Abstract
Left-sided maternal cradling has been widely reported in human populations. In this paper, I review the evidence of laterality in maternal cradling and infant positional biases in non-human primates. The review revealed some evidence of population-left sided cradling in great apes but little consistency in bias was found among Old and New World monkeys. Very little data have been reported in prosimians. I further describe how asymmetries in either maternal cradling or infant positional biases may explain individual and species differences in hand preference.
Keywords: handedness, laterality, maternal cradling, nipple preferences
INTRODUCTION
There is considerable theoretical and empirical debate over the mechanisms that influence the development of handedness in humans, particularly right-handedness. Many genetic models have been proposed to explain both the development (Annett, 1985, 1999; Laland et al., 1995; McManus and Bryden, 1992; Yeo and Gangestad, 1993, 2002) and evolution of human right handedness (Corballis, 1997, 2002). In contrast to genetic models of handedness, others have proposed nongenetic models of handedness that largely focus on the role of early biological or environmental factors such as the position in utero of the infant (Previc, 1991), early orienting asymmetries (Michel, 1981), or social learning (Provins, 1997). In particular, it has been reported that the intrauterine environment is asymmetric in many different respects (Previc, 1991). Some of the asymmetries derive from maternal anatomy, whereas others appear to be intrinsic to the fetus itself. For example, developing fetuses as young as 3 mo gestation show pronounced right-sided asymmetries in thumb-sucking (Hepper et al., 1991). There is also evidence that the intrauterine position of the fetus during the last trimester of pregnancy correlates with head orienting asymmetries of the infant at birth (Hopkins and Rönnqvist, 1998). Thus, certain sources of lateralization in the prenatal environment, and particularly specific intrauterine factors, could influence the development of motoric lateralization such as handedness or early orienting asymmetries.
At present, there is no empirical evidence that purely supports either genetic or nongenetic models of human handedness. For example, although hand preferences clearly run in human families, no gene or set of genes has been linked to the expression of hand preference. Thus, the evidence of heritability of hand preference in humans does not necessarily mean that a genetic mechanism is the cause.
In contrast to human hand preference, historically laterality in other animals, and specifically nonhuman primates, has not been considered to be under genetic control. Warren (1980) championed this view, claiming that human hand preference is determined by genetic factors, while limb preferences of primates and rodents were acquired via nongenetic mechanisms, notably incidental learning and conditioning. Warren (1980) based his claim on the observation that hand or paw preference is bimodally distributed in nonhuman species, while human hand preferences are skewed to the right. The differences in the distributions of hand or paw preferences needed explanation, and Warren (1980) proposed that nongenetic factors account for nonhuman handedness, while genetic factors account for human handedness. Since Warren’s (1980) publication, numerous population-level asymmetries have been demonstrated in vertebrates (Rogers, 2002; Rogers and Andrew, 2002; Verstynen et al., 2001; Vallortigara and Bisazza, 2002). Even in rodents, previously believed to exhibit limb preferences only at the individual but not population-level, a weaker population level right-handedness has been reported (Waters and Denenberg, 1994; Tang and Verstynene, 2002). Recently, there have been reports of population-level hand preferences in nonhuman primates for a host of behavioral measures (Bradshaw and Rogers, 1993; Hook, in press; Hook-Costigan and Rogers, 1997; Hopkins, 1996; MacNeilage et al., 1987; Rogers and Andrew, 2002; Ward and Hopkins, 1993). The evidence of population-level asymmetries in nonhuman animals does not support Warren’s position that there are consistent differences in the distribution of hand preference between humans and nonhumans and therefore calls into question the basis for his arguments of differential mechanisms of expression. Further, evidence of population-level handedness does not need to be a prerequisite for the potential genetic basis for limb preferences.
Few researchers have examined biological or nonbiological factors that might influence the expression of hand or paw preferences in nonhuman species, which makes it difficult to evaluate whether similar mechanisms may influence the development of laterality in nonhuman species versus humans. Numerous studies suggest that directional biases in paw preferences cannot be selectively bred for in rodents (Collins, 1985; Signore et al., 1991; contra Bisazza et al., 2000; Waters and Denenberg, 1994) but can be easily modified by changing the biases of the environment in which the rodents are raised (Collins, 1975).
There is also evidence that rats will lever press with a specific paw based on the paw used by a model rat that they observed (Collins, 1988), which lends some support to theories proposing social learning as the means by which handedness in transmitted across generations. In nonhuman primates, there is some evidence that hand preferences run in families (Hopkins et al., 1994; Matoba et al., 1991) but not all researchers have reported significant associations (Boesch, 1991; Brooker et al., 1981; Byrne and Byrne, 1991; Westergaard and Suomi, 1997), and in most studies the independent effect of genetic from nongenetic factors cannot be easily dissociated (Hopkins, 1999; Hopkins et al., 2001). In other words, the evidence of heritability has mostly come from family studies in which no differential rearing or cross-fostering comparisons are made in the phenotypic expression of handedness. Taken together, the data suggest that hand preferences run in families but they do not strongly support a genetic basis for heritability of hand preference in nonhuman primates.
I proffer a developmental and evolutionary theory of handedness in primates that is based on the potential role that early mother-infant behaviors play in the development of individual and specific differences in hand preference. In particular, I argue that phylogenetic and individual differences in hand preference, including intra- and inter-familial variation, might be explained or facilitated by asymmetries in mother-infant interactions, specifically maternal cradling bias, infant head position and infant nipple preference. Some authors have emphasized that environmental and experiential factors, such as interactions with parents and students, could influence the direction and the strength of manual laterality in humans (Provins, 1997); however, an explicit description of the role of cradling has not been suggested to explain the development of human hand preference despite considerable research on the topic of lateral bias in maternal infant cradling.
Salk (1960) was the first to suggest that humans exhibit a left cradling bias for infants and the finding has been replicated in various human cultures (Damerose and Vauclair, 2002). Salk (1973) reported no association between maternal hand preference and maternal cradling bias, which may have led some to believe that the asymmetries in maternal cradling bias are due to biases related to expression of specific emotional states (Sieratzki and Woll, 1996). Whether this is the case or not is a matter of debate beyond the scope of this paper (Bencie and Sieratzki, 2002; Turnbull et al., 2001).
Instead I propose that asymmetries in the early mother-infant interaction may influence the development of hand preference in the offspring. I further argue that variation in mother-infant interactions between births can influence the concordance rates in offspring hand preference. Finally, I argue that a prolongation of interbirth-intervals in higher primates led to greater consistency in asymmetries in mother-infant interactions between offspring, which resulted in consistency in hand use within families.
