Abstract
Rationale
Dopaminergic neurotransmission is critically involved in many aspects of complex behavior and cognition beyond reward/reinforcement and motor function. Mental and behavioral disorders associated with major disruptions of dopamine neurotransmission, including schizophrenia, Attention Deficit/Hyperactivity Disorder, Parkinson’s Disease, Huntington’s Disease and substance abuse, produce constellations of neuropsychological deficits in learning, memory and attention in addition to other defining symptoms.
Objective
To delineate the role dopaminergic D1-like and D2-like receptor subtypes play in complex brain functions.
Methods
Monkeys (N=6) were trained on cognitive tests adapted from a human neuropsychological assessment battery (CANTAB; CAmbridge Neuropsychological Test Automated Battery). The battery included tests of spatial working memory (self-ordered spatial search task, SOSS), visuo-spatial associative memory and learning (visuo-spatial paired associates learning task, vsPAL) and motivation (progressive ratio task, PR). Tests of motor function (bimanual motor skill task, BMS; rotating turntable task, RTT) were also included. Effects of the dopamine D2-like antagonist raclopride (10-56 μg/kg, i.m.) and the D1-like antagonist SCH23390 (SCH; 3.2-56 μg/kg, i.m.) on cognitive performance were then determined.
Results
Deficits on PR, RTT and BMS performance were observed after both raclopride and SCH23390. Spatial working memory accuracy was reduced to a greater extent by raclopride than by SCH which was unexpected, given prior reports on the involvement of D1 signaling for spatial working memory in monkeys. Deficits were observed on vsPAL performance after raclopride, but not after SCH23390.
Conclusions
The intriguing results suggest a greater contribution of D2-like over D1-like receptors to both spatial working memory and object-location associative memory.
Keywords: Alzheimer’s Disease, Parkinson’s Disease, Substance Abuse, Working Memory, ADHD, Motor Function, Aging
Introduction
Dopaminergic signaling in the central nervous system (CNS) plays a clear role in many aspects of complex behavior and cognition. Mental and behavioral disorders associated with major disruptions of dopamine (DA) neurotransmission include normal aging, schizophrenia, Attention Deficit/Hyperactivity Disorder (ADHD), Parkinson’s Disease (PD), Huntington’s Disease (HD) and substance abuse. These conditions are associated with neuropsychological impairments of learning, memory, sustained attention and motor function, and these deficits are thought to be related to alterations in dopamine neurotransmission. Positive associations of cognitive performance with dopamine transporter (DAT), dopamine D2-like and/or D1-like receptor binding in the striatum, have been reported in PD (Duchesne et al. 2002; Muller et al. 2000), HD (Backman et al. 1997; Lawrence et al. 1998) and in methamphetamine abusers (Volkow et al. 2001b). Dopamine receptor binding in prefrontal cortical regions is also correlated with cognitive performance on tasks of executive function in PD (Rinne et al. 2000). Reduced orbito-frontal activity has been correlated with low D2-like receptor binding in the striatum of methamphetamine abusers (Volkow et al. 2001a).
Neuropsychological deficits of learning, memory and attention were not originally considered primary therapeutic targets in pharmacological treatments in these disorders. Success in treating primary symptoms pharmacologically (i.e., motor function in PD, hallucinations/delusions in schizophrenia, etc) has permitted a current focus on mnemonic and attentional symptoms. The relative lack of treatment success with traditional approaches in substance abuse has driven research to study attentional and associative mechanisms that may be co-opted by drug exposure. Further understanding of the role dopaminergic signaling plays in aspects of complex behavior and neuropsychological brain functioning may assist with the development of novel therapeutic measures. The importance of understanding mechanisms subserving higher cognitive function is important well beyond traditional mental disorders. For example relative attentional, learning and executive inability, as opposed to outright dysfunction may have a significant effect on the performance of physicians (Perry and Crean 2005).
Given the diversity of cognitive and behavioral impairments associated with disrupted DA neurotransmission in a variety of clinical populations, developing nonhuman models to assess D1 and D2 receptor functions on a range of well-defined cognitive tasks is of substantial importance. Previous studies of the role of dopaminergic signaling in nonhuman primate behavioral models have primarily focused on reinforcement processes (Bergman et al. 1991; Campbell et al. 1999; Gasior et al. 2004; Sinnott et al. 1999; Tidey and Bergman 1998; Woolverton and Virus 1989), and on a single assay of spatial memory, the two-alternative delayed response (DR) task or its oculo-motor counterpart (Arnsten et al. 1994; Arnsten et al. 1995; Sawaguchi 2001). The oculo-motor version of the spatial delayed response task has provided evidence for an optimal range of D1 signaling in primate prefrontal cortex for spatial working memory (Williams and Goldman-Rakic 1995). Because of the restricted cognitive range of such studies, important questions remain regarding the behavioral and pharmacological selectivity of dopaminergic signaling in complex cognition. If other tasks, accessing other cognitive domains are not evaluated it is not possible to conclude that a given manipulation is specific to a single cognitive domain such as “spatial working memory”.
Previous research has reported impaired performance on tasks of spatial working memory following administration of a D1-like antagonist (Arnsten et al. 1994; Sawaguchi 2001). It was hypothesized that the D1-like antagonist SCH23390 would impair performance on the SOSS task, which is a more complex spatial working memory task. It was further predicted that the D2-selective antagonist raclopride would preferentially impair performance on the vsPAL task due to previous reports of a loss of D2 expression in inferior temporal regions (Joyce et al. 1998) which have been associated with object-in-place memory in monkeys (Malkova and Mishkin 2003), and findings of impaired memory after and D2-like antagonists (Arnsten et al. 1995; Mehta et al. 2001). Effects on PR, RTT and BMS tasks are predicted to be similar for the two compounds on the basis of prior results with drug- or food-reinforced responding (Nader et al. 2002; Woolverton and Virus 1989) and motor function (Weed and Gold 1998) assays in monkeys.
The present investigation was designed to identify differential contributions made by DA receptor classes to multiple aspects of cognitive performance in rhesus monkeys. Dopaminergic manipulation was conducted via acute challenge with the D1-like antagonist SCH23390 and the D2-like antagonist raclopride. Monkeys were assessed on tests that have been previously shown to be selectively affected or differentially sensitive to a number of acute pharmacological challenges in rhesus monkeys including cholinergic (Katner et al. 2004a; Taffe et al. 1999; Taffe et al. 2002c), glutamatergic (Taffe et al. 2002a; Taffe et al. 2002c) and serotonergic (Taffe et al. 2002b; Taffe et al. 2003b) manipulations. Performance is also affected by neurotropic viral infection (Gold et al. 1998; Weed et al. 2004), alcohol consumption (Katner et al. 2004b), the aging process (Taffe et al. 2003a) and experimental changes in motivational status (Taffe 2004; Weed et al. 1999). The present study aimed to determine the effects of systemic challenge with dopaminergic compounds across a range of behaviors. The results can therefore contribute to the development of more refined psychotherapeutics. The overall goal is to differentiate DA receptor contributions to multiple aspects of cognition and thus contribute to a more comprehensive understanding of the role of D1-like and D2-like receptors in cognitive and motor functions. The data obtained may offer further insight into cognitive declines related to neurological and psychiatric disorders, as well as determining the manner in which D1-like and D2-like receptors contribute to normal cognitive function.
