Abstract
Purpose
To compare the age-, and gender-specific testability rates for the Amblyopia Treatment Study (ATS) HOTV Visual Acuity Testing Protocol using the Electronic Visual Acuity (EVA) Tester in African-American and Hispanic preschool children.
Design
Population-based, cross-sectional study
Methods
Measurement of presenting monocular distance visual acuity using the ATS-HOTV protocol was attempted in all African-American and Hispanic children aged 30–72 months from the population-based Multi-Ethnic Pediatric Eye Disease Study (MEPEDS). Children able to be tested monocularly in both eyes were considered “able.” Age-, gender- and ethnicity-specific testability rates were calculated. Comparisons of testability among different groups were performed using chi-square analyses and the Cochran trend test.
Results
Testing was attempted on 3126 children (1471 African-American, 1655 Hispanic; 50% female). Overall, 84% (83% African-American, 85% Hispanic; 86% female, 82% male) were testable. Older children were more likely to successfully complete testing than younger children (p<0.0001). Age-specific testability in children 30–36 months, 37–48 months, 49–60 months, and 61–72 months of age was 39%, 84%, 98%, and 100%, respectively. After stratifying by age, there were no ethnicity-related differences in children testable (p=0.12). Girls (86%) were slightly more likely to be testable than boys (82%) (p>0.003).
Conclusions
Monocular threshold visual acuity testing using the ATS-HOTV protocol on the EVA tester can be completed by most African-American and Hispanic preschool children, particularly those older than 36 months of age. This protocol may therefore be used in minority preschool children as an integral part of the diagnosis and management of amblyopia and other forms of visual impairment.
Keywords: visual acuity, Electronic Visual Acuity Tester, HOTV optotypes, ATS visual acuity protocol, children, preschool, population-based study
Introduction
Visual acuity is the most commonly used clinical measure for determining whether amblyopia or other forms of visual impairment are present. Because it is sensitive to uncorrected refractive error and many abnormalities that affect the eye and visual pathways, its quantification is an integral part of the diagnosis and management of eye disorders. While the need to obtain an accurate and reliable measurement of visual acuity in young children has long been recognized and various methods of assessment are available, there is no recognized standard for testing visual acuity in preschool children.
In an effort to provide standardization of visual acuity measurements across multiple sites for its randomized clinical trials on amblyopia in young children, members of the Pediatric Eye Disease Investigator Group developed a computerized visual acuity testing protocol using isolated HOTV optotypes with surround bars.1, 2 Designed for children 3 to 7 years of age, the Amblyopia Treatment Study (ATS) HOTV visual acuity testing protocol is currently being used in pediatric vision research studies and also in clinical practice. Two small clinical studies of children 3 to 7 years1, 2 and a larger non-population-based study of 41- to 60-month-old children3 have reported high testability rates using this visual acuity protocol.
The Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) is a population-based cross-sectional study designed to determine age-specific prevalence rates and risk factors for strabismus, amblyopia, and refractive error in African-American, Asian-American, Hispanic, and non-Hispanic White children 6 months through 72 months of age. In this study, the ATS-HOTV protocol is the method used for visual acuity assessment. The objective of this paper is to report testability rates for completing monocular threshold visual acuity measurements using the ATS-HOTV visual acuity testing protocol presented on the Electronic Visual Acuity (EVA) Tester in a large population-based cohort of African-American and Hispanic preschool children aged 30 to 72 months of age.
METHODS
Study Population
The study population consisted of African-American and Hispanic children, aged 30 to 72 months, living within 35 census tracts in and around the city of Inglewood in Los Angeles County, California. Details of the study design and sampling plan have been described previously.4 In brief, after conducting a door-to-door census of all dwelling units within the targeted census tracts, eligible children (aged 6–72 months) whose parents consented to participate were scheduled for a MEPEDS eye examination.
Clinical Examination Procedures
At the examination center, participants underwent a comprehensive eye examination performed by MEPEDS pediatric eye care providers who were trained and certified using standardized protocols.4 Presenting monocular distance visual acuity with existing refractive correction (if any) was measured using the ATS single-surround HOTV letter protocol1 presented on the Electronic Visual Acuity Tester2 for all participants aged 30 to 72 months. Testing was attempted on all children, including those with developmental delay or disability.