Laterality for Maternal Cradling & Infant Positional Biases
Several investigators have reported evidence of laterality in cradling biases in nonhuman primates, which are summarized in Table I (Damerose and Vauclair, 2002). It was not always clear whether a distinction was made by some authors between maternal cradling and infant positional biases (Table I). Manning et al. (1994) studied the largest sample of great ape subjects (n = 52) and reported a left bias for infant cradling in chimpanzees and gorillas. For all great apes, 71% (67 of 95) showed a left-sided bias in cradling and/or infant position. Dienske et al. (1995) and Hopkins et al. (1993) could not completely replicate the findings by Manning and Chamberlain (1990) in chimpanzees, but the behavioral coding schemes differed between the studies and the sample sizes were much smaller. Manning and Chamberlain (1990) recorded the side bias of the infant (i.e., the infants’ head position on the mothers’ ventrum while Dienske et al. (1995) and Hopkins et al. (1993) explicitly recorded the active arm/hand cradling biases of the mother. In contrast to Dienske et al. (1995) and Hopkins (1993), Toback (1999) found a significant left-sided cradling bias in a sample of 14 chimpanzees, with 11 chimpanzees preferring the left hand and 3 preferring the right hand. Fisher et al. (1982) reported that 3 female gorillas tended to cradle their offspring on the left side, though there is no individual data in the paper. Rogers and Kaplan (1995) reported that 3 orangutans carried their infants on the right-side and 1 carried her offspring on the left side. When considering the data separately for each great ape species, only the chimpanzees (z = 2.83, p < .01) and gorillas (z = 3.83, p < .001) exhibit a population-level left side cradling bias. The results are less clear in orangutans and gibbons.
Table I.
Distribution of laterality in maternal cradling and infant positional biases in primates
| Author (s) | Genus | #L | #R | Condition |
|---|---|---|---|---|
| Maternal cradling/Infant position | ||||
| Manning et al. (1994)a | Gorilla | 13 | 2 | Captive |
| Pan | 16 | 4 | Captive | |
| Pongo | 4 | 4 | Captive | |
| Hylobates | 7 | 2 | Captive | |
| Lockard (1984) | Gorilla | 1 | 0 | Captive |
| Fischer et al. (1982) | Gorilla | 3 | 0 | Captive |
| Hopkins et al. (1993)b | Pan | 10 | 5 | Captive |
| Dienske et al. (1995) | Pan | 4 | 5 | Captive |
| Toback (1999) | Pan | 11 | 3 | Captive |
| Rogers and Kaplan (1995) | Pongo | 1 | 3 | Wild |
| Tanaka (1989) | Macaca | 3 | 5 | Captive |
| Tomaszycki et al. (1998) | Macaca | 21 | 20 | Captive |
| Westergaard et al. (1998) | Cebus | 12 | 4 | Captive |
| Fagot (1993) | Papio | 2 | 2 | Captive |
| Damerose and Hopkins (2002) | Papio | 7 | 3 | Captive |
| Nipple preference | ||||
| Nishida (1993) | Pan | 18 | 10 | Wild |
| Dienske et al. (1995) | Pan | 5 | 4 | Captive |
| Deets and Harlow (1970) | Macaca | 2 | 2 | Captive |
| Erwin et al. (1975) | Macaca | 18 | 28 | Captive |
| Tanaka (1989) | Macaca | 21 | 19 | Captive |
| Tomaszycki et al. (1998) | Macaca | 25 | 15 | Captive |
| Hiraiwa (1981) | Macaca | 7 | 6 | Captive |
| Fagot (1993) | Papio | 1 | 3 | Captive |
| Damerose and Hopkins (2002) | Papio | 7 | 2 | Captive |
| Rogers and Kaplan (1998) | Callithrix | 7 | 8 | Captive |
Note. observational conditions are coded as captive or wild but wild-caught individuals comprised many of the subjects that were observed in captivity. L (left) and R (right) refer to the position of the infant on the mother’s ventrum.
This includes data published by Manning and Chamberlain (1990).
This includes additional data not published in Hopkins et al. (1993).
In Old and New monkeys and prosimians, the data are less clear in terms of population-level asymmetries. Tanaka (1989) reported a bimodal distribution in the cradling bias of 20 Japanese macaque mother-infant dyads with 9 preferring the right hand and 11 preferring the left hand. Similarly, Tomaszycki et al. (1998) found no evidence of population-level cradling biases in a sample of 41 rhesus macaques. Twenty-one of them preferred to cradle with the left hand and 20 preferred the right. For a sample of 10 baboons, Damerose and Hopkins (2002) reported that 7 females had a left-side bias and 3 had a right-side bias. In New World monkeys, Rogers and Kaplan (1998) found no evidence of population-level asymmetries in infant carrying by either the mother or father. Finally, an unpublished report indicated that Galago and Otolemur exhibit nipple preferences (Pinger et al., 1988), while there is no preferences in Lemur catta (Ward, unpublished data).
Infant Nipple Preferences
In a sample of 32 wild chimpanzees, Nishida (1993) reported a left side bias in nipple preference with approximately 64% of the sample showing the pattern. Tomaszycki et al. (1998) examined nipple preference in 40 captive infant Macaca mulatta for the first 6 weeks of life. Of the 40 infants, 25 preferred the left nipple and 15 preferred the right. Overall, there was a left nipple preference, and the bias was particularly strong for the first 3 weeks. After 3 weeks, they preferred both nipples equally often. In contrast to Tomaszycki et al. (1998), Tanaka (1989) found no evidence for population-level nipple preferences in 20 captive, Japanese macaque mother-infant dyads over the first 6 mo of life. Moreover, Tanaka (1989) reported that the nipple preferences did not develop until after 3 weeks of life, a finding opposite those of Tomaszycki et al. (1998). Tanaka (1989) also reported that nipple preferences of the previous and subsequent infants alternated between birth seasons, though the cradling bias of the mother did not. Tanaka (1989) hypothesized that size differences in the nipple contributed to the alternation in nipple preference between infants. Like Tanaka (1989), Hiraiwa (1981) reported no evidence of population-level nipple preferences in a sample of 16 infant Macaca fuscata. Six of them preferred the right nipple, 7 preferred the left nipple, and 3 had no preference. Lindburg (1971) reported a population-level right nipple preference in a sample of 23 wild Macaca mulatta. Although he provided no individual data Lindburg (1971) reported that on 79% of observations, they contacted the right nipple. Erwin et al. (1975) examined nipple preferences in 56 Macaca nemestrina and reported that 82% showed a definitive nipple preference within the first month of life. Twenty-eight preferred the right nipple and 18 preferred the left, a difference that did not reach traditional levels of statistical significance (i.e., p < .05). Erwin et al. (1975) also reported anecdotal evidence of nipple preference in 3 sets of twins including 2 pairs of pig-tailed macaques and 1 pair of baboons. One pig-tailed macaque twin pair showed complementary nipple preference while for the other pair, both infants showed a preference for the same nipple. For the baboon twins, one infant showed a nipple preference and the other did not.
Deets and Harlow (1970) reported significant nipple preferences in 4 singleton and 4 sets of twin rhesus monkeys. For the 4 singleton offspring, 2 preferred the left nipple and 2 preferred the right. In all 4 sets of twins, members of the dyads had opposite nipple preferences. More recently, Rogers and Kaplan (1998) examined nipple preferences in 15 common marmosets derived from 6 sets of twins and 1 set of triplets. Among the twins, all 6 pairs displayed disconcordant nipple preferences with one infant preferring the left and one preferring the right nipple. Among the triplets, 2 preferred the right nipple and 1 preferred the left. There are significant positive correlations between parental carrying bias and nipple preferences. There is no relationship between teat preferences in relation to the offspring’s hand preference for touching objects and holding food, a result that differs from those reported by Hopkins et al. (1993) in chimpanzees. The data indicating opposite nipple preferences in twin marmosets are consistent with data in twin macaque monkeys (Deets and Harlow, 1970; Erwin et al., 1975; Nakamichi, 1983) and langurs (Winkler and Prestel, 1989), albeit with much smaller samples sizes in these latter species.