Materials and Methods
Animals
Six young adult male rhesus macaques (Macaca mulatta) served as subjects. The monkeys were approximately 3- 4 years of age and weighed 3.8-6.4 kg at the beginning of the study. Animals were individually housed and fed in the home cage after completion of the daily testing session. The animals’ normal diet (Lab Diet 5038, PMI Nutrition International; 3.22 kcal of metabolizable energy (ME) per gram) was supplemented with fruit or vegetables seven days per week and water was available ad libitum in the home cage at all times. The animals’ normal chow allotment (~200-250 g/day) was individually adjusted and modestly restricted 5 days per week to ensure consistent behavioral responding. Adequate growth rates within 1 SD of published norms for free ranging (Rawlins et al. 1984) and laboratory housed (Schapiro and Kessel 1993) male rhesus monkeys were maintained. Individual M-F minimum chow amounts ranged from 95-155 g/day and maximum chow amounts ranged from 115-180 g/day through the course of these studies. Prior experience with this food restriction protocol produces a mean growth rate of 0.06 kg/month prior to puberty and 0.14 kg/month thereafter for animals 2-8 years of age. The subjects had received acute doses of ketamine (5-20 mg/kg) no less than semiannually for routine care and acute doses of raclopride, methylphenidate, scopolamine and d-amphetamine in prior behavioral studies. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al. 1997) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. All animals were trained on the behavioral testing battery which has been previously described (Taffe et al. 2004; Weed et al. 1999).
Behavioral Testing
Performance was evaluated using a battery of tests designed to model specific cognitive domains in rhesus monkeys (Taffe et al. 2004; Weed et al. 1999). The battery included tests of learning (visuo-spatial paired-associate learning; vsPAL), memory (self-ordered spatial search; SOSS), sustained attention and reinforcer efficacy (progressive ratio; PR), bimanual motor coordination (bimanual motor skill ; BMS) and unimanual motor tracking (rotating turntable; RTT). Monkeys were tested 5 days/week (Monday- Friday) on the task battery, completing either PR and SWM and RTT, vsPAL or PR and RTT on alternate days and BMS each day. This schedule was adapted slightly from the typical schedule used in prior studies (Katner et al. 2004a; Taffe et al. 2002a) because of the anticipated short duration of action of the test compounds. The order of the three session types relative to the testing week, and therefore each treatment condition, was randomized across subjects.
Apparatus
For behavioral testing, animals were transferred to transport cages similar to the home cage and were tested either in the colony room or in smaller isolated rooms. Our experience using this paradigm shows that any potential differences in performance attributable to testing location are much smaller than individual differences. This is perhaps because of the motivational control and within-task observing response requirements minimize gross distraction or because the isolated rooms are not insulated, thus some degree of auditory distraction is still possible. The transport cage was placed in front of a touch-sensitive computer monitor and all animals had been previously trained to reach out of the cage to touch the location on the screen at which visual stimuli were presented to obtain a food pellet reward. The test battery consisted of five alternating behavioral tasks. Three of the tasks (vsPAL, SOSS, PR) are part of the non-human primate CAmbridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK). BMS required the animal to extract raisins from holes in a transparent polycarbonate board and RTT required the animal to retrieve pellets from a rotating turntable. Comprehensive descriptions of the individual tasks and the procedural details have been previously reported (Taffe et al. 2002c; Taffe 2004; Taffe et al. 2004; Weed et al. 1999); however a brief description follows. The time required to train the tasks for these particular subjects was highly consistent with data presented in those prior methodological reports.
Progressive-Ratio (PR) Schedule of Reinforcement
The PR task was designed to measure sustained attention and reinforcer efficacy. Subjects were required to respond to a single colored rectangle presented in the center of the screen for pellet reinforcement. The response requirement started at 1 touch and incremented by arithmetic progression within blocks of 8 reinforcers and by geometric progression between blocks of 8. The session was terminated after 10 minutes, or earlier if 3 minutes elapsed following a response. The interval between session start and the last response emitted was recorded as the time-to-last-response measure. Sustained attention is thought to be indexed by total number of responses made and reward efficacy is traditionally indexed by number of reinforcers acquired.
Bimanual Motor Skill Task (BMS)
The BMS task was designed to measure fine motor coordination. A transparent polycarbonate board (10 cm wide × 25 cm high × 2.75 cm thick) drilled with 15 holes (spaced 13 mm apart in a 3 horizontal × 5 vertical array) was filled with raisins and mounted perpendicular to the door of the transport cage. Subjects were required to push the raisin out of the hole with one finger before retrieving it with the opposite hand, thus entailing bimanual dexterity. The time elapsed to retrieve all 15 raisins was recorded as a change from percentage baseline performance in retrieval latency.
Rotating Turntable Task (RTT)
The RTT task was designed to assess unimanual motor coordination, procedural learning and visual tracking of moving objects. A 58cm opaque white plastic disk containing short radial slots at the edge was mounted to a motor controlled from 0-150 rpm by rheostat. Subjects were required to retrieve 6 of 10 pellets from the slots for a “pass”. The trial is considered failed if the subject failed to retrieve or dropped 5 of 10 pellets. Turntable speed is increased after passed trials and decreased after failed trials in a titration procedure. The final maximum speed for a given session was the highest “passed” speed prior to three failed attempts.
Self-Ordered Spatial Search (SOSS)
The SOSS task was designed to measure spatial working memory. Two, three or four small colored rectangles (boxes) were displayed on the screen in positions randomly allocated from 16 possible locations. Subjects were required to select all boxes without revisiting a box once it had been touched. A session consisted of 30 trials grouped into 6 blocks by trial type as follows: 5 (2 boxes), 5 (3 boxes), 5 (4 boxes), 5 (3 boxes), 5(4 boxes), 5 (2 boxes). Accuracy scores were calculated for each trial type by dividing the number of correctly completed trials by the number of trials in which there was at least one response. Trial latency is the average time a subject takes to touch all boxes for correctly completed trials.