ATS-HOTV Visual Acuity Testing Protocol Using the EVA Tester
The testing protocol has been described previously.1, 2 In brief, it consists of an initial screening to obtain an approximate visual acuity threshold, followed by a first threshold determination phase, a reinforcement phase, and a second threshold determination phase. The visual acuity score is the smallest logMAR level passed in the two threshold phase scores. The test provides visual acuity scores in 1-logMAR line increments from 20/800 to 20/16.
The EVA tester consists of a programmed hand-held device using the Palm operating system that communicates with a personal computer and a 17-inch monitor.2 The program on the hand-held device runs the visual acuity testing protocol with the system presenting isolated high-contrast black-and-white HOTV optotypes framed by crowding bars spaced a half-letter width around the letter on the monitor (Figure 1).
Figure 1.
Amblyopia Treatment Study HOTV Visual Acuity Testing Protocol Presented on the Electronic Visual Acuity (EVA) Tester
Visual Acuity Procedure
Children were seated comfortably at a test distance of 3 meters from the monitor. The child was provided with a laminated lap card containing the surrounded HOTV letters. For younger children, and any children who might have difficulty comprehending the task, monocular testing was preceded by a binocular pretest at near, which if passed was followed by a binocular pretest at 3 m. Monocular threshold testing at 3m was then performed for those able to perform the pretests.
For pretesting, the examiner sat approximately 40 to 50 cm from the child, and explained the task while holding up another card with large-sized surrounded HOTV optotypes. With both eyes open, the child was instructed to identify an individual letter on the examiner’s card either verbally or by using the lap card for matching. It was sometimes necessary to teach younger children the “matching game” when they were not immediately able to perform matching correctly. Children who remained unable to comprehend or perform the matching task at near were considered “unable” and visual acuity testing ended. Children who were successful at near, were then directed to shift their attention to the monitor at 3m and a distance pretest using 20/125 or 20/400 letters was administered. With both eyes open, the child was instructed to identify the demonstration letter on the monitor either verbally or by pointing to the identical optotype on the hand-held matching card. Children not able to perform this task despite instruction, coaching, and encouragement were considered “unable.” All children who successfully completed the near and distance pretests proceeded to have monocular threshold visual acuity measured.
For monocular threshold testing, the right eye was tested first and then the left eye, with the fellow eye occluded with an adhesive patch or with occluding glasses in the rare instances when a child refused to wear the patch. Children who knew their letters were still encouraged to refer to their lap card during testing.
Children who completed monocular testing and received a visual acuity score for both right and left eyes were considered “able” or “testable.” Children who were judged “unable” during binocular pretesting were considered “unable” or “nontestable,” as were those who underwent monocular testing but could not cooperate with completion of testing with both eyes.
Children initially unable to perform testing the day of their examination had the opportunity to be retested at a later point in the examination, either as part of quality-control procedures, or with refractive correction at the end of the examination. Children unable to perform testing on the day of their initial examination were sent home with a training card to return for a visual acuity retest on another day.
The testability rates (i.e., “ables”) reported herein are based only on the initial attempt to measure monocular visual acuity; successful measures at a same-day or subsequent-day retest were not included in the analyses.
Data Analysis
Age-, gender- and ethnicity-specific testability rates were calculated. Comparisons of testability among different groups were performed using chi-square analyses and the Cochran trend test (SAS software 9.1, SAS institute, Inc., Cary, NC). Testability rates were plotted against age by gender and by ethnicity with locally weighted regression lines using S-PLUS 7.0 (Insightful Corporation, Seattle, Washington). The asymptotic confidence intervals for proportions were calculated using the normal approximation to the binomial distribution.
RESULTS
Measurement of presenting monocular visual acuity was attempted on 3126 children 30 to 72 months of age. Of these, 1471 were African-American and 1655 were Hispanic; 50% were female. An educational level of attainment of a high school diploma or greater was reported by 41% of the mothers and 46% of the fathers of the Hispanic children and 85% and 93% of the African-American mothers and fathers, respectively. Major demographic characteristics of the participants are summarized in Table 1.
Table 1.