Summary and Potential Outcomes
There are two central findings based on the extant data on maternal cradling and infant nipple preferences in nonhuman primates. First, female chimpanzees and gorillas show a left-sided cradling bias. Great ape infants also appear to show a left-sided positional bias on the female, and it appears that wild infant chimpanzees show a left nipple preference. In contrast to Pongidae, Cercopithecinae, Platyrrhini and Prosimii show no population-level biases for maternal cradling. Moreover, infants from these 3 taxa appear to show no population-level positional biases or nipple preferences, though individual preferences are clearly evident. In short, there is some suggestion of taxonomic differences in maternal cradling bias, infant positional biases and infant nipple preferences. Secondly, maternal cradling bias is inversely correlated with offspring hand preferences in chimpanzees and capuchins but not in marmosets. To date, analysis of this potential association has not been conducted in a large sample of Old World monkeys but at least one report (Damerose and Hopkins, 2002) indicates that baboons resemble chimpanzees and capuchins. Finally, among twins, clear disconcordance in nipple preferences emerge in the offspring.
Two fundamental questions arise from these observations: 1) why do the biases exist? and 2) what is the potential outcome of this early bias?
Origins of Laterality in Maternal Cradling and Infant Positional Biases
It is not clear why the left-sided asymmetries in maternal cradling, infant position and nipple preference exists in some ape species—chimpanzee and gorilla—but not other primate species. In addition, it is not clear whether individual differences in nipple preferences, maternal cradling bias or infant position are derived from the female or the infant or some interaction between them. In other words, it is not clear whether an inherent bias by the mother is imposed on the offspring or the mother accommodates an inherent bias by the infant. Several possible hypotheses might explain these results. With respect to the taxonomic differences in early mother-infant biases, there are several possible explanations but the paucity of existing data makes any explanation speculative.
First, neonatal (birth to 90 days) chimpanzees have significantly stronger grips for the right hands and feet versus the left cheiridia (Fagot and Bard, 1995). In addition, neonatal chimpanzees show a right-sided bias in leading limb during locomotion (Chorazyna, 1976; Hopkins, Bard, and Griner, 1997, Cunningham, Forsythe, and Ward, 1989). The greater strength and postural development for right limbs may result in the infants holding onto the left side of the mother more than the right when engaged in ventro-ventral clinging. The inherent reflexive strength bias in motor development may lead to infants positioning themselves more so on mother’s left side. This could subsequently lead to a left nipple preference or left maternal cradling biases or both. The potential absence of these kinds of neonatal asymmetries, at the population-level, in other nonhuman primates would explain the bimodal distributions in maternal cradling biases and infant nipple preference. Unfortunately, there is no data on early strength or grasping asymmetries in neonatal Old or New World monkeys and prosimians.
In addition, neonatal chimpanzees show a right-sided bias in head orientation while sleeping (Hopkins and Bard, 1995). Thus, the natural position of the head is oriented to the right, which resembles human babies (Michel, 1981). The orientation bias could influence the development of motor skills or influence the behavior of the mother in specific ways that might bias their early environment. Specifically, the right-sided head orientation asymmetry could lead to differential stimulation in hand-eye coordination for the right hand and eye (Michel, 1981). For example, neonatal chimpanzees show a right-side bias in hand-to-mouth activity (Hopkins and Bard, 1993) which might be due to the head orientation asymmetry leading to easier access to the right versus to the left digits. In chimpanzees, asymmetries in hand-to-mouth and head orientation within the first 3 mo of life predict hand preferences at 3–5 yr (Hopkins and Bard, 2000). Similarly, Westergaard et al. (1998) reported that lateral bias in the position of the infants head when riding dorsally on the mother predicted subsequent hand preferences in simple reaching at 24 and 48 weeks of age in capuchins. Alternatively, the head orientation asymmetry by the baby may cause the mothers to cradle the offspring on one side or the other. For example, in humans head orientation of doll-babies influences cradling biases (Ginsburg et al., 1979). Dolls with heads oriented to the left are typically held on the right-side by the females while dolls with the head oriented to the right are cradled on the left-side by human subjects (contra Bundy, 1979; Saling and Tyson, 1981). A similar process may take place at a population-level in chimpanzees and gorillas, but not orangutans, Old and New World monkeys or prosimians. Measures of asymmetry of the kind that have been obtained in humans and chimpanzees are lacking for neonatal monkeys and prosimians
Of course, the extant data in nonhuman primates does not rule out the possibility that the left-sided bias in cradling is linked to the position of the heart, as originally proposed by Salk (1960, 1973). One problem with this explanation is that the position of the heart is shifted slightly to the left, in all primate species investigated, thus the Salk theory would only apply to great apes (and possibly humans). The extant data also does not rule out the possibility that left-sided cradling is mediated by a preference of females to view their offspring with the left eye (Manning and Chamberlain, 1991). Finally, it is possible that the asymmetries in cradling or nipple preferences are associated with the hand preference of the individual. Thus, for example, right-handed individuals may want to keep the right hand free for various tasks or locomotion, which would leave the left one available for cradling the offspring. In human subjects, Salk (1973) argued that hand preference was unrelated to infant cradling bias but there was no objective measure of hand preference in his sample. Notwithstanding, in Salk’s (1973) paper, the subjects responses to the question “Why did you cradle your baby on the left-side?” were illuminating. Right-handers justified left-cradling by the fact that it frees the right hand in case they needed to perform an action, like protecting the baby. In response to the same question, left-handers responded that they cradled on the left-side because the left hand was more sensitive and thus more appropriate to better feel any movement of the baby.
Potential Consequences of Maternal Cradling and Infant Positional Biases
Individual Differences in Hand Preference
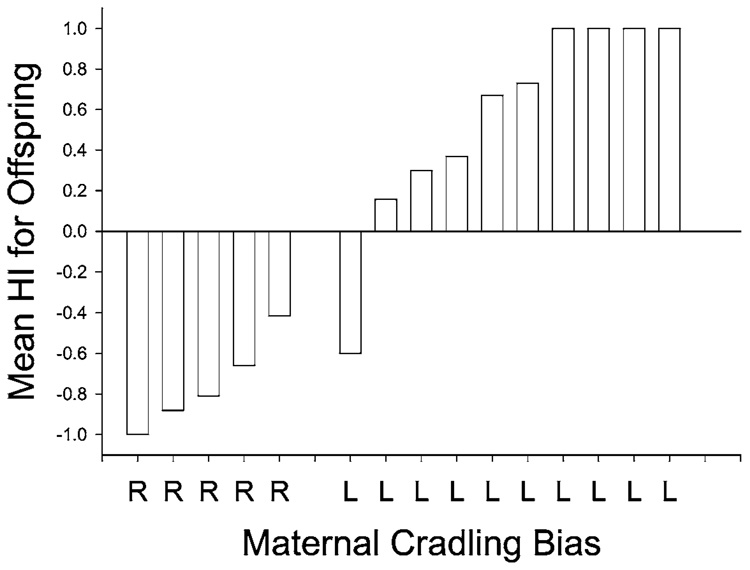
There are 3 studies on the association between early mother-infant interactions and the development of hand preferences in nonhuman primates. Hopkins et al. (1993) reported a negative association between maternal cradling bias and infant hand preferences for simple reaching. Depicted in Fig. 1 are the mean handedness scores for 15 offspring born to 10 females that preferred to cradle their offspring on the left and 5 that preferred to cradle on the right (Hopkins et al., 1993). We assessed maternal cradling bias for the first 2 weeks of life. We assessed infant hand preferences for simple reaching when the offspring were ca. 3 years old. With the exception one dyad, there is an inverse relationship between maternal cradling bias and infant hand preference. In other words, females that cradle their infants on the left side have right-handed offspring for simple reaching. In contrast, females that cradle their offspring on the right side have left-handed offspring for simple reaching. Interestingly, female hand preferences for 2 measures of hand use, including bimanual feeding and coordinated bimanual actions (Hopkins, 1994, 1995a,b), do not correlate with offspring hand preference. Thus, the maternal cradling bias appears to be the only variable that predicts the outcome of infant hand preference.