Visuo-Spatial Paired Associates Learning (vsPAL)
The vsPAL task was designed to measure associative and procedural learning, and memory. Subjects were required to learn and remember the spatial location of visual patterns. In brief, colored abstract stimuli were displayed in one of four possible target locations and the subject was required to touch this sample stimulus, which then disappears. After a one second screen blank, the same pattern reappeared (choice phase) in two or four locations on the screen (the original location plus one or more novel locations). The subject was required to touch the stimulus that is presented in the same location as the sample item to obtain a reinforcer. Subjects were allowed up to 5 additional attempts to successfully complete the set of stimulus-location associations in a given trial, thus measuring incremental learning. Each session consisted of 20 trials in sequential blocks including 10 × 2-stimulus trials and 10 × 4-stimuli trials. Performance was measured by percent correct on the initial-attempt to complete a trial and the percent correct of trials successfully completed within the allowed attempts (overall completion). The initial-attempt score is interpreted as a memory measure and the difference between overall completion rates and initial attempt rates are interpreted as a measure of learning. Additional measures of performance include the initial-attempt choice accuracy (proportion of correct choices on the first attempt, task completion (proportion of trials which both sample and choice responses were made), total number of correct choices, total number of incorrect choices, mean sample latency (response time for all observing responses) and mean choice latency (response time for all correct choice responses.
Acute Drug Challenge Procedure
Animals received drug injections of dopamine-like antagonists raclopride and SCH23390 (each compound dissolved with physiological saline to obtain a constant injection volume of 0.1 ml/kg) on Tuesdays and Fridays, vehicle (physiological saline, 0.1 ml/kg) injections on Thursdays and no injections on Mondays and Weds. The monkeys were first challenged with the D2-like antagonist raclopride in an ascending dose order (10, 17.8, 32, 56 μg/kg, i.m.) with a constant injection volume of 0.1 ml/kg. Prior to initiating the second drug challenge, a washout period of a minimum of 3 weeks was employed that consisted of physiological saline, 0.1 ml/kg injected three days per week (Tue, Thur, Fri) until performance following injections was equivalent to non-injection performance. Following the washout period, the same monkeys were challenged with the D1-like antagonist SCH23390 in an ascending dose order (3.2, 10, 17.8, 32, 56 μg/kg, i.m.) with a constant injection volume of 0.1 ml/kg. Each dose of raclopride and SCH23390 was evaluated once for SOSS, PAL or PR, twice for RTT and three for BMS. The pretreatment intervals were as illustrated in Figure 1. The mean performance of an individual for all sessions run under a given treatment condition was generally used for analyses. One exception was made to this rule because preliminary inspection of the data suggested a clear pharmacokinetic effect for each drug. That is, BMS dose-response functions were similar for the shorter two session types (Figure 1: Session 2, 3) but no effect was observed for the longer session type. Consistent with this interpretation, the earlier-run 2-stimulus trials of the vsPAL task appeared affected whereas the 4-stimulus trials appeared unaffected. Thus, it was concluded that behaviorally relevant levels of challenge drug were no longer present by approximately 55-60 minutes post-injection. The BMS data for the longest session and 4-stimulus trial data for vsPAL were therefore not analyzed, nor presented. One individual (#413) participated in the raclopride study but was not available for the SCH23390 study.
Figure 1. Session Schedule.
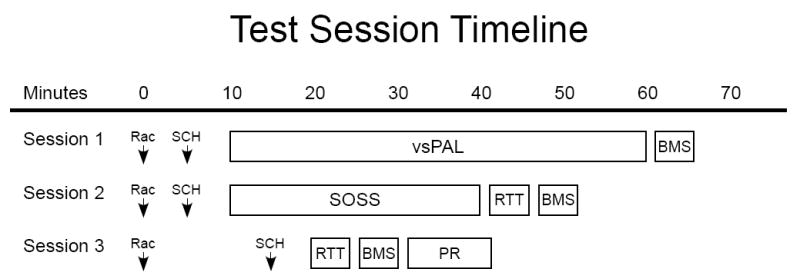
The approximate timing for the three types of behavioral sessions run in rotation on successive test sessions is outlined. The effective pre-treatment intervals for each of the test compounds varied slightly depending on the behavioral task, as illustrated. The challenge studies for raclopride and SCH23390 were run sequentially with a minimum 3 week interval between studies. Preliminary analyses suggested the each compound lost significant behavioral activity after about 60 min, see Materials and methods for data treatment.
Data Analysis
All statistical analyses were conducted using GB-STAT v7.0 for Windows (Dynamic Microsystems, Inc., Silver Spring MD, and the criterion for significance in all tests was p ≤ 0.05. Data for the behavioral tasks were analyzed by repeated measures analysis of variance (ANOVA). Variables analyzed include the number of reinforcers acquired, the total number of responses, the response rate and the time to last response in the PR task, the retrieval time in the BMS task and the threshold speed of the turntable at which 6/10 pellets could be retrieved in the RTT procedure. Data analyzed for the SOSS task included the percent correct trials and the overall response latency for correct trials for each of three difficulty conditions, using a 2-way ANOVA). Performance measures analyzed for the vsPAL task included the initial-attempt trial completion success, overall trial completion success, percent of correct choices on first attempt, percent task completed, total number of correct choices, total number of incorrect choices, sample latency and choice latency. The initial-attempt trial completion and overall trial completion measures were analyzed with a 2-way ANOVA, which was necessary in order to demonstrate the learning effect.
Significant effects in the one way ANOVAs were followed up with post hoc tests using the Dunnett procedure. Post hoc exploration of significant effects in the two-way designs was conducted using the Tukey-Kramer procedure including all possible pair-wise comparisons.
Results
Progressive Ratio Task
Raclopride significantly decreased the number of reinforcers acquired [F5,25 = 8.2; p < 0.001], the total number of responses [F5,25 = 5.6; p < 0.01], the response rate [F5,25 = 4.5; p < 0.01] and the time-to-last-response [F5,25 = 3.0; p < 0.05] in the PR task as is shown in Figure 2. Post hoc analysis confirmed that raclopride significantly altered all PR measures relative to vehicle levels after the 56 μg/kg dose was administered and the total number of responses were significantly reduced compared with vehicle following the 17.8 and 32 μg/kg doses. SCH23390 also significantly decreased the number of reinforcers acquired [F6,24 =5.8; p < 0.001], the total number of responses [F6,24 = 3.6; p < 0.05], the response rate [F6,24 = 3.9; p < 0.01] and the time-to-last-response [F6,24 =3.2; p < 0.05]. Post hoc analysis confirmed that SCH23390 significantly altered all PR measures relative to vehicle levels after the 56 μg/kg dose was administered and the number of reinforcers acquired was significantly lower than vehicle following the 32 μg/kg dose.
