Abstract
Previous studies have reported sex and estrous cycle dependent differences in the reinstatement of cocaine-seeking triggered by cocaine injections or drug-paired cues. However, the relationship between estradiol or progesterone levels and cocaine-seeking in a reinstatement model of relapse has not been explored. Thus, we examined changes in plasma hormone levels during cocaine-taking and cocaine-seeking behaviors in gonadally intact female rats. Rats self-administered cocaine (0.5 mg/kg/infusion) during daily 2-h sessions, followed by extinction. For reinstatement, cocaine (0, 5, or 10 mg/kg, i.p.) was administered 30 min prior to testing. Vaginal smears and blood samples were collected prior to and during chronic cocaine self-administration, extinction, and reinstatement testing. Relative to nonestrous females, females in estrus showed greater responding during self-administration, extinction, and during cocaine-primed reinstatement. The highest progesterone levels were noted at the time of lowest cocaine-seeking (proestrus) and the lowest levels of progesterone occurred at the time of highest cocaine-seeking (estrus). In contrast, plasma estradiol levels did not show any clear pattern with cocaine-seeking. These data from an animal model of relapse supports recent clinical evidence that progesterone reduces subjective craving in cocaine-dependent women. Overall, these results suggest that progesterone administration may be a useful intervention for reducing the incidence of relapse.
Keywords: progesterone, female, estrous cycle, cocaine, self-administration, reinstatement
1. Introduction
Epidemiological evidence suggests that significant sex differences exist in psychostimulant addiction. While men are more likely to have a cocaine abuse or dependence disorder (Brady and Randall, 1999), women begin using cocaine at an earlier age (Weiss et al., 1997), progress more rapidly from casual use to dependence (McCance-Katz et al., 1999; Westermeyer and Boedicker, 2000), and have higher rates of cocaine use than men (Griffin et al., 1989). In terms of relapse to cocaine use following a period of abstinence, women tend to have shorter cocaine-free periods (Griffin et al., 1989) and are more likely to relapse following stressful life events or depression (Back et al., 2005; Elman et al., 2001; McKay et al., 1996). Recent research has also demonstrated brain activation differences between abstinent men and women in response to events that can precipitate drug-craving and relapse, including exposure to cocaine-paired cues (Kilts et al., 2004) or stress-related imagery (Li et al., 2005), events that can precipitate drug-craving and relapse. While psychosocial factors likely contribute to these differences, considerable preclinical data suggests that biological factors may also play a significant role in sex differences of cocaine-related behaviors.
Similar to humans, preclinical studies have revealed sex differences in cocaine-taking and cocaine-seeking in laboratory animals. Compared to males, female rats more rapidly acquire cocaine self-administration (Hu et al., 2004; Lynch and Carroll, 1999), exhibit higher breaking points on progressive ratio schedules of reinforcement (Carroll et al., 2002; Hecht et al., 1999; Roberts et al., 1989), display greater responding on short access (i.e., 2-h) schedules (Fuchs et al., 2005; Kippin et al., 2005) and greater cocaine intake on extended access (i.e., > 6-h) schedules (Lynch and Taylor, 2004; Roth and Carroll, 2004). Recent data from animal models of relapse have demonstrated attenuated conditioned-cued reinstatement of cocaine-seeking in females (Fuchs et al., 2005), but enhanced cocaine-primed reinstatement relative to males (Kippin et al., 2005; Lynch and Carroll, 2000). Moreover, differences in self-administration (Hecht et al., 1999; Lynch et al., 2000; Roberts et al., 1989) and reinstatement (Fuchs et al., 2005; Kippin et al., 2005) behaviors vary as a function of the estrous cycle, with the greatest effects seen during the estrous phase, when levels of estrogen and progesterone are relatively low (Festa and Quinones-Jenab, 2004). While some evidence indicates a lack of effects of sex, gonadectomy, and gonadal hormones on cocaine self-administration (Caine et al., 2004), taken as a whole, previous studies suggest that sex differences in cocaine-related behaviors may be mediated, at least in part, by cyclic hormonal changes.