Fig. 1.
Mean HI for offspring handedness for simple reaching and female cradling biases in a sample of 15 chimpanzees. HI = handedness index and is derived following the formula HI = (#R−#L)/(#R+#L). Positive values reflect right-hand biases and negative values left-hand biases.
Westergaard et al. (1998) reported a similar finding in capuchins, though the measures were slightly different because capuchins carry offspring dorsally rather than ventrally, as in the case in many other primate species. Westergaard et al. (1998) recorded the head position of 14 infants during the first 2-weeks of life when riding dorsally on the backs of their mothers. At 24 and 48 weeks, they assessed hand preferences for simple reaching in the same monkeys. There is an inverse correlation between the lateral biases in head orientation and hand preference for reaching (Westergaard et al., 1998). In contrast, Rogers and Kaplan (1998) examined nipple preferences, maternal carrying and paternal cradling in adult marmosets and the handedness for holding food by the offspring at 5–8, 10–12 and 22 mo of age. There is no significant correlation between nipple preference, parental carrying biases and the offspring hand preferences. The findings from Rogers and Kaplan (1998) are not consistent with my theory, but it is important to recognize that paternal cradling in marmosets may induce some inconsistency in the development of offspring handedness, whereas it may not be the case in species in which the female is the sole caregiver for the infant. In my reanalysis of the Rogers and Kaplan (1998) data (Tables II and III), paternal carrying biases are negatively correlated with the offspring hand preferences at 5–8 mo (r = −.732, p < .05), 10–12 (r = −.626, p < .05) and 22 mo (r = −.581, p < .05). Maternal carrying biases did not strongly correlate with offspring hand preferences. The result cannot easily be attributed to differences in maternal and paternal carry rates because the percentage of time males carried the offspring was 45% for males versus 55% for the females. The result reinforces the notion that the role that males play in infant carrying in nonhuman primates may need special consideration in my model.
It is not clear what mechanism accounts for the associations between cradling bias or nipple preference and the development of hand preference. This is due, in part, to the fact that nipple preferences are mildly correlated with both cradling biases and carrying biases (Dienske et al., 1995; Rogers and Kaplan, 1998; Tomaszycki et al., 1998). A possible explanation may be that biases in head orientation, head position, nipple preference or cradling leads to differential stimulation of one hand or the other. One might tend to think that the development of hand preference would be contingent upon the hand that is free from the function of clinging but it is just as likely that the hand used to cling may receive greater motor and neurological stimulation than the free hand does.
Birth Order Effects
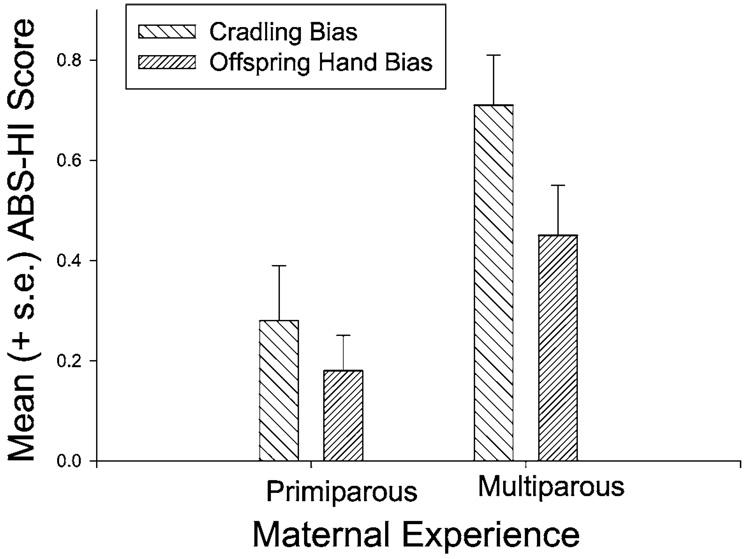
Hopkins and Dahl (2000) reported a significant association between birth order and hand preference in chimpanzees. The incidence of left-handedness is significantly higher in first- and latter-born (defined as chimpanzees with parities ≥6) versus middle-born chimpanzees (chimpanzees with parities between 2 and 5). The incidence of left-handedness in relation to birth order in chimpanzees is mirrored by higher incidences of either stillbirths or spontaneous abortions at the same parities (Hopkins et al., 2000). Moreover, the higher incidence of left-handedness in latter born chimpanzees is associated with low levels of estrogen during pregnancy, whereas the higher incidence left-handedness in first-born offspring is not. The finding suggests that the factor that influences left-handedness in latter-born chimpanzees may be associated with prenatal hormones while the increased incidence of left-handedness in first-borns may be due to other, unspecified perinatal events. A potential perinatal event may be inconsistent cradling or nipple preferences expressed by first-borns or primiparous females. For example, Tanaka (1997) reported that nipple preferences are less well developed in offspring born to primiparous females. Similarly, Tomaszycki et al. (1998) reported increased lateral bias in maternal cradling bias in association with increasing maternal experience. In chimpanzees, there is a similar trend with primiparous females showing less pronounced cradling biases versus those of multiparous females (Hopkins et al., 1993; Fig. 2). If we assume that early mother-infant experiences have a long-lasting effect on hand preference, then this observation may explain the higher incidence of non-right handedness in first-born offspring.
Fig. 2.
Mean absolute HI scores for cradling bias in primiparous and multiparous females. Mean absolute HI scores for reaching in offspring born to primiparous and multiparous females.
Heritability of Hand Preference in Full- and Maternal Half-siblings
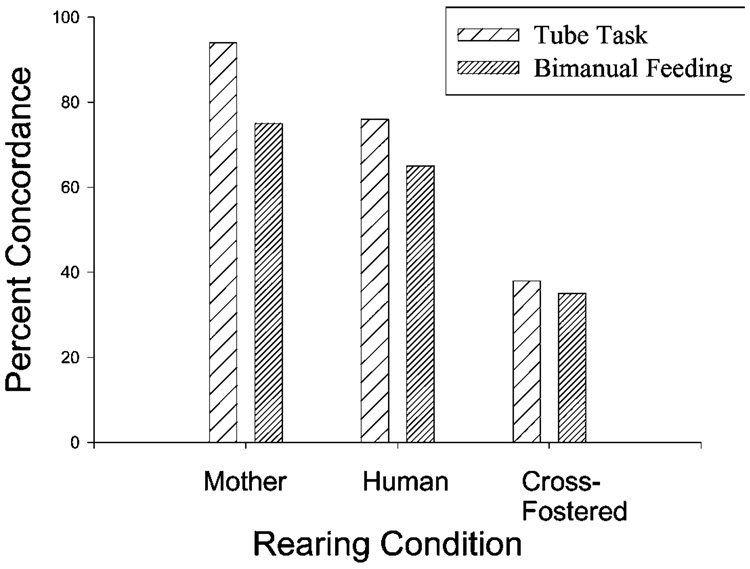
In captive chimpanzees, full- and maternal half-siblings raised in the same environment exhibit concordant hand preferences that are significantly greater than chance for ≥2 measures of hand preference (Hopkins, 1999; Fig. 3). The effects are not evident when considering paternal half-siblings, which suggests that the determining factor in hand preference is derived from the female. Moreover, there are non-significant or weakly significant concordances in hand preference between offspring and their biological parents, suggesting that the subjects are not acquiring their hand preferences by modeling the hand preferences of their parents. Significant concordance rates among siblings are not restricted to captive chimpanzees. Matsuzawa et al. (2001) reported that hand preference for nut-cracking behavior in 9 wild chimpanzee siblings dyads are perfectly concordant; 9 of 9 pairs had the same preference. Interestingly, maternal hand preference was not associated with offspring hand preference, and studies in captive chimpanzees.