Demographic Characteristics of Multi-Ethnic Pediatric Eye Disease Study Participants
| Age | Hispanic/Latino (n=1655) | African American (n=1471) | Total (n=3126) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 30–36 months | 291 (18%) | 255 (17%) | 546 (17%) |
| 37–48 months | 463 (28%) | 389 (26%) | 852 (27%) |
| 49–60 months | 468 (28%) | 430 (29%) | 898 (29%) |
| 61–72 months | 433 (26%) | 397 (27%) | 830 (27%) |
|
| |||
| Gender | |||
|
| |||
| Male | 828 (50%) | 724 (49%) | 1552 (50%) |
| Female | 827 (50%) | 747 (51%) | 1574 (50%) |
Overall, 84% (83% African-American, 85% Hispanic; 86% female, 82% male) of the children were testable. Age-specific testability in children ages 30–36 months, 37–48 months, 49–60 months, and 61–72 months was 39%, 84%, 98%, and 100%, respectively (Table 2). Older children were more likely to complete testing successfully than younger children (P trend <0.0001). Testability rates increased steadily with age up to 41 months of age; by 42 months testability was 85% or greater, and after 46 months testability was 93% or greater (Figure 2).
Table 2.
Multi-Ethnic Pediatric Eye Disease Study Participants By Age, Gender, and Ethnicity Able to Complete Monocular Threshold Testing of Both Eyes Using the Amblyopia Treatment Study HOTV Visual Acuity Testing Protocol Presented on the Electronic Visual Acuity Tester
| Age in months
No. of participants |
30–36
n=546 |
37–48
n=852 |
49–60
n=898 |
61–72
n=830 |
|---|---|---|---|---|
| Gender | Number of Participants % (95% CI) | |||
| Female | 110
41 (35,47) |
368
87(84,90) |
455
98 (97,100) |
422
100 (99,100) |
| Male | 101
36 (30,42) |
344
80 (76,84) |
427
98 (97,99) |
403
99 (98,100) |
| Ethnicity | Number of Participants % (95% CI) | |||
| Hispanic/Latino | 110
38 (32,43) |
401
87 (84,90) |
459
98 (97,99) |
432
100 (99,100) |
| African-American | 101
40 (34,46) |
311
80 (76,84) |
423
98 (97,100) |
393
99 (98,100) |
| Total | 211
39 (35,43) |
712
84 (81,86) |
882
98 (97,99) |
826
100 (99,100) |
CI = 95% confidence interval
Figure 2.
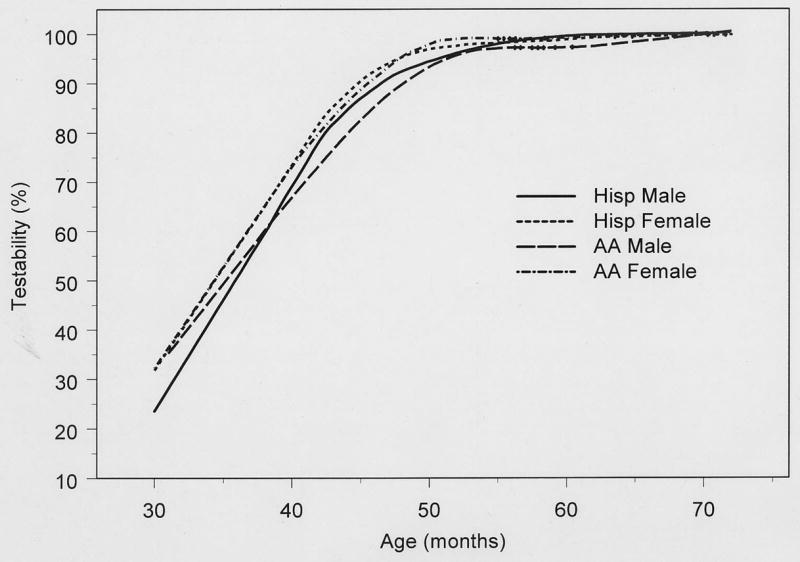
Testability of MEPEDS Participants by Month of Age: Monocular Threshold Testing Using the Amblyopia Treatment Study HOTV Visual Acuity Testing Protocol Presented on the Electronic Visual Acuity Tester
Table 2 summarizes the results for testability stratified by age, ethnicity, and gender. An ethnicity-related difference in testability (P=0.007) was found only for ages 37–48 months, with a greater proportion of Hispanic children successfully tested. Overall, there was no difference in testability between African-American and Hispanic children after adjusting for age (P = 0.12). However, after adjusting for age, there was a small but significant gender-related difference in testability (p=0.003); girls were more often able to complete testing (86%) than boys (82%). With age stratification, this difference was statistically significant (P=0.009) only in the 37 to 48 months age group.