Several studies have demonstrated alterations in cocaine-motivated behaviors following ovariectomy and/or exogenous hormone administration. For example, estradiol administration reverses ovariectomy-induced decreases in self-administration (Hu et al., 2004; Jackson et al., 2006; Lynch et al., 2001; but see Grimm and See, 1997) and cocaine-primed reinstatement (Larson et al., 2005). On the other hand, acute progesterone treatment has been found to reverse estradiol's effects on the acquisition of cocaine self-administration (Jackson et al., 2006) and cocaine-primed reinstatement (Anker et al., 2006). These results suggest that estrogen and progesterone have opposing effects on the modulation of cocaine-seeking behavior. However, most previous studies have utilized ovariectomized female rats, which affects more than a single hormone. Moreover, exogenous replacement does not mimic the temporal pattern of ovarian hormone fluctuations found in human subjects.
We have previously utilized vaginal cytology procedures for the determination of estrous cycle phases (Fuchs et al., 2005; Kippin et al., 2005). However, measurement of ovarian hormone levels provides valuable information on estrous status, since chronic cocaine disrupts the estrous cycle in rats (Grimm and See, 1997; King et al., 1993; King et al., 1990) and the menstrual cycle in monkeys (Mello et al., 1997). Direct assessment of hormone levels also allows for comparison of hormone levels with behavioral responding. The relationship between estradiol or progesterone levels with cocaine self-administration and reinstatement of drug-seeking has been minimally studied. Therefore, the purpose of the present study was to examine plasma ovarian hormone levels during self-administration, extinction, and cocaine-primed reinstatement of cocaine-seeking in intact (non-ovariectomized) female rats.
2. Methods and Materials
2.1. Subjects
Female, Sprague-Dawley rats (n =30, initial weight 250-275 g; Charles River, Wilmington, MA, USA) were individually housed in a temperature- and humidity-controlled vivarium on a 12-h light-dark cycle (lights on 6AM-6PM). Animals were given water ad libitum and were maintained on 25 g of standard rat chow (Harlan, Indianapolis, IN, USA) per day for the duration of each experiment. Rats were acclimated to handling and allowed to adapt for a minimum of three days prior to the start of the experiment. All experimental procedures (except for initial lever response training) occurred between 7AM and 5PM. Housing and care of the rats were carried out in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
2.2. Lever response training
Rats were trained to lever press in standard operant conditioning chambers (30 × 20 × 20 cm) linked to a computerized data collection program (MED-PC, Med Associates, Inc., St. Albans, VT, USA). The chambers were equipped with two retractable levers, a stimulus light above each lever, a food pellet dispenser between the levers, a speaker linked to a programmable tone generator (ANL-926, Med Associates), and a house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan. Forty-eight hours prior to surgery, rats were food deprived overnight and trained to lever press on a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Noyes, Lancaster, NH, USA) during a 15-h overnight training session in the absence of explicit conditioned stimulus (CS) presentation (i.e., active lever presses resulted in the delivery of a food pellet only). Lever presses on an inactive lever were recorded, but had no programmed consequence. Following lever response training, food dispensers were permanently removed from the test chambers.
2.3. Surgery
Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (33 and 0.665 mg/kg, respectively, IP) followed by equithesin (0.25 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP). Surgical procedures were conducted using aseptic techniques. Catheters were constructed using previously described methods (Fuchs et al., 2004) and consisted of external guide cannulae with screw-type connectors (Plastics One Inc., Roanoke, VA, USA), Silastic tubing (10 cm; ID = 0.64 mm; OD = 1.19 mm; Dow Corning, Midland, MI, USA), prolite polypropylene monofilament mesh (2 cm diameter, Atrium Medical Corporation, Hudson, NH, USA), and cranioplastic cement. A small incision was made on the back and chest of the rat 5 mm above the area where the jugular vein enters the rib cage. The external guide cannula exited from the incision on the rat's back and the open end of the Silastic tubing was passed subcutaneously to the area of the jugular vein. The free end of the tubing was inserted 33 mm into the right jugular vein and secured with 4.0 silk sutures. Both incisions were sutured with 4.0 sterile surgical thread.