Fig. 3.
Concordance in hand preference in full and maternal half-sibling chimpanzees for two separate measures of hand use including bimanual feeding and the TUBE task (Hopkins, 1994, 1995b).
An explanation for the finding of Matsuzawa et al. (2001) is that females show consistent hand preferences for maternal cradling biases or infant position or both for all offspring. Consistency on the part of the females’ maternal behavior would result in consistent hand preference among offspring. Preliminary data from our laboratory support this assumption. For 3 female chimpanzees, we assessed maternal cradling bias for 2 separate offspring and found consistent lateral biases in all 3 females. In other words, they preferred to cradle their offspring on the same side from one generation to the next.
This observation has implications for the comparison of hand preferences in other nonhuman primate species. Specifically, in some Old World monkey species infant nipple preferences alternate from one birth season to another. Tanaka (1989) proposed that the offspring alternate nipple preferences because of size differences in the female’s nipple at the time of birth of each new infant. In Old World monkeys, the older sibling is not always completely weaned from the female at the time of birth of a new offspring; thus, the nipple preferred by the older sibling remains larger than the non-preferred nipple. When the new infant is born, they presumably develop a preference for the previously, non-preferred nipple because it is smaller and easier to mouth.
If the nipple preferences/maternal cradling bias of the offspring facilitate the development of hand preference, then evidence of heritability of hand preference in non-ape species would mandate testing offspring from more than one generation. In fact, if this theoretical explanation is correct, then it would be predicted that heritability of hand preference among immediately adjacent Old or New World monkey siblings would be significantly disconcordant while significant concordances would be occur in alternating generations. This explanation assumes that each female gave birth on a yearly basis. For females that do not give birth yearly but instead bi-annually, adjacent siblings would likely be concordant for hand preference. Similarly, among some New World monkeys and prosimians in which twinning is common, a high degree of disconcordance in hand preference would be predicted among full- and maternal half-siblings because each would develop nipple preferences that would lead to opposite hand preferences (cf. Rogers and Kaplan, 1998). At present, there is evidence to support the hypothesis, but more data are needed. Milliken et al. (1989) reported that 2 of 3 pairs of ring-tailed lemur twins were disconcordant for hand preference. Further, Singer and Schwibbe (1999) reported that 6 of 7 tamarin twins were disconcordant for hand preference. Lastly, for species with long interbirth intervals and prolonged infancy, high rates of concordance in handedness like those in chimpanzees would be predicted.
Accordingly, significant association in hand preference is predicted for siblings but not necessarily between offspring and their biological parents. Predicting significant associations in hand preference between offspring and parents, particularly maternal effects, are contingent upon whether the females cradle or carry their offspring due to inherent biases in their hand preference. In chimpanzees, there is no association between female maternal cradling biases and their hand preference, nor is there a strong association between maternal and offspring hand preference (Hopkins, 1999).
Salk (1973) argued that hand preferences are unrelated to maternal cradling biases in humans (Salk, 1973). The lack of a significant association between maternal cradling bias and hand preference in humans and chimpanzees may not apply to other species and needs to be tested. Perhaps maternal cradling biases in other species are mediated by maternal hand preference, which would lead to the prediction of significant positive associations between both maternal and offspring hand preference as well as significant concordance rates among siblings.
Explaining Possible Specific Differences in the Evolution of Hand Preference
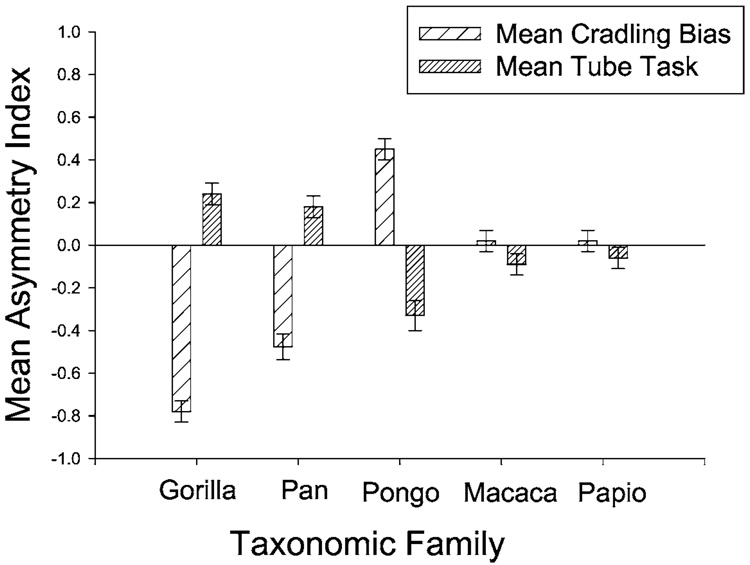
The suggestive evidence of taxonomic differences in cradling biases and/or nipple preferences may explain taxonomic differences in the expression of hand preference if it is assumed that the early mother-infant interactions have long-lasting effects on the development of hand use. Species that show left-sided cradling biases tend have more right-sided biases for hand use (Fig. 4). In contrast, in species that cradle on the right or show no cradling bias, hand preferences are skewed to the left or do not show a bias. The mechanism for individual expression of hand preference is the same in all species, i.e., early mother-infant interactions. That which varies between species is the degree to which population-level asymmetries are manifest for each behavioral trait, i.e., cradling versus hand preference. The theoretical prediction that I propose is that primate species that show population-level asymmetries in early mother-infant behaviors, such as cradling, nipple preferences or head position, will similarly show population-level hand preferences. The direction of the population-level asymmetries maternal in cradling and offspring hand use would be opposite in direction.
Fig. 4.
Mean cradling and handedness scores for 6 different primate species tested on the TUBE task.
Conclusions and Implications for Human Handedness and Cradling
Current evidence suggests that asymmetries in early mother-infant interactions are evident in nonhuman primates. There is good evidence for a left-side asymmetry in maternal cradling in gorillas and chimpanzees. There are too few studies in other apes, Old and New World monkeys and prosimians to evaluate population-level asymmetries in early mother-infant interactions, but individual biases are clearly evident and may have an impact on the development of handedness in their offspring. Notwithstanding these limitations, there are several general implications of the nonhuman primate findings for understanding human lateral bias in cradling and other behaviors.
