 2.Altschuler, D. L., and F. Ribeiro-Neto. 1998. Mitogenic and oncogenic properties of the small G protein Rap1b. Proc. Natl. Acad. Sci. USA 95:7475-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, W. T., L. A. Quilliam, and J. A. Cooper. 2004. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 167:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, J. L. 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31:680-686. [DOI] [PubMed] [Google Scholar]
- 5.Bos, J. L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17:123-128. [DOI] [PubMed] [Google Scholar]
- 6.Caloca, M. J., H. Wang, and M. G. Kazanietz. 2003. Characterization of the Rac-GAP (Rac-GTPase-activating protein) activity of beta2-chimaerin, a ‘non-protein kinase C’ phorbol ester receptor. Biochem. J. 375:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cass, L. A., and J. L. Meinkoth. 2000. Ras signaling through PI3K confers hormone-independent proliferation that is compatible with differentiation. Oncogene 19:924-932. [DOI] [PubMed] [Google Scholar]
- 8.Fikaris, A. J., A. E. Lewis, A. Abulaiti, O. M. Tsygankova, and J. L. Meinkoth. 2006. Ras triggers ataxia-telangiectasia-mutated and Rad-3-related activation and apoptosis through sustained mitogenic signaling. J. Biol. Chem. 281:34759-34767. [DOI] [PubMed] [Google Scholar]
- 9.Fujita, H., S. Fukuhara, A. Sakurai, A. Yamagishi, Y. Kamioka, Y. Nakaoka, M. Masuda, and N. Mochizuki. 2005. Local activation of Rap1 contributes to directional vascular endothelial cell migration accompanied by extension of microtubules on which RAPL, a Rap1-associating molecule, localizes. J. Biol. Chem. 280:5022-5031. [DOI] [PubMed] [Google Scholar]
- 10.Gao, Q., A. Kumar, L. Singh, J. M. Huibregtse, S. Beaudenon, S. Srinivasan, D. E. Wazer, H. Band, and V. Band. 2002. Human papillomavirus E6-induced degradation of E6TP1 is mediated by E6AP ubiquitin ligase. Cancer Res. 62:3315-3321. [PubMed] [Google Scholar]
- 11.Gao, Q., S. Srinivasan, S. N. Boyer, D. E. Wazer, and V. Band. 1999. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol. Cell. Biol. 19:733-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goretzki, P. E., A. Frilling, D. Simon, and H. D. Roeher. 1990. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 118:48-63. [DOI] [PubMed] [Google Scholar]
- 13.Ishida, D., K. Kometani, H. Yang, K. Kakugawa, K. Masuda, K. Iwai, M. Suzuki, S. Itohara, T. Nakahata, H. Hiai, H. Kawamoto, M. Hattori, and N. Minato. 2003. Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell 4:55-65. [DOI] [PubMed] [Google Scholar]
- 14.Johansson, F. K., H. Goransson, and B. Westermark. 2005. Expression analysis of genes involved in brain tumor progression driven by retroviral insertional mutagenesis in mice. Oncogene 24:3896-3905. [DOI] [PubMed] [Google Scholar]
- 15.Jones, A. C., M. M. Shyamsundar, M. W. Thomas, J. Maynard, S. Idziaszczyk, S. Tomkins, J. R. Sampson, and J. P. Cheadle. 1999. Comprehensive mutation analysis of TSC1 and TSC2—and phenotypic correlations in 150 families with tuberous sclerosis. Am. J. Hum. Genet. 64:1305-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitayama, H., Y. Sugimoto, T. Matsuzaki, Y. Ikawa, and M. Noda. 1989. A ras-related gene with transformation suppressor activity. Cell 56:77-84. [DOI] [PubMed] [Google Scholar]
- 17.Knauf, J. A., B. Ouyang, E. S. Knudsen, K. Fukasawa, G. Babcock, and J. A. Fagin. 2006. Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J. Biol. Chem. 281:3800-3809. [DOI] [PubMed] [Google Scholar]
- 18.Kooistra, M. R., N. Dube, and J. L. Bos. 2007. Rap1: a key regulator in cell-cell junction formation. J. Cell Sci. 120:17-22. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatkowski, D. J., and B. D. Manning. 2005. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum. Mol. Genet. 14:R251-R258. [DOI] [PubMed] [Google Scholar]
- 20.Li, Y., S. Asuri, J. F. Rebhun, A. F. Castro, N. C. Paranavitana, and L. A. Quilliam. 2006. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 281:2506-2514. [DOI] [PubMed] [Google Scholar]
- 21.Logsdon, C. D., D. M. Simeone, C. Binkley, T. Arumugam, J. K. Greenson, T. J. Giordano, D. E. Misek, R. Kuick, and S. Hanash. 2003. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 63:2649-2657. [PubMed] [Google Scholar]
- 22.Maheshwar, M. M., J. P. Cheadle, A. C. Jones, J. Myring, A. E. Fryer, P. C. Harris, and J. R. Sampson. 1997. The GAP-related domain of tuberin, the product of the TSC2 gene, is a target for missense mutations in tuberous sclerosis. Hum. Mol. Genet. 6:1991-1996. [DOI] [PubMed] [Google Scholar]
- 23.Maillet, M., S. J. Robert, M. Cacquevel, M. Gastineau, D. Vivien, J. Bertoglio, J. L. Zugaza, R. Fischmeister, and F. Lezoualc'h. 2003. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat. Cell Biol. 5:633-639. [DOI] [PubMed] [Google Scholar]