Of the 235 children who were initially unable and subsequently attempted the retesting protocol, including a return visit retest, if indicated, 134 (57%) were able to complete testing successfully. Successful retesting occurred in 47% (76/129), 80% (52/65), and 67% (6/9) of the children aged 30–36 months, 37–48 months, and 49–60 months, respectively.
DISCUSSION
We found high testability rates overall for monocular threshold visual acuity testing using the ATS-HOTV testing protocol presented on the EVA tester in a large population-based sample of Hispanic and African-American preschool children. Four of 5 children aged 30 to 72 months were able to complete testing successfully and no ethnicity-related differences were found.
The number of children able to complete testing was age-dependent. Only about 40% of children 30 to 36 months of age were able to complete testing; however, nearly all children older than 48 months of age were testable. The rate of testability of visual acuity in young children is known to be a function of age, with children 4 years of age and older able to respond to most preschool visual acuity tests.5 Indeed, at least 93% of all children in our cohort were testable after 46 months of age (data not shown).
The percentage of MEPEDS children able to complete monocular visual acuity testing was generally as good as, or better than, reported previously. Our testability rates were somewhat higher, particularly in the 3-year-old age group, than those found by Holmes and colleagues1 who reported testability rates of 67% in 21 children 3 years old, 87% in 60 children 4 years old, and 94% in 32 children 5 years old using the same protocol presented on the Baylor-Video Acuity Tester (BVAT) (Medtronic Xomed Solan Ophthalmics, Jacksonville FL). Because the protocol is identical for the child regardless of whether the BVAT or EVA tester is used for letter presentation, no difference in testability would be expected. However, there could be some unknown factor related to differences in test administration. BVAT testing required the examiner to recall the protocol from memory, choose the correct order of acuity levels for presentation, and manually record the child’s responses on a paper form, whereas the testing protocol and presentation of letters is automated and results are recorded electronically when using the EVA tester. Alternatively, the difference in testability may simply be related to the specific children who made up the considerably smaller sample with a possibility that the age distribution of their 3-year-old children was younger than that of ours.
Moke et al. 2 reported testability rates of 85% (n=27), 94% (n=35), and 100% (n=71) in mostly White and developmentally normal 3-, 4-, and 5-to 7-year-old children, respectively. Although many of these children had been tested previously using isolated surrounded HOTV letters and therefore one might anticipate better performance, their testability rates are almost identical to our rates of 84%, 98%, and 100% found in 3-, 4-, and 5-year-old MEPEDS participants naïve to the test optotypes and protocol used in the study.
Using the same criterion for testing as our study, the Vision in Preschoolers (VIP) Study Group reported they successfully tested 93% of 3.5-year-old, 97% of 4-year-old, and 99% of 5-year-old children enrolled in Head Start Programs.3 While our testability rates of 98% for 4-year-olds and 100% for 5-year-olds are essentially the same as found in the VIP study, their 93% testability rate for children aged 3.5 years is higher than the 84% rate we found for our 3-year-old children. This higher rate could be related to several factors. First, the VIP study excluded children with developmental delay whereas we did not exclude these children. Second, unlike our study, they did not test any children in the 37- to 40-month-old age range, resulting in a different age distribution and probably an older mean age. Lastly, their children had prior experience with surrounded HOTV letters from a previous vision screening and ours did not.
Indeed, prior experience and practice appear to enhance performance for some preschool children. Eighty percent of the 65 MEPEDS children in the 37 to 48-month-old age range who attempted the retesting protocol after initially being unable to complete testing, were successful at a second test administration, either that same day or on a subsequent day no more than 3 months later. Retest success was age-dependent in that fewer (57%) of the 30 to 36-month-old children were testable with repeat administration. These rates, however, may not provide a true estimate of children testable at a second test administration because some of our initially non-testable children did not return for their scheduled second attempt at testing.
The small but statistically significant difference in testability with girls more often able to complete testing than boys, was significant only in 3-year-old children. This gender-related difference has not been reported in the aforementioned studies. We suspect that slight behavioral and maturational differences, on average, between the 3-year-old girls and boys in our study population, may affect the sustained attention and cooperation necessary for successful completion of visual acuity testing. Indeed, differences between boys and girls in terms of mental development and maturity can be identified as early as 1 year of age and remain at age 4 years.6
Because all testing was conducted by eye care professionals with considerable experience in working with young children and who were highly motivated to capture all data required for the MEPEDS vision examination, the testability rates for the preschool children reported herein are likely to represent the upper limit of testability in the populations studied. Testing performed in vision screening settings or by lay volunteers or other persons with less experience in working with children might result in lower testability rates.