Cradling
Because, the left-sided bias observed in some great apes, particularly those most closely-related to humans, is consistent with maternal cradling biases in human populations (Salk, 1973); the bias in humans might have strong evolutionary origins with other primate species. Why the cradling is left-sided is unclear but the original hypothesis of Salk (1973) does not seem warranted given the data in nonhuman primates. Specifically, if displacement of the heart to the left side was the sole reason for cradling on the left side, then all primates should show the bias because they all have hearts displaced slightly leftward in the thorax. Whether the bias is due to specialization of the right hemisphere in regulating infant affect, which results in a left-eye preference, as has been proposed in humans, remains unclear (Manning and Chamberlain, 1991). Several studies suggest that monkeys prefer to view emotionally relevant stimuli with the left eye (Ifune et al., 1984; Rogers et al., 1994), but none of them included tests of the preference in the context of mother-infant preferences.
Secondly, from an evolutionary perspective, the overwhelming lack of paternal influence in raising infants in many nonhuman primate species may explain the well-documented sex differences in lateral bias for infant cradling in humans. Several studies in humans showed that females have a left-sided cradling bias while males do not (Damerose and Vauclair, 2002). If we assume that a similar sex difference is evident in chimpanzees and gorillas, which would be difficult to determine because most males show little infant cradling, then whatever social or genetic factors selected for left-sided cradling was retained in human evolution as manifest in the extant sex differences seen in modern humans. Social factors do not appear to be a likely explanation because males with considerable experience raising children still do not show a left-sided cradling bias (Bundy, 1979), thus, genetic or other nongenetic mechanisms seem more likely.
Thirdly, I found no published study on infant nipple preferences in women who chose to breast-feed their offspring. Many women, particularly primiparous individuals, have difficulty in breast-feeding, and one apparent problem is that the infants show nipple preferences that must be overcome with time in order to have lactational let-down for both breasts (Stables and Hewitt, 1995). Multiparous females are less prone to these problems but still report that strong infant head preferences can create or contribute to unilateral breast-feeding problems. Our understanding of these issues is anything but clear, but additional research seems warranted in order to understand how these factors are related and whether they can have an influence on the development of handedness in their offspring. The descriptive data suggest that early mother-infant asymmetries are being derived from infant head orientation.
Handedness
My model does not rule out any of the existing genetic models of handedness, but it suggests that the potential genetic mechanisms may be acting on different behaviors or that gene-environment interactions may explain handedness. One interpretation from the data is that the heritable trait is for the arm/hand used for infant cradling or early infant postural or positional behaviors, not offspring handedness in relation to parental handedness. There is evidence that cradling bias runs in families (Manning and Denman, 1994) and that the effects are stronger for maternal-female offspring associations than maternal-male offspring, paternal-female or paternal-male offspring associations. As with all existing genetic models, the pattern of heritability for cradling bias can be explained by nongenetic factors, such as social learning; however, if we assume that a gene (or set of genes) is at work, then the gene would be acting on the cradling biases between mothers and their daughters, i.e., sex specific.
As an alternative, the model may be part of a gene-environment interaction that results in heritability in handedness. For example, Deng and Rogers (2002) described a gene-environment interaction model to explain the development of visual preference in chicks. Accordingly, genes that encode for left or right morphological and embryonic positional features interact with exposure to light, which influences the development and lateralized functions of the visual system. In humans, and perhaps in other primates, genes that code for left or right morphological characteristics influence the eventual cradling or positional biases expressed by the infants. One example might be left-sided genetic coding for the position of the heart which results in the offspring being cradled on the left side. This subsequently results in the offspring developing right-handedness. Variation in the timing and duration of exposure to the early positional biases, including cradling, would presumably influence the adult manifestation of handedness in different primates.
The model that I propose also addresses or potentially explains several aspects of human handedness that are often interpreted as supporting a purely genetic explanation. First, human right-handedness is often claimed to be a universal human trait, present in all cultures (Lockard et al., 1979). Interestingly, left-sided cradling is also often considered a universal human trait. Thus, perhaps the 2 oppositely lateralized human behaviors co-evolved in a complementary manner. Secondly, handedness studies in twins have often reported higher incidences of left-handedness and disconcordant handedness compared to siblings who are not twins. The evidence in nonhuman primates clearly shows that nipple preferences are highly disconcordant in twins and, at least some preliminary data suggest that hand preference in nonhuman primate twins are often disconcordant. Thus, the model can explain some phenotypic variation in handedness in human and nonhuman primates. Thirdly, in behavioral genetic studies of handedness in humans, the mother effect is often reported. The is the evidence that there is a higher proportion of right-handed offspring born to mixed-handed parents when the mother is right-handed and the father is left-handed versus the opposite conditions. If right-handed mothers cradle their offspring on left side and left-handed mother do so less often, then this could explain the mother effect.
SUMMARY
In summary, the proposed theory links the development and evolution of handedness in primates to early lateral biases in mother-infant relations. Rather than consider handedness as a uniquely human attribute with links to the evolution of bipedalism, language or other allegedly uniquely human attributes (see Bradshaw & Rogers, 1993), this theory proposes that the mechanisms that govern the expression of handedness in primates are the same and is strongly linked to asymmetries that are present during early mother-infant interactions that are common to many primate species (e.g., cradling or offspring transport, nipple preference and consequential head orientation). Species and individual differences that are observed in various primates can be explained by characteristics of this early mother-infant interactions and how they related to social and ecological variables.
ACKNOWLEDGEMENT
This work was supported in part by NIH grants RR-00165, NS-42867, NS-36605 and HD-38051. I thank Eric Damerose and Dr. Jacques Vauclair for discussion of various aspects of the paper. The comments of two anonymous reviews are also greatly appreciated. Correspondence and reprint requests should be addressed to Dr. William Hopkins, Division of Psychobiology, Yerkes National Primate Research Center, 954 Gatewood Road, Atlanta, Georgia 30322. E-mail: Lrcbh@rmy.emory.edu or whopkins@berry.edu.