Figure 2. Progressive Ratio.
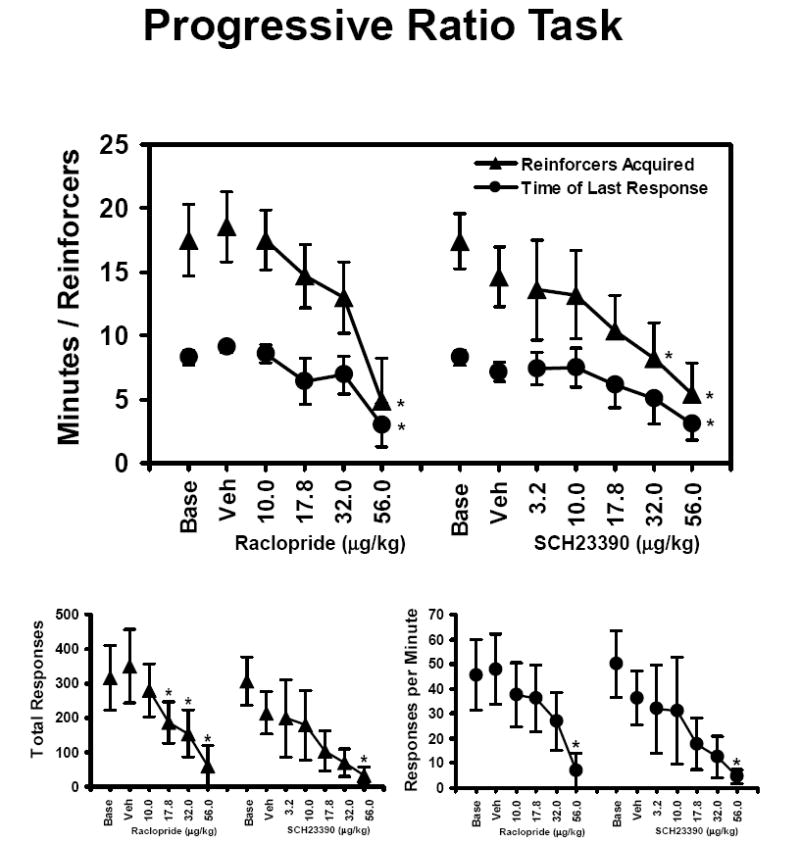
The mean effect of acute doses of raclopride (N = 6; ± SEM) and SCH23390 (N = 5; ± SEM) on performance of the PR task is expressed in terms of the number of reinforcers acquired, the time of last response, the total number of responses and the response rate. A significant difference from the vehicle condition is indicated by *. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
Bimanual Motor Skill and Rotating Turntable Tasks
The speed of raisin retrieval in the BMS task was significantly slowed by both raclopride and SCH23390 as is illustrated in Figure 3A. The mean baseline retrieval latency was 19.9 sec (SEM = 3.7) in the raclopride condition and 20.2 sec (SEM = 2.5) in the SCH23390 condition. The repeated measures ANOVA confirmed a significant main effect of drug treatment condition for the raclopride [F5,25 = 3.0; p < 0.05] and SCH23390 [F6,30 = 5.4; p < 0.001] challenges. Post hoc exploration of this effect confirmed that significantly longer retrieval times were observed following the 56 μg/kg dose of each compound.
Figure 3. Bimanual Motor Skill and Rotating Turntable Tasks.
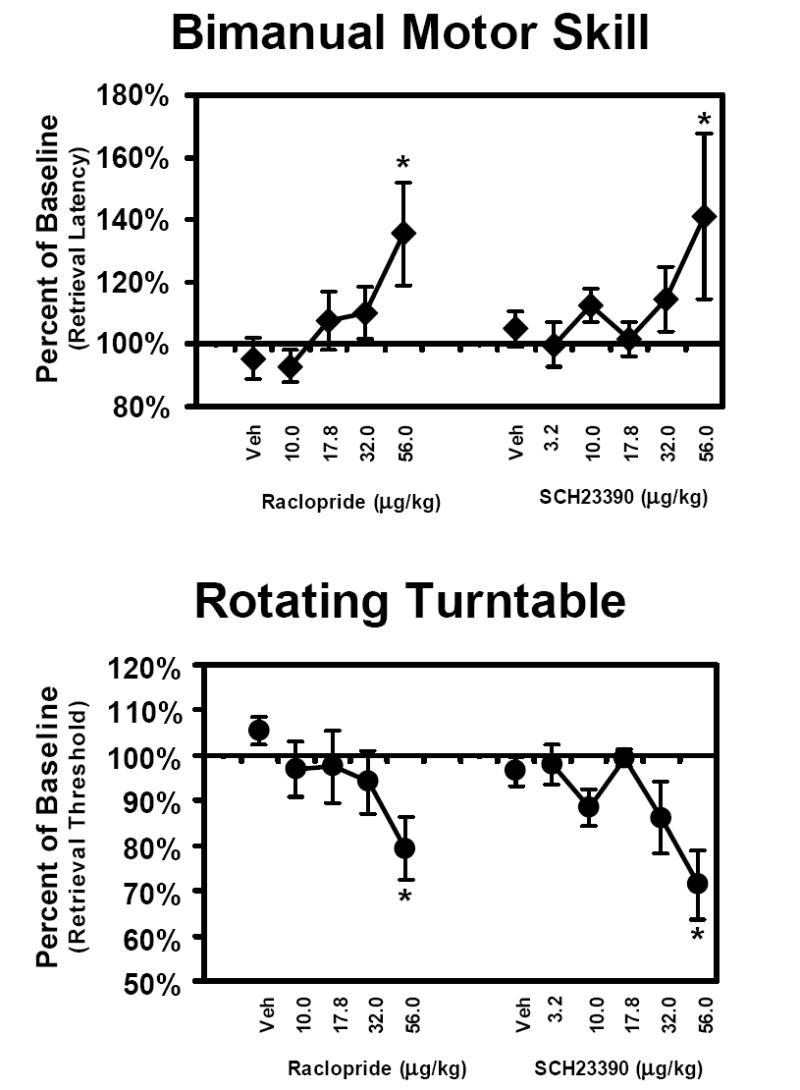
The mean effect of acute doses of raclopride (N = 6; ± SEM) and SCH23390 (N = 5; ± SEM) on performance of the BMS and RTT tasks. Data are expressed as a proportion of individual baseline performance levels. A significant difference from the vehicle condition is indicated by * and Veh indicates vehicle injection sessions.