To maintain catheter patency, catheters were flushed once daily for 4 days after surgery with 0.1 ml each of an antibiotic solution of cefazolin (100 mg/ml; Schein Pharmaceuticals, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ, USA) and heparinized saline. For the duration of the experiment, each subject then received 0.1 ml of heparinized saline (10 U/ml) immediately prior to self-administration sessions and the cefazolin and 70 U/ml heparinized saline regimen following each session. Stylets were inserted into the catheters when the rats were not connected to infusion pumps. To verify catheter patency, rats occasionally received a 0.12 ml infusion of methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
2.4. Cocaine self-administration
Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% sterile saline; cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2-h sessions according to an FR 1 schedule of reinforcement. At the start of each session, the catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in a steel spring leash (Plastics One Inc., Roanoke, VA, USA). The house light signaled the initiation of the session and remained illuminated throughout the entire session. Lever presses on the active lever resulted in a 2-s activation of the infusion pump (0.5 mg/kg cocaine per 0.05 ml infusion) and a 5-s presentation of a stimulus complex, consisting of activation of the white stimulus light above the active lever and the tone generator (78 dB, 2 kHz). This cocaine bolus dose was selected based on our previous data showing that both males and females readily acquire self-administration and do not display differences in cocaine intake at this dose (Fuchs et al., 2005; Kippin et al., 2005). After each infusion, responses on the active lever had no consequences during a 20-s time-out period. During the sessions, responses on the inactive lever were recorded, but had no programmed consequences. Daily cocaine self-administration continued until each rat had obtained the self-administration criterion of ten sessions with at least ten infusions per session.
2.4. Extinction and reinstatement of cocaine-seeking
Following cocaine self-administration and before the first reinstatement test, rats underwent daily 2-h extinction sessions. During each session, responses on both levers were recorded, but had no consequences. Once active lever pressing extinguished to a criterion of a minimum of seven extinction sessions with ≤ 25 active lever responses per session for 2 consecutive days, animals underwent three cocaine-primed reinstatement tests. Using similar procedures, we have found equivalent levels of responding following all three cocaine-primed reinstatement tests (Berglind et al., 2006; Kippin et al., 2005; Ledford et al., 2003). Immediately prior to each 2-h reinstatement test, rats received an injection of cocaine hydrochloride (5 or 10 mg/kg, i.p.) or vehicle (0.9% physiological saline). The order of the cocaine-primed reinstatement tests was counterbalanced according to the average number of lever presses across the last three cocaine self-administration sessions and further extinction sessions occurred between reinstatement tests until extinction criteria were re-established (i.e. ≤ 25 active lever responses per session for 2 consecutive days). During the reinstatement test session, responses on both levers were recorded, but had no programmed consequences.
2.5. Estrous cycle monitoring
In order to ascertain the role of estrous cycle and ovarian hormones on cocaine-seeking behavior, vaginal lumen samples (immediately prior to and following the session) and whole blood (immediately following the session) was collected at the following time points: the day before self-administration (Pre-SA), the 7th day of cocaine self-administration (SA), the first day of extinction training (EXT), and on each reinstatement test. To provide points of reference for categorization, vaginal lumen samples were also collected the day before and the day after these time points. To habituate females to the vaginal cytology procedure, vaginal smears were taken daily 3 days prior to the Pre-SA baseline. Vaginal lumen samples were collected using a gentle sweeping motion with sterile cotton-tipped applicators. Smears were placed on glass slides, stained using Quick-Dip Hematology Stain (Mercedes Medical, FL, USA), examined using a light microscope set to 10x magnification and classified according to previously published criteria (Marcondes et al., 2002). The proestrus phase was defined as the presence of more than 75% nucleated epithelial cells. The estrous phase (note: vaginal estrus as opposed to behavioral estrus) was defined as the presence of more than 75% anucleated cornified epithelial cells. The diestrus I (also known as metestrus) phase was defined as the presence of approximately equal proportions of nucleated epithelial cells, anucleated cornified epithelial cells and leukocytes. The diestrus II phase was defined as a minimum amount of cells, including leukocytes and occasional epithelia. Due to the low number of animals from which diestrus smears were obtained, a lack of behavioral differences between females in the diestrus I and II states, and similar ovarian hormone profile (i.e., low estrogen and moderate levels of progesterone), females in the two diestrus phases were combined in statistical analyses and are hereafter referred to as diestrus I/II.