We conclude from our findings that the ATS-HOTV visual acuity testing protocol presented on the EVA tester may be used successfully in the majority of preschool children to determine monocular threshold visual acuity as demonstrated in this population-based cohort of minority children aged 30 to 72 months. Our results support those from two previous small studies of clinical patients1, 2 and a non-population-based study of 41- to 60-month-old children.3 Eighty-four percent of all children 30 to 72 months old are testable. Testability is strongly dependent on age with 98% of children testable by 4.0 years of age. While there are no ethnicity-related differences in testability, girls are somewhat more likely to be testable than boys, particularly at 37 to 48 months of age. A highly useable testing protocol for visual acuity determination in the general preschool population, the ATS-HOTV protocol is not only suitable for use in clinical trials and epidemiologic studies, but also for the clinical management of vision disorders in preschool children seen in clinical practice.
Acknowledgments
This study was supported by grants EY14472 and EY03040 from the National Eye Institute, Bethesda, Maryland and an unrestricted grant from the Research to Prevent Blindness, New York, NY. The authors indicate no financial conflict of interest. Involved in the design of the study (S.C., K.T., S.A., M.B., R.V.); all authors (S.C., K.T., S.A., M.B., R.V., Y.W., A.D.) were involved in the collection, management, analysis, and interpretation of the data and the preparation, review and final approval of the manuscript. The protocol and Health Insurance Portability and Accountability Act-compliant informed consent forms were approved by the Institutional Review Board (IRB)/Ethics Committee of the Los Angeles County University of Southern California Medical Center, and the parent or guardian of each study participant gave written informed consent.
APPENDIX I THE MULTI-ETHNIC PEDIATRIC EYE DISEASE STUDY GROUP
University of Southern California
Rohit Varma, MD, MPH (Principal Investigator); LaVina Abbott (2002–2005); George Ayala (2005–2006); Stanley P. Azen, PhD; Tal Barak, OD; Mark Borchert, MD; Jessica Chang, OD; Felicia K. Chen, OD; Susan Cotter, OD, MS (Co-Principal Investigator); Jennifer Deneen, MPH; Jackie Diaz; Anne DiLauro, MPH; Jill Donofrio, MPH (2003–2005); Claudia Dozal (2003–2004); Athena Foong; James Gardner; Jackson Lau, OD; Jesse Lin, MS; George Martinez; Roberta McKean, PhD; Kisha Milo, Carlos Moya; Sylvia Paz, MS (2002–2005); Ana Penate; Claudia Salazar; Erin Song, OD; Kristina Tarczy-Hornoch, MD, DPhil; Mina Torres, MS; Natalia Uribe, OD; Ivania Verrico; Ying Wang, MS; Peng Zhao, MS; Amy Zhu.
Battelle Survey Research Center
Charles Aders (2003–2006); Candace Kwong, MPH; Nancy Noedel; Michael Preciado; Karen Tucker, MA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holmes JM, Beck RW, Repka MX, Leske DA, Kraker RT, Blair RC, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 2.Moke PS, Turpin AH, Beck RW, Holmes JM, Repka MX, Birch EE, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 3.Vision in Preschoolers Study Group. The electronic visual acuity tester: testability in preschool children. Optom Vis Sci. 2004;81:238–44. doi: 10.1097/00006324-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Varma R, Deneen J, Cotter S, Paz SH, Azen SP, Tarczy-Hornoch K, et al. The Multi-ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–62. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 5.Fern KD, Manny RE. Visual acuity of the preschool child: a review. Am J Optom Physiol Opt. 1986;63:319–45. doi: 10.1097/00006324-198605000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Nordberg L, Rydelius PA, Zetterstrom R. Psychomotor and mental development from birth to age of four years; sex differences and their relation to home environment. Children in a new Stockholm suburb. Results from a longitudinal prospective study starting at the beginning of pregnancy. Acta Paediatr Scand Suppl. 1991;378:1–25. doi: 10.1111/j.1651-2227.1991.tb12034.x. [DOI] [PubMed] [Google Scholar]