The threshold speed at which animals could retrieve 6 of 10 reinforcers from the rotating turntable was slowed by each compound as shown in Figure 3B. The mean retrieval threshold was 66.7 rpm (SEM = 10.0) for the raclopride study and 86.5 rpm (SEM = 9.4) for the SCH23390 study. The repeated measures ANOVA confirmed a significant main effect of raclopride [F5,25 = 3.5; p < 0.05] and SCH23390 [F6,30 = 12.5; p < 0.001] with the post hoc test attributing the effect to the 56 μg/kg dose of each compound.
SOSS
Successful trial completion in the SOSS task was significantly reduced by trial difficulty for both the raclopride [F2,10 = 72.0; p < 0.001] and SCH23390 [F2,8 = 22.9; p < 0.001] challenge studies, as is depicted in Figure 4. The percentage of trials completed successfully was significantly decreased by the administration of raclopride [F5,25 = 9.3; p < 0.001]. The Tukey-Kramer post hoc test confirmed a specific effect on 3-box performance following the 56 μg/kg dose. There was a trend for SCH23390 to reduce the percentage of trials completed [F6,24 = 2.4; p = 0.062]. In neither study was any significant interaction between the two main factors observed. There were no significant main effects of trial difficulty on response latency in the raclopride study, however there was a significant main effect of drug treatment condition raclopride [F5,25 = 3.8; p < 0.05] and an interaction between trial difficulty and drug treatment [F10,50 = 3.3; p < 0.01]. Post hoc evaluation of these effects with the Tukey-Kramer procedure confirmed that response latency was significantly slowed for 3-box trials following the 56 μg/kg dose. Response latency in the SCH23390 study was significantly affected by trial difficulty [F2,8 = 5.4; p < 0.05] and drug treatment condition [F6,24 = 2.8; p < 0.05]. Further post-hoc exploration with one-way ANOVA (drug condition) for each of the three trial-difficulty types confirmed a significant effect of drug condition on response latency for 2-box trials [F6,24 = 3.5; p < 0.05] and a non-significant trend for increased response latency for 3-box trials [F6,24 = 2.5; p = 0.052]. A significant increase over vehicle in 2-box response latency following the 56 μg/kg dose of SCH23390 was confirmed with the Dunnett procedure.
Figure 4. Self-Ordered Spatial Search.
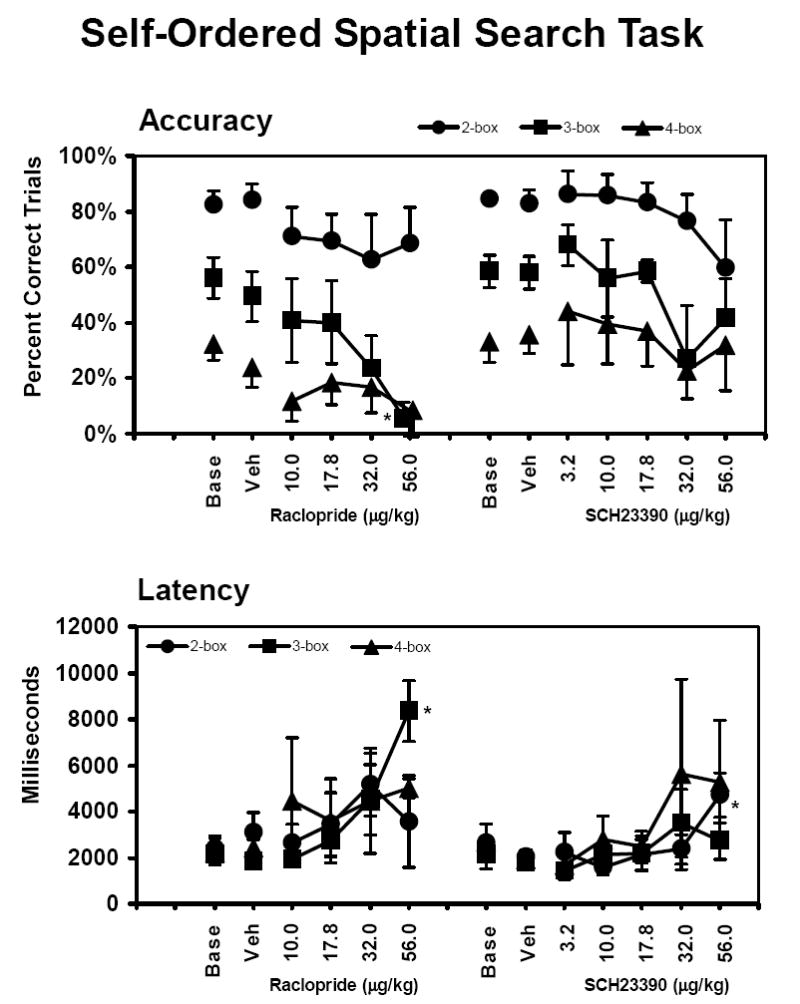
The mean effect of acute doses of raclopride (N = 6; ± SEM) and SCH23390 (N = 5; ± SEM) on performance of the SOSS task are presented in terms of the percentage of trials correctly completed and the response latency for correct trials. The data are presented separately for each of three trial difficulty levels. A significant pair-wise difference from the vehicle condition is indicated by * and complete analysis details are provided in the text. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
vsPAL
The predicted learning effect was observed as animals performed significantly more trials correct when permitted repeated attempts relative to their first attempt accuracy in both raclopride (main effect of completion measure, initial vs. overall, [F1,5 = 24.7; p < .01] and SCH23390 studies ([F1,4 = 12.0; p < .05]) as is illustrated in Figure 5. Acute challenge with raclopride interfered with trial completion success (main effect of drug condition; [F5,25 = 3.1; p < .05], an effect which interacted with completion measure [F5,25 = 3.4; p < .05]. Post hoc exploration with the Tukey-Kramer procedure confirmed a significant reduction in initial, but not overall, trial completion success following the 17.8 and 56 μg/kg doses of raclopride. A parallel main effect of drug condition was observed [F5,25 = 2.9; p < .05] when considering the percentage of choices made accurately on the first attempt. The Dunnett post-hoc test confirmed that this was due to a significant reduction in performance following the 17.8 and 56 μg/kg doses. Raclopride also significantly affected the latency to respond to the sample stimuli [F5,25 = 2.8; p < .05], the total number of correct responses [F5,25 = 2.8; p < .05] and the percent of the task completed [F5,25 = 7.1; p < .001]. Post hoc exploration of these effects confirmed a significant increase in sample latency, a decrease in total correct responses and a decrease in the percent of the task completed following the 32 and 56 μg/kg doses of raclopride. A significant effect of SCH23390 treatment was observed only for the percent of the task completed [F6,24 = 3.8; p < .01] and the Dunnett test confirmed a significant reduction relative to the vehicle condition for the 56 μg/kg doses of SCH23390.