2.6. Radioimmunoassay
For determination of plasma levels of estradiol and progesterone, 1.2 ml of whole blood was collected through the catheter using 1000 U heparin. Fluid levels were replaced with an equivalent amount of filtered 0.9% physiological saline and there was a minimum of 3 days between each blood sample. Plasma was isolated by centrifugation (10,000 rpm at 4°C for 20 min) and stored at -80°C until assayed. Plasma progesterone (ng/ml) and estradiol (pg/ml) were determined using radioimmunoassay (Diagnostic Systems Laboratories, DSL-3900 and DSL-4800, Webster, TX, USA) according to the manufacturer's directions.
2.7. Data analysis
Lever responses, cocaine intake (mg/kg), and plasma hormone levels were analyzed using one-way analyses of variance (ANOVA) with estrous cycle phase as the between-subjects factor. For reinstatement testing, lever responses, plasma progesterone and estradiol levels were analyzed using a two-way ANOVA with estrous cycle phase and cocaine-priming dose as between subject factors. All post hoc analyses were conducted using Tukey's with the alpha set at 0.05. Data are expressed as the mean ± S.E.M.
3. Results
3.1. Cocaine self-administration, extinction, and reinstatement
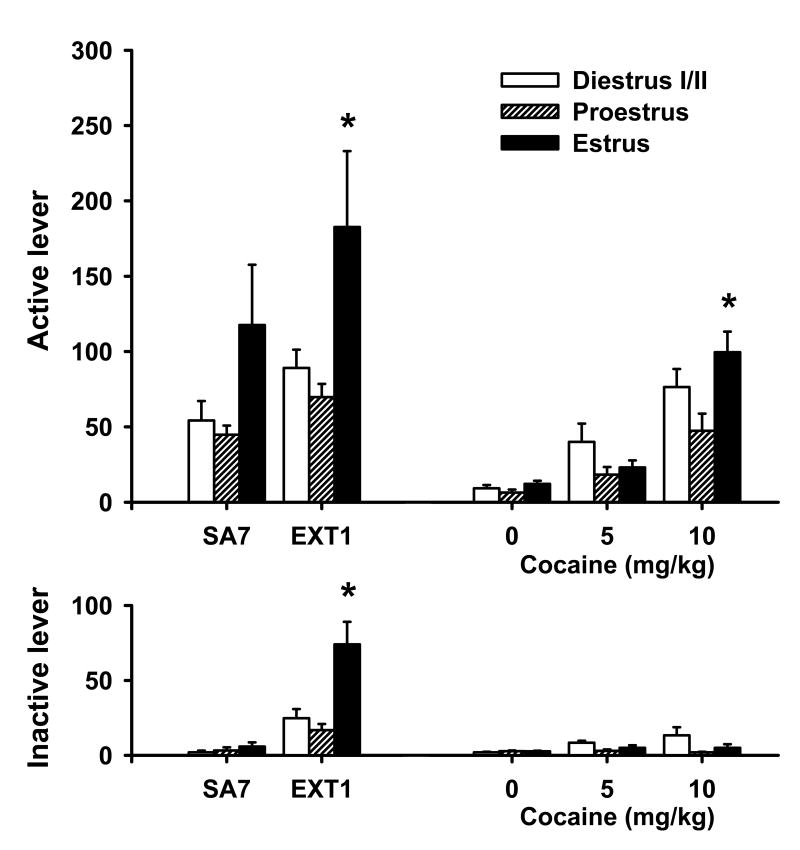
Animals readily acquired cocaine self-administration and maintained stable lever responding during the maintenance phase of self-administration. When animals were assessed for estrous cycle phase on day 7 of cocaine self-administration, females in estrus displayed elevated levels of active lever responding (Fig. 1) and cocaine intake, relative to females in other phases of the estrous cycle; however, this effect did not attain significance. Cocaine intake (mean ± S.E.M.) for the 2-h session on day 7 was: diestrus I/II = 16.45 ± 0.64 mg/kg, proestrus = 14.78 ± 1.62 mg/kg, and estrus = 19.52 ± 2.78 mg/kg.
Fig. 1.
