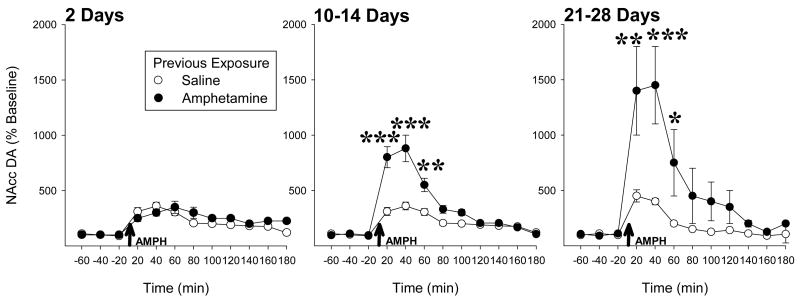
The past 30 years have witnessed a substantial and growing interest in drug sensitization, particularly relating to the psychoactive and appetitive properties of psychostimulant and opiate drugs. It is now well established that rats repeatedly exposed to amphetamine, for example, will exhibit an enhanced - sensitized - locomotor response when subsequently challenged with the drug some time later. It is also well established that in these rats, the ability of amphetamine to increase extracellular levels of dopamine (DA) in forebrain is also enhanced. There have been many reports of such effects with a number of abused drugs from these two drug classes (for references and critical reviews of the preclinical literature, see Kalivas and Stewart, 1991; Vanderschuren and Kalivas, 2000; Vezina, 2004). Of particular note, these effects are long lasting in the rat: up to one year following drug exposure for locomotion (Paulson et al., 1991) and up to three months for DA overflow (Hamamura et al., 1991) - the longest withdrawal periods tested. Indeed, amphetamine-induced nucleus accumbens DA overflow increases with time after exposure to the drug (Figure 1).
Figure 1.
Previous exposure to amphetamine sensitizes its ability to increase nucleus accumbens DA overflow in a time-dependent manner. Rats in different groups were administered five injections of amphetamine (1.0 mg/kg, i.p.) or saline, one injection every third day, and tested 2, 10–14 or 21–28 days later. On the test, in vivo microdialysis was used to estimate extracellular levels of DA in the nucleus accumbens before and after all rats were challenged with amphetamine (1.0 mg/kg, i.p.; arrows). Note that while no evidence for sensitization is evident 2 days following drug exposure, enhanced responding is greatest 21–28 days after exposure, the longest withdrawal period tested in this experiment. n/group=5–7. *, p<0.05, **, p<0.01, ***, p<0.001, significant differences between groups detected by Scheffé post hoc comparisons following ANOVA.
Given the critical role played by the mesoaccumbens DA pathways in the generation of appetitive behaviors including the pursuit and self-administration of abused drugs, it follows that altered activity in these pathways could affect the appetitive behavioral output of the organism. The incrementing and long lasting changes in nucleus accumbens DA overflow observed in rats following exposure to sensitizing drug regimens suggest that such effects on behavior can be long lasting. Such possibilities have fueled a large number of systems, cellular and molecular biological level investigations of the mechanisms that might underlie altered reactivity in midbrain DA neurons and those systems they interact with. On the other hand, while there is general agreement that drug sensitization can be demonstrated in some preparations, there has been less consensus as to whether and how sensitization might impact the generation of appetitive behaviors either within or between species.
This special issue highlights evidence obtained in rodents, non-human primates and humans that supports a role for sensitization in the generation of states that promote or are related to enhanced drug taking. This evidence is reviewed in the context of different data sets, interpretations and models from the clinical and pre-clinical literature with the goal of understanding and integrating sometimes apparently disparate views.
Drug addiction is characterized by compulsive drug taking that persists despite escalating costs and adverse consequences. Over the past 20 years, sensitization of the appetitive effects of drugs has emerged as a possible mechanism underlying enhanced drug use (Robinson and Berridge, 1993, 2003). However, a review of the pre-clinical literature reveals findings apparently for and against this view. It is now well established that manipulations leading to sensitization of locomotion and nucleus accumbens DA overflow also facilitate the acquisition of drug taking behaviors and increase the work animals will emit to self-administer a drug (Vezina, 2004). On the other hand, other procedures known to lead to an escalation of drug intake, such as providing rats with daily 6-h vs 1-h access to the drug, are not associated with sensitization of locomotion or nucleus accumbens DA overflow (Ahmed et al., 2003). Rather, this escalation in drug taking has been attributed to incremental recruitment of opponent processes (Koob and Le Moal, 1997). In their review of the effects of nicotine exposure, Vezina et al. (2007; this issue) note that the effects observed following drug exposure are determined by at least two interrelated factors: the intensity of the drug exposure regimen and the time of testing following exposure. They propose a view of drug addiction that integrates opponent-process and sensitization components of drug exposure, with the former observed soon after exposure to sufficiently intense drug regimens when withdrawal symptoms are prevalent, and the latter appearing long after exposure once drug withdrawal symptoms have abated. Roberts et al. (2007; this issue) explore further the conditions that lead to enhanced motivation to self-administer drug. Using the progressive ratio schedule of reinforcement, they found that cocaine self-administration using a number of access procedures does not in and of itself lead to enhanced work output. Interestingly, rats given access to binge-abstinence access procedures modeling patterns of cocaine use in humans do exhibit progressively increasing motivation to self-administer cocaine. These findings have important clinical implications because they begin to identify those characteristics of active drug exposure (as opposed to more commonly tested passive drug exposure) that lead to progressive increases in the motivation to seek and self-administer drugs.