Figure 5. visuo-spatial Paired Associates Learning.
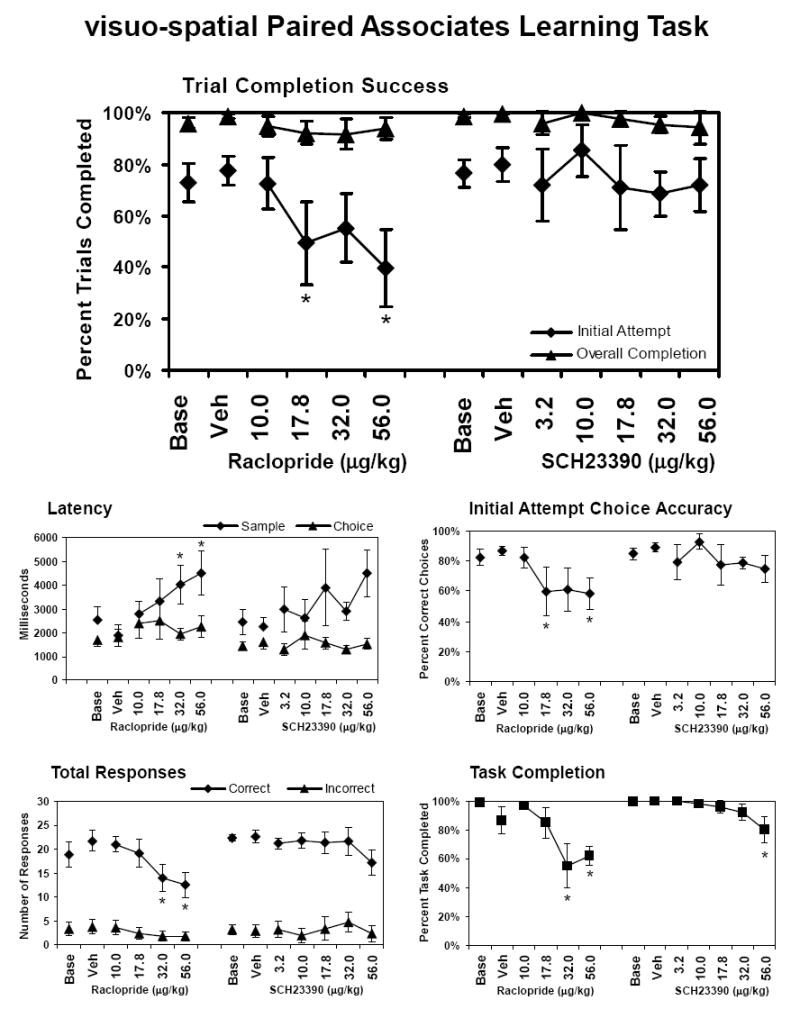
The mean effect of acute doses of raclopride (N = 6; ± SEM) and SCH23390 (N = 5; ± SEM) on performance of the 2-stimuli/2-locations vsPAL trials. The data presented in the top panel include the proportion of trials successfully completed on the initial attempt and the overall completion success. The lower panels present the response latency for sample and choice responses, the per-response choice accuracy on the first attempt at each trial, the total number of correct and incorrect responses and the percent of the task that was completed. A significant difference from the vehicle condition is indicated by *. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
Discussion
The present work shows specific deficits in learning, memory, attention and motor domains following D1 and D2 drug challenges. Similar deficits in performance on the progressive ratio (PR), bimanual motor skill (BMS) and rotating turntable (RTT) tasks were impaired by both raclopride and SCH23390. Clear differences emerged however in the context of the memory tasks. Perhaps the most surprising finding was that the SCH23390 challenge did not significantly impair performance on the SOSS task, thought to assess spatial working memory capabilities; however, performance in this task was impaired by raclopride. The D2-like, but not the D1-like, antagonist also interfered with performance of the visuo-spatial paired associate learning (vsPAL task), suggesting that visuo-spatial associative memory depends more on D2-like function than it does on D1-like neurotransmission, a finding consistent with the a priori hypothesis.
The effects of the DA antagonist compounds on the PR task were clear, and consistent with prior work. That is, schedule controlled responding for shock termination (Bergman et al. 1991), cocaine (Campbell et al. 1999; Woolverton and Virus 1989) and food (Nader et al. 2002; Woolverton and Virus 1989) are similarly decreased by D1-like and D2-like antagonist treatment in monkeys. Those results are confirmed and extended here since all measures of PR performance, were similarly affected (Figure 2). This outcome suggests a similar impact of each compound on motivation, sustained attention motor response generation/execution as these have traditionally been applied to PR measures; it is always possible that such distinctions do not hold in the present model. With respect to motivational aspects of PR performance, studies show that nigro-striatal dopaminergic projections (Ljungberg et al. 1991; Schultz et al. 1993a; Schultz et al. 1993b) and D2-expressing cholinergic interneurons (Watanabe and Kimura 1998) may encode motivation and reward value in a plastic manner. In particular Watanabe and colleagues show that learned, reward-associated alterations in striatal neuron discharge rates are attenuated by local application of both D1-like and D2-like antagonists (Watanabe and Kimura 1998). Thus, it is likely that both the D1-like and D2-like antagonist effects on PR performance are likely to be specific for motivational aspects of the task.
The current results also extend prior findings of deficits in motor execution and production. As with the PR response rate measure, the BMS and RTT results illustrate a consistent involvement of D1-like and D2-like neurotransmission in both bimanual coordinated responses and unimanual tracking and retrieval. Together with a prior report, showing that effects of D1-like and D2-like antagonists were similar in slowing reaction time (Weed and Gold 1998), these results confirm the critical involvement of both receptor subtypes in many aspects of motor function. The relatively minimal impact of raclopride and SCH23390 on response latencies in the memory procedures potentially contrasts with this conclusion. However, drug challenges may selectively slow sample versus choice latency in the vsPAL procedure in some cases (present raclopride study, Taffe et al. 2002c), but not others (Katner et al. 2004a), as well as selectively slow choice latency on 2-box over 4-box trials in the SOSS procedure (Taffe et al. 2002a). In all cases the drugs significantly slowed BMS performance. Thus the response latencies across these tasks are likely determined by different cognitive demands and processes. Together this evidence suggests that impaired response latencies in the memory procedures most likely reflect changes in sustained attention, speed/accuracy bias or mnemonic certainty as opposed to motor capability.