Estrous cycle effects on active (top) and inactive (bottom) lever responding (mean ± SEM) on day 7 of cocaine self-administration (SA7, n = 8, 9 and 13 for Diestrus I/II, Proestrus and Estrus, respectively), day 1 of extinction (EXT1, n = 16, 9 and 5 for Diestrus I/II, Proestrus and Estrus, respectively), and during cocaine-primed reinstatement (0 mg/kg, n = 8, 9 and 9; 5 mg/kg; n = 5, 9 and 13; or 10 mg/kg; n = 9, 9 and 8 for Diestrus I/II, Proestrus and Estrus, respectively). During self-administration, active lever responses resulted in the delivery of a cocaine infusion (0.5 mg/kg/infusion). Each reinforced lever press was followed by a 20-s time out period, during which active lever responses had no programmed consequences. Responses during extinction and reinstatement had no programmed consequences. Females during the estrous phase showed greater responding on the active lever compared to females in proestrus on EXT1 and the 10 mg/kg cocaine-primed reinstatement test and on the inactive lever on EXT1 (*p < 0.05).
Similar to the maintenance phase of cocaine self-administration, females in estrus displayed enhanced cocaine-seeking behavior on the first day of extinction training (Fig. 1). One-way ANOVA revealed significant estrous cycle main effects for both active [F(2,29) = 6.47, p = 0.005] and inactive [F(2,29) = 10.97, p < 0.001] lever responding. Post hoc analyses of these data revealed that females in estrus exhibited greater active and inactive lever responding, relative to females in diestrus I/II or proestrus (p < 0.05).
Priming injections of cocaine produced a dose-dependent reinstatement of cocaine-seeking as seen by increased responding on the previously cocaine-paired lever (Fig. 1). No order effects were found across the three reinstatement tests. A 3 × 3 ANOVA of active lever responding revealed significant main effects for dose [F(2,70) = 52.12, p < 0.001] and estrous cycle [F(2,70) = 6.02, p < 0.005], as well as a significant dose by estrous cycle interaction [F(4,70) = 3.41, p < 0.05]. Post hoc analyses of these data revealed significantly greater active lever responding for animals in the 10 mg/kg treatment group relative to the 5 mg/kg or vehicle groups, as well as for diestrus I/II and estrous females relative to proestrus females (ps < 0.05). Similar analyses of inactive lever responding only revealed a significant main effect for estrous cycle [F(2,70) = 3.92, p < 0.05], with post hoc analyses revealing greater inactive responding for diestrus I/II females relative to females in proestrus (p < 0.05). The dose main effect and dose by estrous cycle interaction effect were not significant.
One-way ANOVA for the 10 mg/kg treatment group revealed significant estrous cycle main effects for active lever responding [F(2,25) = 4.44, p < 0.05], with post hoc analyses showing significantly higher active lever responding for estrous females relative to proestrus females (p < 0.05). Similar analyses for the vehicle or 5 mg/kg reinstatement test sessions, or for inactive lever responding on any test session, showed no significant main effects of estrous cycle phase.
3.2. Plasma progesterone and estradiol