The clinical literature supporting a role for sensitization in human drug use has also been equivocal. This has led to arguments that drug sensitization as a mechanism for drug abuse is of limited value as it does not extend to the human case. For example, imaging studies using PET and fMRI techniques have generally shown a profile in limbic regions of reduced rather than augmented responses in cocaine addicted patients compared to controls (Volkow et al., 1997). On the other hand, recent evidence from PET studies in humans suggests that individual differences in drug-induced dopamine release correlate positively with the personality trait of novelty seeking and drug-induced wanting, that acute dopamine depletion decreases drug craving and work emitted to obtain the drug, and that repeated amphetamine administration leads to increased dopamine release. In his review of these findings, Leyton (2007; this issue) proposes that the differential ability of human studies to demonstrate drug sensitization may reflect the effects of different drug exposure regimens and withdrawal periods in non drug abusing and drug abusing human subjects as well as the ability of drug-paired and drug-unpaired cues to differentially influence drug-induced DA responsivity in these two groups.
Caprioli et al. (2007; this issue) make a strong case for a critical contribution by non-pharmacological environmental factors to the effects of drugs. Correctly noting that drug exposure does not occur in a vacuum, they review evidence obtained mostly in rats supporting three major ways environmental conditions and cues can alter responsiveness to addictive drugs. These include the effects of prior adverse life experiences, the formation of associations between previously neutral cues and drug effects, and the impact of the environmental circumstances immediately surrounding exposure to a drug. The findings reviewed indicate that non-pharmacological environmental factors are amenable to study in animal models and exert complex effects. As such, they have important and far reaching implications for evaluating and understanding findings in human subjects previously exposed to sensitizing drug regimens.
Interestingly, the enhanced amphetamine-evoked DA release observed in humans previously exposed to the drug is not only quantitatively greater in ventral striatum but also extends to more dorsal striatal regions (Boileau et al., 2006). In their review, Porrino et al. (2007; this issue) explore and characterize this topographical shift in experiments using cerebral glucose utilization in non-human primates. They also found that chronic exposure to cocaine self-administration leads to a long lasting and orderly expansion of targeted regions from ventral to dorsal striatum and showed as well that a similar progression in magnitude and spatial extent occurs in cortical areas that project to these striatal regions. These findings suggest a growing influence during the sensitization process of drugs like cocaine in areas mediating the generation of drug taking behaviors as well as an expansion into cortical areas contributing to higher order cognitive processing.
A number of findings demonstrate that drug sensitization has long lasting effects on behavior and cognition that go beyond changes in locomotor activity and drug taking. In their review, Featherstone et al. (2007; this issue) present compelling evidence that the amphetamine-induced sensitized state models the positive symptoms and cognitive deficits observed in schizophrenia. They describe a number of rodent experiments showing that exposure to sensitizing amphetamine injection regimens produces reduced prepulse inhibition and disrupted latent inhibition as well as impairments in tasks that require sustained attention, extradimensional shifts and reversal learning. Given that schizophrenics show increased co-morbidity for drug abuse and that a sizable number develop this co-morbidity following the onset of the disease, it is conceivable that sensitized neuronal states underlying schizophrenia are responsible for increased predisposition for substance abuse in these individuals. Castner and Williams (2007; this issue) further evaluate and characterize the hallucinatory-like behavior and impaired higher cognitive function observed in nonhuman primates previously exposed to sensitizing regimens of amphetamine injections. They present evidence suggesting that the prefrontal cortex is a critical site for the disruption of D1 DA receptor signaling and cognitive function observed in amphetamine sensitized animals and that disruption of cognitive function may play a major role in increased vulnerability to drug addiction. On the basis of these and other findings, they argue that the specific targeting and repeated activation of prefrontal cortical D1 DA receptors may produce enduring benefits for those suffering from addiction.
Collectively, the evidence presented in this special issue indicates that drug sensitization is a potent and long lasting form of plasticity that can be observed in the rodent, non-human primate and human. This evidence supports the view that sensitization in brain DA pathways and those systems they interact with contributes importantly to drug addiction and psychopathology in animals and humans. Clearly sensitization is a complex form of plasticity. Understanding its impact on behavior will require continued consideration and evaluation of multiple output measures and different theories of motivated behavior as well as an appreciation for the contribution of non-pharmacological factors to the effects of drugs.
Acknowledgments
Supported by a grant (DA09397) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced accumbens dopamine levels. J Neurochem. 2003;86:102–13. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: A [11C]raclopride/PET study in healthy volunteers. Arch Gen Psychi. 2006;63:1386–95. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Badiani A. Modeling the role of environment in addiction. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.029. this issue. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV. From vice to virtue: Insights from sensitization in the nonhuman primate. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.026. this issue. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.025. this issue. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Akiyama K, Akimoto K, Kashihara K, Okumura K, Ujike H, Otsuki S. Co-administration of either a selective D1 or D2 dopamine antagonist with methamphetamine prevents methamphetamine-induced behavioral sensitization and neurochemical change, studied by in vivo intracerebral dialysis. Brain Research. 1991;546:40–6. doi: 10.1016/0006-8993(91)91156-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.027. this issue. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioural depression and persistent behavioural sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–92. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LK, Smith HR, Nader MA, Beveridge TJR. The effects of cocaine: A shifting target over the course of addiction. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.040. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Morgan D, Liu Y. How to make a rat addicted to cocaine. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.028. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Persp Neuro-Psychopharmacol Biol Psychi. 2007;31 doi: 10.1016/j.pnpbp.2007.08.038. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-F, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]