The significant deficits observed following raclopride, without significant effect of SCH23390, on self-ordered spatial search (SOSS) performance was unexpected. As a caveat however, it must be appreciated that a non significant trend for an effect of SCH23390 was observed, thus any contrasing results with raclopride must be interpreted in terms of relative impact rather than present/absent. Arnsten and colleagues (1994) have shown that young adult monkeys’ performance of the delayed-response (DR) task designed to assess spatial working memory is impaired by SCH23390. Interestingly, the impairment in young monkeys was produced at doses which were noticeably sedating but did not impair performance on a fine motor task. In another study, the D2-like agonist quinpirole impaired DR performance at low doses (attributed to D2 autoreceptor activity) but improved performance at higher doses (Arnsten et al. 1995). The former effect was ameliorated by raclopride but not SCH23390, whereas the latter effect was reversed by both raclopride and SCH23390, suggesting a role of D1-like signaling. Sawaguchi and Goldman-Rakic (1994) have provided more specific evidence using local application of compounds to frontal cortex of monkeys performing an oculomotor version of the delayed response task (ODR). In their study D1-like antagonists SCH23390 and SCH31996 and non-selective antagonist haloperidol all significantly impaired task performance, unlike the D2-selective antagonists raclopride and sulpiride.
A possible model to explain the effects of D1-like versus D2-like neurotransmission is suggested by the identification of D2-like signalling with response-preparation for memory-guided saccade responses (Wang et al. 2004) and D1-like signalling with the retention interval (Sawaguchi 1997). This might suggest a more retention-specific role of D1-like receptors; in SOSS the cognitive load is imposed by the number of locations to be remembered rather than parametric manipulation of retention interval. Interestingly Collins and colleagues found that while excitotoxic lesions of prefrontal cortex of marmosets impaired SOSS performance, prefrontal catecholamine lesions induced by 6-hydroxydopamine did not (Collins et al. 1998). The catecholamine-depleted group was, however, impaired on acquisition of spatial delayed response suggesting on the face of it that the SOSS may access very different cognitive constructs than delayed response. Of course experimental manipulation effects on acquisition and stable performance of tasks are frequently uncorrelated. In addition to this methodological feature, further experiments in the marmosets demonstrated that the excitotoxic lesion impairment of SOSS was abolished if perseverative responding was prevented and remained consistent even when the memory requirement was removed from the task (Collins et al. 1998). Perseverative responding was not increased by either drug treatment in the present study thus it appears that cross-laboratory comparisons are complex, even when the task appears similar in form.
Alternately it may be the case that the effects reflect wider circuitry of the brain. For example delayed match-to-sample, traditionally viewed as a hippocampally-dependent task in monkeys, activates dorsolateral prefrontal cortex in addition to hippocampal regions (Porrino et al. 2005). Conversely, a conjoint lesion of hippocampus and amygdala impairs spatial delayed-response in a manner dependent on retention interval (Zola-Morgan and Squire 1985). Tasks thought to assess working memory appear to activate fronto-parietal (Schweinsburg et al. 2005) as well as fronto-hippocampal (Karlsgodt et al. 2005) circuitry in humans. Evidence also suggests that working memory tasks enhance dopamine release in both frontal and temporal regions (Aalto et al. 2005). Furthermore, in human brain, D2-like receptor mRNA is highly expressed in hippocampus which has low expression of D1-like receptor mRNA (Hurd et al. 2001). Conversely D1-like mRNA is at high levels in cortex, especially medial orbitofrontal cortex, where D2-like mRNA is low. Thus it is plausible that D2-like receptor mediated functions of medial temporal structures predominate in determining performance on both SOSS and vsPAL
The finding that raclopride significantly impaired visuo-spatial paired associate learning (vsPAL) performance where SCH23390 did not is consistent with a growing picture of the dependence of object-location associative memory on specific regions of the temporal lobe memory system. Evidence from prospective studies following elderly individuals with questionable dementia strongly suggest that the capabilities necessary for performance of the vsPAL task decline early in the progression of AD (Blackwell et al. 2004; Fowler et al. 1997; 2002; Swainson et al. 2001). Consistent with this, a preliminary study suggested that aged monkeys may be particularly impaired on this task relative to young adult monkeys (Taffe et al. 2003a). We have also shown that intact muscarinic and nicotinic cholinergic, as well as NMDA receptor glutamatergic, neurotransmission is critical for young adult monkeys to perform the task (Katner et al. 2004a; Taffe et al. 2002c). Here we show that normal D2-like, but not D1-like, dopaminergic neurotransmission is also required in nonhuman primates for object-location associative memory. The appearance of a significant reduction in performance at a dose (17.8 μg/kg) where most other test battery measures were unaffected also supports a specific role for D2-like signalling in vsPAL performance. The pattern of impaired memory but not incremental learning is more similar to the effects of NMDA glutamatergic or nicotinic cholinergic blockade and less similar to muscarinic cholinergic blockade.
The vsPAL finding is consistent with evidence suggesting that object-in-place memory such as required by the vsPAL task, may depend specifically on parahippocampal cortical regions (Angeli et al. 1993; Malkova and Mishkin 2003; Parkinson et al. 1988) which normally express high levels of D2 receptors (Goldsmith and Joyce 1994). This regional specificity of the effect is supported by reports that D2-like binding in hippocampal regions is correlated with verbal memory in AD (Kemppainen et al. 2003), where striatal D1-like or D2-like binding in AD is not correlated (Kemppainen et al. 2000). Given that there was not even a trend for SCH23390 to affect vsPAL performance here, the present work reemphasizes the likely priority of temporal versus frontal cortical mechanisms in object-location associative memory.
In summary the present results suggest that the D2-like dopamine receptor antagonist raclopride has a range of detrimental effects on complex behavior indicating D2 involvement in cognitive constructs such as spatial working memory, visuo-spatial associative memory, fine motor coordination and motor tracking in addition to reinforcer efficacy and sustained attention. The D1-like antagonist SCH23390 produced similar effects on non-mnemonic tasks however significant effects on memory tasks were not observed. This intriguing discrepancy from prior work which asserted the primacy of D1 signalling in spatial working memory tasks suggests the relationships between DA receptor subtypes and various memory capabilities may be much more complex than previously appreciated.
Acknowledgments
This work was supported by USPHS grants DA13390 and MH61692. This is publication #16573-NP from The Scripps Research Institute.