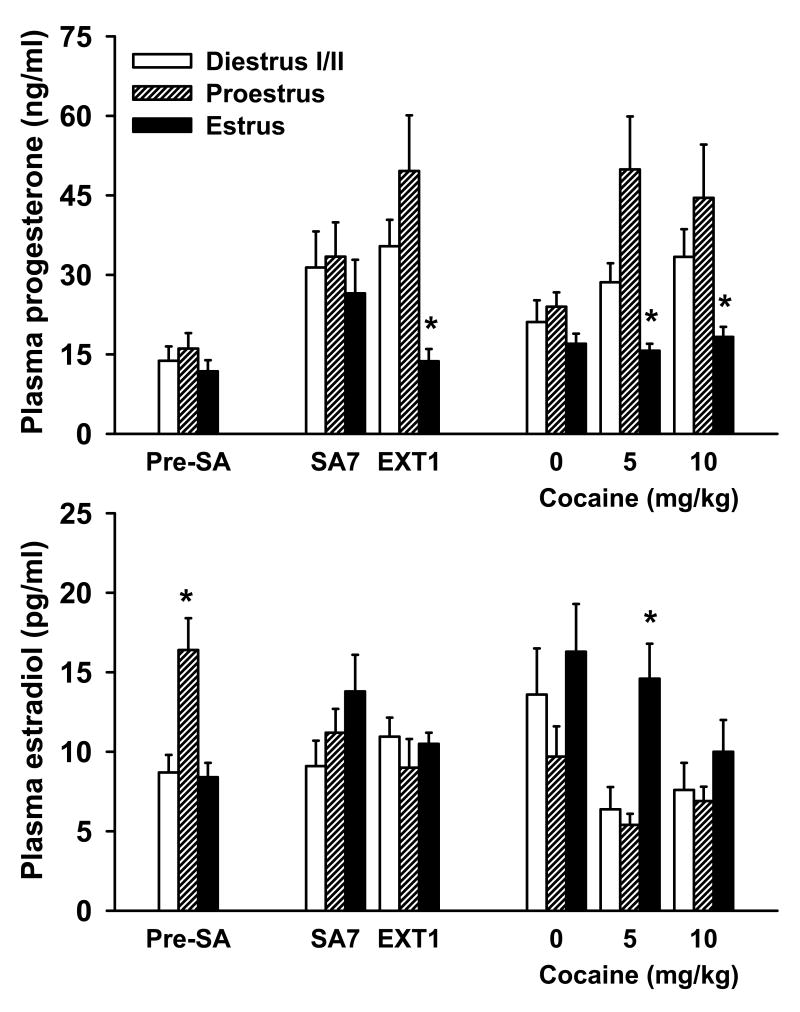
Prior to cocaine self-administration, peak plasma levels of estradiol and progesterone were noted for females in proestrus (Fig. 2). While a one-way ANOVA of plasma progesterone levels failed to reveal a significant main effect, there was a significant estrous cycle main effect for plasma estradiol levels [F(2,29) = 10.23, p < 0.001], with post hoc analyses showing significantly greater estradiol levels for females in proestrus, relative to females in estrus and diestrus I/II (p < 0.05). Thus, prior to any cocaine exposure, females showed a typical estradiol surge at proestrus.
Fig. 2.
Plasma levels of progesterone (top) and estradiol (bottom) as a function of estrous cycle phase prior to cocaine self-administration (pre-SA, n = 10/phase), on day 7 of cocaine self-administration (SA7, n = 8, 9 and 13 for Diestrus I/II, Proestrus and Estrus, respectively), day 1 of extinction (EXT1, n = 16, 9 and 5 for Diestrus I/II, Proestrus and Estrus, respectively), and during cocaine-primed reinstatement (0 mg/kg, n = 8, 9 and 9; 5 mg/kg; n = 5, 9 and 13; or 10 mg/kg; n = 9, 9 and 8 for Diestrus I/II, Proestrus and Estrus, respectively). Prior to chronic cocaine self-administration, females during the proestrus phase showed higher levels of estradiol compared to females in estrus or disestrus I/II (*p < 0.05). On extinction day 1 and during reinstatement, females in estrus showed significantly lower levels of progesterone that females in proestrus (*p < 0.05).
After one week of daily cocaine self-administration, neither plasma progesterone or estradiol levels differed across the estrous cycle, suggesting a disruption of normal estrous cycle hormone regulation. However, on the first day of extinction, one-way ANOVA revealed a significant estrous cycle main effect for plasma progesterone levels [F(2,29) = 3.98, p < 0.05], and post hoc analysis of these data revealed that females in estrus exhibited lower plasma progesterone levels than females in proestrus (p < 0.05). In contrast, as seen on self-administration day 7, plasma estradiol remained relatively constant across the estrous cycle.
For cocaine-primed reinstatement, a 3 × 3 ANOVA of plasma progesterone levels revealed significant main effects for dose [F(2,70) = 3.83, p < 0.05] and estrous cycle phase [F(2,70) = 12.95, p < 0.001], with post hoc analyses showing significantly reduced progesterone levels for animals in the vehicle group relative to the 10 mg/kg treatment group, as well as for estrous and diestrus I/II females relative to proestrus females, and estrous females relative to diestrus females (ps < 0.05). The dose by estrous cycle interaction was not significant. Similar analyses for plasma estradiol levels revealed significant main effects for dose [F(2,70) = 4.96, p < 0.05] and estrous cycle [F(2,70) = 7.55, p = 0.001], with post hoc comparisons indicating higher estradiol levels for animals in the vehicle treatment group relative to the 10 mg/kg group, and for diestrus I/II or estrous females as compared to proestrus females (ps < 0.05). The dose by estrous cycle interaction was not significant.