- 24.McLeod, S. J., A. H. Li, R. L. Lee, A. E. Burgess, and M. R. Gold. 2002. The Rap GTPases regulate B cell migration toward the chemokine stromal cell-derived factor-1 (CXCL12): potential role for Rap2 in promoting B cell migration. J. Immunol. 169:1365-1371. [DOI] [PubMed] [Google Scholar]
- 25.Meinkoth, J. L. 2004. Biology of Ras in thyroid cells. Cancer Treat. Res. 122:131-148. [DOI] [PubMed] [Google Scholar]
- 26.Miller, M. J., L. Rioux, G. V. Prendergast, S. Cannon, M. A. White, and J. L. Meinkoth. 1998. Differential effects of protein kinase A on Ras. effector pathways. Mol. Cell. Biol. 18:3718-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra, S., S. M. Smolik, M. A. Forte, and P. J. Stork. 2005. Ras-independent activation of ERK signaling via the torso receptor tyrosine kinase is mediated by Rap1. Curr. Biol. 15:366-370. [DOI] [PubMed] [Google Scholar]
- 28.Nagai, H., M. Negrini, S. L. Carter, D. R. Gillum, A. L. Rosenberg, G. F. Schwartz, and C. M. Croce. 1995. Detection and cloning of a common region of loss of heterozygosity at chromosome 1p in breast cancer. Cancer Res. 55:1752-1757. [PubMed] [Google Scholar]
- 29.Pang, X. P., J. M. Hershman, M. Chung, and A. E. Pekary. 1989. Characterization of tumor necrosis factor-alpha receptors in human and rat thyroid cells and regulation of the receptors by thyrotropin. Endocrinology 125:1783-1788. [DOI] [PubMed] [Google Scholar]
- 30.Polakis, P. G., B. Rubinfeld, T. Evans, and F. McCormick. 1991. Purification of a plasma membrane-associated GTPase-activating protein specific for rap1/Krev-1 from HL60 cells. Proc. Natl. Acad. Sci. USA 88:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruitt, K., A. S. Ulku, K. Frantz, R. J. Rojas, V. M. Muniz-Medina, V. M. Rangnekar, C. J. Der, and J. M. Shields. 2005. Ras-mediated loss of the pro-apoptotic response protein Par-4 is mediated by DNA hypermethylation through Raf-independent and Raf-dependent signaling cascades in epithelial cells. J. Biol. Chem. 280:23363-23370. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro-Neto, F., A. Leon, J. Urbani-Brocard, L. Lou, A. Nyska, and D. L. Altschuler. 2004. cAMP-dependent oncogenic action of Rap1b in the thyroid gland. J. Biol. Chem. 279:46868-46875. [DOI] [PubMed] [Google Scholar]
- 33.Rubinfeld, B., S. Munemitsu, R. Clark, L. Conroy, K. Watt, W. J. Crosier, F. McCormick, and P. Polakis. 1991. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell 65:1033-1042. [DOI] [PubMed] [Google Scholar]
- 34.Tsygankova, O. M., E. Feshchenko, P. S. Klein, and J. L. Meinkoth. 2004. Thyroid-stimulating hormone/cAMP and glycogen synthase kinase 3beta elicit opposing effects on Rap1GAP stability. J. Biol. Chem. 279:5501-5507. [DOI] [PubMed] [Google Scholar]
- 35.Tsygankova, O. M., A. Saavedra, J. F. Rebhun, L. A. Quilliam, and J. L. Meinkoth. 2001. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol. Cell. Biol. 21:1921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasko, V., M. Ferrand, J. Di Cristofaro, P. Carayon, J. F. Henry, and C. de Micco. 2003. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J. Clin. Endocrinol. Metab. 88:2745-2752. [DOI] [PubMed] [Google Scholar]
- 37.Verhoef, S., R. Vrtel, L. Bakker, I. Stolte-Dijkstra, M. Nellist, J. H. Begeer, J. Zaremba, S. Jozwiak, A. M. Tempelaars, D. Lindhout, D. J. Halley, and A. M. van den Ouweland. 1998. Recurrent mutation 4882delTT in the GAP-related domain of the tuberous sclerosis TSC2 gene. Hum. Mutat. Suppl. 1:S85-S87. [DOI] [PubMed] [Google Scholar]
- 38.Wang, H., and M. G. Kazanietz. 2002. Chimaerins, novel non-protein kinase C phorbol ester receptors, associate with Tmp21-I (p23). Evidence for a novel anchoring mechanism involving the chimaerin C1 domain. J. Biol. Chem. 277:4541-4550. [DOI] [PubMed] [Google Scholar]
- 39.Wang, Z., T. J. Dillon, V. Pokala, S. Mishra, K. Labudda, B. Hunter, and P. J. Stork. 2006. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol. Cell. Biol. 26:2130-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White, M. A., C. Nicolette, A. Minden, A. Polverino, L. Van Aelst, M. Karin, and M. H. Wigler. 1995. Multiple Ras functions can contribute to mammalian cell transformation. Cell 80:533-541. [DOI] [PubMed] [Google Scholar]
- 41.Williamson, C., A. A. Pannett, J. T. Pang, C. Wooding, M. McCarthy, M. N. Sheppard, J. Monson, R. N. Clayton, and R. V. Thakker. 1997. Localisation of a gene causing endocrine neoplasia to a 4 cM region on chromosome 1p35-p36. J. Med. Genet. 34:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, L., L. Chenwei, R. Mahmood, K. van Golen, J. Greenson, G. Li, N. J. D'Silva, X. Li, C. F. Burant, C. D. Logsdon, and D. M. Simeone. 2006. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 66:898-906. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Z., R. S. Mitra, B. S. Henson, N. S. Datta, L. K. McCauley, P. Kumar, J. S. Lee, T. E. Carey, and N. J. D'Silva. 2006. Rap1GAP inhibits tumor growth in oropharyngeal squamous cell carcinoma. Am. J. Pathol. 168:585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]