References
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–7. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli SJ, Murray EA, Mishkin M. Hippocampectomized monkeys can remember one place but not two. Neuropsychologia. 1993;31:1021–30. doi: 10.1016/0028-3932(93)90030-4. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–51. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–39. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Robins-Wahlin TB, Lundin A, Ginovart N, Farde L. Cognitive deficits in Huntington’s disease are predicted by dopaminergic PET markers and brain volumes. Brain. 1997;120(Pt 12):2207–17. doi: 10.1093/brain/120.12.2207. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Spealman RD. Behavioral effects of D1 and D2 dopamine receptor antagonists in squirrel monkeys. J Pharmacol Exp Ther. 1991;258:910–7. [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17:42–8. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Rodefer JS, Carroll ME. Effects of dopamine receptor antagonists (D1 and D2) on the demand for smoked cocaine base in rhesus monkeys. Psychopharmacology (Berl) 1999;144:381–8. doi: 10.1007/s002130051021. [DOI] [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. Ilar J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates. Effects Of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci. 1998;10:332–54. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Duchesne N, Soucy JP, Masson H, Chouinard S, Bedard MA. Cognitive deficits and striatal dopaminergic denervation in Parkinson’s disease: a single photon emission computed tomography study using 123iodine-beta-CIT in patients on and off levodopa. Clin Neuropharmacol. 2002;25:216–24. doi: 10.1097/00002826-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized neuropsychological tests in the early detection of dementia: prospective findings. J Int Neuropsychol Soc. 1997;3:139–46. [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Gasior M, Paronis CA, Bergman J. Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J Pharmacol Exp Ther. 2004;308:249–59. doi: 10.1124/jpet.103.052795. [DOI] [PubMed] [Google Scholar]
- Gold LH, Fox HS, Henriksen SJ, Buchmeier MJ, Weed MR, Taffe MA, Huitron-Resendiz S, Horn TF, Bloom FE. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J Med Primatol. 1998;27:104–12. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith SK, Joyce JN. Dopamine D2 receptor expression in hippocampus and parahippocampal cortex of rat, cat, and human in relation to tyrosine hydroxylase-immunoreactive fibers. Hippocampus. 1994;4:354–73. doi: 10.1002/hipo.450040318. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat. 2001;22:127–37. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Myers AJ, Gurevich E. Dopamine D2 receptor bands in normal human temporal cortex are absent in Alzheimer’s disease. Brain Res. 1998;784:7–17. doi: 10.1016/s0006-8993(97)01005-6. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Shirinyan D, van Erp TG, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–31. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology (Berl) 2004a;175:225–40. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, Taffe MA. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004b;28:873–83. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen N, Laine M, Laakso MP, Kaasinen V, Nagren K, Vahlberg T, Kurki T, Rinne JO. Hippocampal dopamine D2 receptors correlate with memory functions in Alzheimer’s disease. Eur J Neurosci. 2003;18:149–54. doi: 10.1046/j.1460-9568.2003.02716.x. [DOI] [PubMed] [Google Scholar]
- Kemppainen N, Ruottinen H, Nagren K, Rinne JO. PET shows that striatal dopamine D1 and D2 receptors are differentially affected in AD. Neurology. 2000;55:205–9. doi: 10.1212/wnl.55.2.205. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Weeks RA, Brooks DJ, Andrews TC, Watkins LH, Harding AE, Robbins TW, Sahakian BJ. The relationship between striatal dopamine receptor binding and cognitive performance in Huntington’s disease. Brain. 1998;121(Pt 7):1343–55. doi: 10.1093/brain/121.7.1343. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey midbrain dopamine neurons during delayed alternation performance. Brain Res. 1991;567:337–41. doi: 10.1016/0006-8993(91)90816-e. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–65. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Muller U, Wachter T, Barthel H, Reuter M, von Cramon DY. Striatal [123I]beta-CIT SPECT and prefrontal cognitive functions in Parkinson’s disease. J Neural Transm. 2000;107:303–19. doi: 10.1007/s007020050025. [DOI] [PubMed] [Google Scholar]
- Nader MA, Sinnott RS, Mach RH, Morgan D. Cocaine- and food-maintained responding under a multiple schedule in rhesus monkeys: environmental context and the effects of a dopamine antagonist. Psychopharmacology (Berl) 2002;163:292–301. doi: 10.1007/s00213-002-1202-3. [DOI] [PubMed] [Google Scholar]
- Parkinson JK, Murray EA, Mishkin M. A selective mnemonic role for the hippocampus in monkeys: memory for the location of objects. J Neurosci. 1988;8:4159–67. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Crean RD. A retrospective review of the neuropsychological test performance of physicians referred for medical infractions. Arch Clin Neuropsychol. 2005;20:161–70. doi: 10.1016/j.acn.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ, Turnquist JE. Reproductive performance, population dynamics and anthropometrics of the free-ranging Cayo Santiago rhesus macaques. J Med Primatol. 1984;13:247–59. [PubMed] [Google Scholar]
- Sawaguchi T. Attenuation of preparatory activity for reaching movements by a D1-dopamine antagonist in the monkey premotor cortex. J Neurophysiol. 1997;78:1769–74. doi: 10.1152/jn.1997.78.4.1769. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T. The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci Res. 2001;41:115–28. doi: 10.1016/s0168-0102(01)00270-x. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Kessel AL. Weight gain among juvenile rhesus macaques: a comparison of enriched and control groups. Lab Anim Sci. 1993;43:315–8. [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993a;13:900–13. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E. Reward-related activity in the monkey striatum and substantia nigra. Prog Brain Res. 1993b;99:227–35. doi: 10.1016/s0079-6123(08)61349-7. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11:631–44. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–80. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Bernot TJ, Mckay HL, Davis SA, Roberts JA, Tuszynski MH. Adaptation of a computerized neuropsychological testing battery to the aged macaque. Society for Neuroscience Annual Meeting; New Orleans, LA. 2003a. [Google Scholar]
- Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002a;68:175–87. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Yuan J, Schroeder R, Hatzidimitriou G, Parsons LH, Ricaurte GA, Gold LH. Cognitive performance of MDMA-treated rhesus monkeys: sensitivity to serotonergic challenge. Neuropsychopharmacology. 2002b;27:993–1005. doi: 10.1016/S0893-133X(02)00380-9. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. MDMA exposure alters cognitive and electrophysiological sensitivity to rapid tryptophan depletion in rhesus monkeys. Pharmacol Biochem Behav. 2003b;76:141–52. doi: 10.1016/s0091-3057(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–12. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002c;160:253–62. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer’s type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–33. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–74. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–6. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kimura M. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol. 1998;79:2568–80. doi: 10.1152/jn.1998.79.5.2568. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH. The effects of dopaminergic agents on reaction time in rhesus monkeys. Psychopharmacology (Berl) 1998;137:33–42. doi: 10.1007/s002130050590. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 2004;20:77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32:691–7. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]