Further one-way ANOVAs were conducted for each reinstatement dosing group. No significant estrous cycle main effects were found for plasma progesterone and estradiol levels after vehicle treatment. However, ANOVA for the 5 mg/kg treatment condition showed significant estrous cycle main effects for plasma progesterone [F(2,26) = 9.59, p = 0.001] and estradiol [F(2,26) = 7.60, p < 0.005] levels, with post hoc analyses revealing significantly lower levels for estrous females relative to proestrus females, and for estrous females relative to both diestrus I/II and proestrus females, respectively (ps < 0.05). Finally, a one-way ANOVA for the 10 mg/kg treatment condition revealed significant estrous cycle main effects for plasma progesterone levels [F(2,25) = 3.52, p < 0.05], with post hoc analyses indicating significantly lower plasma progesterone levels for estrous females relative to proestrus females (p < 0.05).
4. Discussion
The present study demonstrates that the estrous cycle influences the level of cocaine-seeking following withdrawal from chronic cocaine self-administration and during cocaine-primed reinstatement. Specifically, estrous females displayed greater extinction responding and reinstatement of cocaine-seeking following a cocaine challenge (10 mg/kg) relative to females in other phases of the estrous cycle. These findings are consistent with our previous report of increased cocaine-paired lever responding in estrous females, relative to non-estrous females and males (Kippin et al., 2005). Furthermore, changes in plasma progesterone, but not estradiol, appear to be inversely related to cocaine-seeking. While significant changes were found in plasma progesterone levels and cocaine-seeking at both extinction and reinstatement, it should be noted that linear regression analysis of progesterone levels and lever responding failed to attain significance (p > 0.05), perhaps due to the limited number of subjects. However, these results further add to growing evidence for estrous cycle and ovarian hormone dependent differences in cocaine-related behaviors (Becker et al., 2001; Festa and Quinones-Jenab, 2004; Sell et al., 2002).
The present findings showed that estrous females display greater cocaine-seeking on day 1 of extinction, as well as a trend towards greater lever responding and drug intake during cocaine self-administration. Previous data has shown that the estrous cycle can modulate cocaineseeking, with females in estrus showing greater responding during cocaine self-administration (Hecht et al., 1999; Lynch et al., 2000). It should be noted that repeated collection of vaginal lumen samples may have affected responding, in that repeated lavaging (although not vaginal swabbing as used in the current study) has been noted to blunt cocaine-stimulated locomotor activity in non-ovariectomized rats, especially females in estrus (Walker et al. 2002). Estrous females on extinction day 1 showed elevated responding on both the active (i.e., previously cocaine-paired) and inactive levers. Increased responding on the inactive lever during extinction likely reflects enhanced utilization of alternative strategies in drug-seeking. Cocaine priming injections in the present experiment increased responding on the active, but not the inactive lever, indicating that the reinstatement of cocaine-seeking was not generalized to previously non-reinforced behaviors. Overall, our results indicate that estrous females possess greater motivation for cocaine and greater vulnerability to cocaine-primed reinstatement of extinguished drug-seeking behavior.
The most unique finding of the current study is the apparent inverse relationship between cocaine-seeking behavior and plasma progesterone levels. Few studies have examined progesterone in relationship to drug-seeking. However, it has been reported that pretreatment with progesterone inhibits cocaine conditioned place preference (Russo et al., 2003) and progesterone, but not estradiol, alters cocaine-induced locomotor behavior in female rats (Niyomchai et al., 2005). Furthermore, congruent with the current findings, progesterone inhibits the potentiating effects of estradiol on acquisition of cocaine self-administration (Jackson et al., 2006). Merely having low progesterone does not equate to greater cocaineseeking, as seen by the fact that levels of progesterone at estrus were fairly equivalent across reinstatement trials. However, higher levels of progesterone may blunt cocaine-seeking under particular circumstances, as seen in the proestrus group at the time of extinction and reinstatement testing following the 10 mg/kg priming dose of cocaine. Previous research has demonstrated that cocaine can enhance plasma progesterone levels in intact female rats, with the greatest effect noted for females in the proestrus phase (Quinones-Jenab et al., 2000). However, this increase was not noted in adrenalectomized animals (Walker et al. 2001), suggesting that this effect may involve cocaine effects on HPA axis activity, either through adrenally-derived progesterone release or HPA axis/ovary interactions. In the current study, the generally higher levels of progesterone seen during and after cocaine self-administration in the non-estrous phases of the cycle may have resulted from adaptive responses to chronic cocaine. However, females in the estrous phase apparently failed to show this increased progesterone response, thus contributing to greater drug-seeking during extinction and reinstatement.