REFERENCES
- Annett M. Left, Right, Hand, and Brain: The Right-Shift Theory. London: Erlbaum; 1985. [Google Scholar]
- Annett M. Handedness and cerebral dominance: The right shift theory. J. Neuropsychiatr. 1999;10:459–469. doi: 10.1176/jnp.10.4.459. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Facchin L, Vallortigara G. Heritability of lateralization in fish: Concordance of right-left asymmetry between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. [DOI] [PubMed] [Google Scholar]
- Bencie W, Sieratzki JS. Leftward cradling bias, prosodic speech and deafness: The deaf are not dumb. J. Genet. Psychol. 2002;163:126–128. doi: 10.1080/00221320209597973. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. Int. J. Primatol. 1991;6:541–558. [Google Scholar]
- Bradshaw J, Rogers LJ. The Evolution of Lateral Asymmetries, Language, Tool use and Intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Bundy RS. Effects of infant head position on side preference in adult handling. Infant Behav. Dev. 1979;2:355–358. [Google Scholar]
- Brooker RJ, Lehman RA, Heinbuch RC, Kidd KK. Hand usage in a colony of bonnet monkeys (Macaca radiata) Behav. Genet. 1981;11:49–56. doi: 10.1007/BF01065827. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Chorazyna H. Shifts in laterality in a baby chimpanzee. Neuropsychologia. 1976;14:381–384. doi: 10.1016/0028-3932(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychol. Rev. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From Hand to Mouth: The Origins of Language. Princeton, NJ: Princeton University Press; 2002. [Google Scholar]
- Collins RL. When left-handed mice live in a right handed world. Science. 1975;187:181–184. doi: 10.1126/science.1111097. [DOI] [PubMed] [Google Scholar]
- Collins RL. On the inheritance of direction and degree of asymmetry. In: Glick S, editor. Cerebral Lateralization in Non-Human Species. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Collins RL. Observational learning of a left-right asymmetry by mice (Mus musculus) J. Comp. Psychol. 1988;102:222–224. doi: 10.1037/0735-7036.102.3.222. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Forsythe C, Ward JP. A report of behavioral lateralization in an infant orangutan. Primates. 1989;30:249–253. [Google Scholar]
- Damerose E, Hopkins WD. Scan and focal sampling: reliability in the maternal cradling and infant nipple preferences of olive baboons, Papio anubis. Anim. Behav. 2002;63:511–518. [Google Scholar]
- Damerose E, Vauclair J. Posture and laterality in human and non-human primates: Asymmetries in maternal handling and infant’s early motor asymmetries. In: Rogers L, Andrew RJ, editors. Comparative Vertebrate Lateralization. Oxford: Oxford University Press; 2002. pp. 306–362. [Google Scholar]
- Deets AC, Harlow HF. Nipple preferences in nursing singleton-and twin-reared rhesus monkey infants. Dev. Psychol. 1970;2:159–162. [Google Scholar]
- Deng C, Rogers LJ. Factors affecting the development of lateralization in chicks. In: Rogers LJ, Andrew JR, editors. Comparative Vertebrate Lateralization. Cambridge: Cambridge University Press; 2002. pp. 206–246. [Google Scholar]
- Dienske H, Hopkins B, Reid AK. Lateralisation of infant holding in chimpanzees: new data do not confirm previous findings. Behaviour. 1995;132:801–809. [Google Scholar]
- Erwin J, Anderson B, Bunger D. Nursing behavior of infant pigtail monkeys (Macaca nemestrina): Preferences for nipples. Percept. Mot. Skills. 1975:592–594. doi: 10.2466/pms.1975.40.2.592. [DOI] [PubMed] [Google Scholar]
- Fagot J, Bard KA. Asymmetric grasping response in neonate chimpanzees (Pan troglodytes) Infant Behav. Dev. 1995;18:253–255. [Google Scholar]
- Fischer RB, Meunier GF, White PJ. Evidence of laterality in the lowland gorilla. Percept. Mot. Skills. 1982;54:1093–1094. [Google Scholar]
- Ginsburg HJ, Fling S, Hope ML, Musgrove D, Andrews C. Maternal holding preferences: a consequence of newborn head-turning response. Child Dev. 1979;50:280–281. [PubMed] [Google Scholar]
- Hepper PG, Shahidullah S, White R. Handedness in the human fetus. Neuropsychologia. 1991;29:1107–1111. doi: 10.1016/0028-3932(91)90080-r. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M. Maternal and alloparental care in a troop of free-ranging Japanese monkeys. Primates. 1981;22:309–329. [Google Scholar]
- Hook MA. The evolution of lateralized motor functions. In: Rogers L, Kaplan G, editors. Comparative Vertebrate Cognition. New York: Kluwer; pp. 325–370. (in press) [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. Int. J. Comp. Psychol. 1997;9:173–207. [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic factors. Dev. Psychobiol. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences in juvenile chimpanzees: Continuity in development. Dev. Psychol. 1995a;31:619–625. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. J. Comp. Psychol. 1995b;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 54 years since Finch (1941) Psychonom. Bull. Rev. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Heritablity of hand preference in chimpanzees: Evidence from a partial interspecies cross-fostering study. J. Comp. Psychol. 1999;113:307–313. doi: 10.1037/0735-7036.113.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bales S, Bennett AJ. Heritability in direction of hand preference in chimpanzees (Pan troglodytes) Int. J. Neurosci. 1994;74:17–26. doi: 10.3109/00207459408987225. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Hemispheric specialization in infant chimpanzees (Pan troglodytes): Evidence for a relation with gender and arousal. Dev. Psychobiol. 1993;26:219–235. doi: 10.1002/dev.420260405. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Evidence of asymmetries in spontaneous head turning in infant chimpanzees (Pan troglodytes) Behav. Neurosci. 1995;109:808–812. doi: 10.1037//0735-7044.109.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. A longitudinal study of hand preference in chimpanzees (Pan troglodytes) Dev. Psychobiol. 2000;34:292–300. [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA, Griner KM. Locomotor adaptation and leading limb asymmetries in neonatal chimpanzees (Pan troglodytes) Int. J. Primatol. 1997;18:104–114. [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Curr. Anthropol. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Dahl JF. Birth order and hand preference in chimpanzees (Pan troglodytes): Implications for pathological models of handedness in humans. J. Comp. Psychol. 2000;114:302–306. doi: 10.1037/0735-7036.114.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Birth order and left-handedness revisited: Some recent findings in chimpanzees (Pantroglodytes) and their implications for developmental and evolutionary models of human handedness. Neuropsychologia. 2000;38:1626–1633. doi: 10.1016/s0028-3932(00)00068-3. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): Evidence in support of the right-shift theory and developmental instability. Psychol. Sci. 2001;12:299–303. doi: 10.1111/1467-9280.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes. A review of findings. Int. J. Primatol. 1993;14:1–25. [Google Scholar]
- Hopkins B, Ronnqvist L. Human handedness: Developmental and eolutionary perspectives. In: Simon F, Butterworth G, editors. The Development of Sensory, Motor and Cognitive Capacities of Early Infancy: From Perception to Cognition. East Sussex, UK: Psychology Press; 1998. pp. 191–236. [Google Scholar]
- Ifune CK, Vermeire BA, Hamilton CR. Hemispheric differences in split-brain monkeys and responding to videotape recordings. Behav. Neural Biol. 1984;41:231–235. doi: 10.1016/s0163-1047(84)90639-3. [DOI] [PubMed] [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene-culture model of human handedness. Behav. Genet. 1995;25:433–445. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- Lindburg DG. The rhesus monkey in North India: An ecological and behavioral study. In: Rosenblum LA, editor. Primate Behavior: Developments in Field and Laboratory Research. Vol. 2. New York: Academic Press; 1971. pp. 1–106. [Google Scholar]
- Lockard J. Handedness in a captive group of lowland gorillas. Am. J. Primatol. 1984;13:356. [Abstract] [Google Scholar]
- Lockard JS, Daley PC, Gunderson V. Maternal and paternal differences in infant carrying: US and African data. Am. Naturalist. 1979;113:235–246. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav. Brain Sci. 1987;10:247–303. [Google Scholar]
- Manning JT, Chamberlain AT. The left-side cradling preference in great apes. Anim. Behav. 1990;39:1224–1227. [Google Scholar]
- Manning JT, Chamberlain AT. Left-side cradling and brain lateralisation. Ethol. Sociobiol. 1991;12:237–244. [Google Scholar]
- Manning JT, Denman J. Lateral cradling preferences in humans (Homo sapiens): Similarities within families. J. Comp. Psychol. 1994;108:262–265. doi: 10.1037/0735-7036.108.3.262. [DOI] [PubMed] [Google Scholar]
- Manning JT, Heaton R, Chamberlain AT. Left-side cradling: Similarities and differences between apes and humans. J. Hum. Evol. 1994;26:77–83. [Google Scholar]
- Matoba M, Masataka N, Tanioka Y. Cross-generational continuity of hand use preference in marmosets. Behaviour. 1991;117:281–286. [Google Scholar]
- Matsuzawa T, Biro D, Humle T, Inoue-Nakamura N, Tonooka R, Yamakoshi G. Emergence of culture in wild chimpanzees: Education by master-apprenticeship. In: Matsuzawa T, editor. Primate Origins of Human Cognition and Behavior. Tokyo: Springer; 2001. pp. 557–574. [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of Neuropsychology. Vol 6. Developmental Neuropsychology, Part 1. Amsterdam: Elsevier; 1992. pp. 115–144. [Google Scholar]
- Michel GF. Right-handedness: A consequence of infant supine head-orientation preference? Science. 1981;212:685–687. doi: 10.1126/science.7221558. [DOI] [PubMed] [Google Scholar]
- Milliken GW, Forsythe C, Ward JP. Multiple measures of hand use in the ring-tailed lemur (Lemur catta) J. Comp. Psychol. 1989;103:262–268. doi: 10.1037/0735-7036.103.3.262. [DOI] [PubMed] [Google Scholar]
- Nakamichi M. The left-sided holding preference is not universal: Evidence from field observations in Madagascar. Ethol. Sociobiol. 1996;17:173–179. [Google Scholar]
- Nishida T. Left nipple suckling preference in wild chimpanzees. Ethol. Sociobiol. 1993;14:45–52. [Google Scholar]
- Pinger C, Lavallee K, Hobbes F, Ward JP. An evaluation of lateralization in infant prosimian primates; Paper presented at the annual meeting of the Canadian Ethological Society; Toronto, Canada. 1988. [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Provins KA. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychol. Rev. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. [DOI] [PubMed] [Google Scholar]
- Rogers LJ. Lateralization in vertebrates: Its early evolution, general pattern, and development. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, editors. Advances in the Study of Behavior. Vol. 31. San Diego: Academic Press; 2002. pp. 107–161. [Google Scholar]
- Rogers LJ, Andrew RJ. Comparative Vertebrate Lateralization. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans, Pongo pygmaeus pygmaeus. Anim. Behav. 1995;51:13–25. [Google Scholar]
- Rogers LJ, Kaplan G. Teat preference for suckling in common marmosets: Relationship to side of being carried and hand preference. Laterality. 1998;3:269–281. doi: 10.1080/713754301. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Ward JP, Stafford D. Eye dominance in the small-eared bushbaby, Otolemur garnetti. Neuropsychologia. 1994;32:257–264. doi: 10.1016/0028-3932(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Salk L. The effects of normal heartbeat sound on the behavior of the newborn infant: implications for mental health. World Ment. Health. 1960;12:168–175. [Google Scholar]
- Salk L. The role of the heartbeat in the relations between mother and infant. Sci. Am. 1973;228:24–29. doi: 10.1038/scientificamerican0573-24. [DOI] [PubMed] [Google Scholar]
- Sieratzki JS, Woll B. Why do mothers cradle their babies on the left? Lancet. 1996;347:1746–1748. doi: 10.1016/s0140-6736(96)90813-2. [DOI] [PubMed] [Google Scholar]
- Signore P, Chaoui M, Nosten-Bertrand M, Perez-Diaz F, Marchaland C. Handedness in mice: Comparison across 11 different strains. Behav. Genet. 1991;21:421–429. doi: 10.1007/BF01065977. [DOI] [PubMed] [Google Scholar]
- Singer S, Schwibbe MH. Right or left, hand or mouth: genera-specific preferences in marmosets and tamarins. Behaviour. 1999;136:119–145. [Google Scholar]
- Stables D, Hewitt G. The effect of lateral asymmetries on breast feeding skills: Can midwives ‘ holding interventions overcome unilateral breast feeding problems. Midwifery. 1995;11:28–36. doi: 10.1016/0266-6138(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Tanaka I. Change of nipple preference between successive offspring in Japanese macaques. Am. J. Primatol. 1989;18:321–325. doi: 10.1002/ajp.1350180406. [DOI] [PubMed] [Google Scholar]
- Tanaka I. Parity-related differences in suckling behavior and nipple preference among free-ranging Japanese macaques. Am. J. Primatol. 1997;42:331–339. doi: 10.1002/(SICI)1098-2345(1997)42:4<331::AID-AJP8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tang AC, Verstynen T. Early life environment modulates ‘handedness’ in rats. Behav. Brain Res. 2002;131:1–7. doi: 10.1016/s0166-4328(01)00330-8. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Stein L, Lucas MD. Lateral preferences in adult embracing: A test of the “hemispheric asymmetry” theory of infant cradling. J. Genet. Psychol. 1995;156:17–21. [Google Scholar]
- Turnbull OH, Rhys-Jones SL, Jackson AL. The leftward cradling bias and prosody: An investigation of cradling preferences in the deaf community. J. Genet. Psychol. 2001;162:178–186. doi: 10.1080/00221320109597959. [DOI] [PubMed] [Google Scholar]
- Toback E. Behavioral laterality in chimpanzees. University of Stirling; 1999. Unpublished doctoral dissertation. [Google Scholar]
- Tomaszycki M, Cline C, Griffin B, Maestripieri D, Hopkins WD. Maternal cradling and infant nipple preferences in rhesus monkeys (Macaca mulatta) Dev. Psychobiol. 1998;32:305–312. doi: 10.1002/(sici)1098-2302(199805)32:4<305::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Bisazza A. How ancient is brain lateralization? In: Rogers LJ, Andrew JR, editors. Comparative Vertebrate Lateralization. Cambridge, UK: Cambridge University Press; 2002. pp. 9–69. [Google Scholar]
- Verstynen T, Tierney R, Urbanski T, Tang A. Neonatal novelty exposure modulates hippocampal volumetric asymmetry in the rat. Neuroreport. 2001;12(14):3019–3022. doi: 10.1097/00001756-200110080-00008. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate Laterality: Current Behavioral Evidence of Primate Asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol. Psychol. 1980;8:351–359. [Google Scholar]
- Waters NS, Denenberg VH. Analysis of two measures of paw preference in a large population of inbred mice. Behav. Brain Res. 1994;63:195–204. doi: 10.1016/0166-4328(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Winkler P, Prestel H. Twins in free-ranging Hanuman langurs (Presbytis entellus) Primates. 1989;30:255–259. [Google Scholar]
- Westergaard GG, Byrne G, Suomi SJ. Early lateral bias in tufted capuchins (Cebus apella) Dev. Psychobiol. 1998;32:45–50. doi: 10.1002/(sici)1098-2302(199801)32:1<45::aid-dev5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Westergaard GG, Suomi SJ. Lateral bias in capuchin monkeys (Cebus apella): Concordance between parents and offspring. Dev. Psychobiol. 1997;31:143–147. [PubMed] [Google Scholar]
- van Lawick-Goodall J. Mother-offspring relationships in free-ranging chimpanzees. In: Morris D, editor. Primate Ethology. Chicago: Aldine; 1967. pp. 287–346. [Google Scholar]
- Yeo R, Gangsted SW. Developmental origins of variation in human hand preference. Genetica. 1993;89:281–296. [Google Scholar]
- Yeo RA, Gangestad SW. Hand preference and developmental instability. Psychobiology. 2002;21:161–168. [Google Scholar]