The mechanisms whereby progesterone affects cocaine-seeking are unclear, although several possibilities exist. Progesterone acts at both progesterone and glucocorticoid receptors and modulates the interaction of glucocorticoids with their receptors (Handa et al., 1994; Strahle et al., 1989). In addition, one of its primary metabolites, allopregnanolone, can potentiate GABAA receptor activation (Bitran et al., 1995). Enhanced GABAergic tone has been suggested to blunt cocaine craving in humans (Brebner et al., 2002). Progesterone interaction with dopamine (DA) likely also plays a critical role, since DA activity has been shown to be affected by ovarian hormonal fluctuations, with peak activity noted during estrus (Becker and Cha, 1989; Xiao and Becker, 1994).
Previous evidence indicates that estradiol modulates cocaine-seeking, including findings that estradiol replacement in gonadectomized females facilitates the acquisition of cocaine self-administration (Hu et al., 2004; Jackson et al., 2006) and can enhance cocaine-primed reinstatement (Anker et al., 2006). However, it should be noted that these results come from studies of estradiol effects on drug-seeking in oviarectomized females, whereby exogenous estradiol is administered in a fashion that does not mimic endogenous phasic physiological levels. While we did not see significant changes in estradiol levels related to cocaine-seeking, it is important to note that the estradiol peak seen during proestrus (evident prior to cocaine self-administration) was not seen during and after chronic cocaine self-administration. This absence of a proestrus estradiol peak is likely due to disrupted estrous cycle activity that occurs following chronic cocaine self-administration. While not anovulatory, rodents and primates (Grimm and See, 1997; Mello et al., 1997) demonstrate disruptions in normal ovulatory cycle activity during and after cocaine self-administration, including repetitive days of estrus and reduced days of proestrus (King et al. 1990). While not measured in the current study, it is also possible that long lasting cocaine-induced changes in prolactin activity (Demaria et al., 2000; Mendelson et al., 1988) may have affected normal ovarian hormone cyclicity.
The current results suggest a relationship between progesterone and cocaine-seeking behavior in our animal model with relevance to cocaine-dependent women. While suggestive, our results are clearly limited, given the complex relationship between ovarian hormone levels and drug-seeking during the cyclical fluctuations of these hormones in intact animals. As in any study examining hormone/behavior relationships, plasma sampling at specific time points can not fully account for delayed alterations in neuronal activity in response to plasma levels of a particular hormone. For example, consistent with research suggesting opposing roles in drug-seeking behavior, estradiol and progesterone have been shown to increase and decrease dopaminergic activity in limbic and striatal neurons, respectively (Fernandez-Ruiz et al., 1990). However, these effects only occurred 4 hours after treatment, suggesting delayed peak responsiveness of these neurons to the effects of ovarian hormones. On the other hand, examination of nigrostriatal and mesolimbic dopaminergic neurons across the estrous cycle demonstrated a rise of activity during estrus and a decline in activity during proestrus (Fernandez-Ruiz et al., 1991). Moreover, the subjective effects of cocaine have been reported to be more intense during the follicular phase relative to the luteal phase in women (Evans et al., 2002; Sofuoglu et al., 1999). The estrous phase of the female rat and the follicular phase in women share the common feature of relatively low and stable levels of both estrogen and progesterone (Festa and Quinones-Jenab, 2004). Congruent with our results, recent clinical studies have reported that progesterone treatment attenuates the subjective effects of cocaine (Sofuoglu et al., 2002; Sofuoglu et al., 2004), an effect found in women, but not men (Evans and Foltin, 2006). Thus, both preclinical and clinical findings now suggest that cyclic fluctuations in ovarian hormone levels contribute to the motivational effects of cocaine. Future studies should be directed towards understanding how progesterone levels may predict relapse to cocaine taking in humans, as well as the use of exogenous progesterone to reduce relapse in the animal model and in cocaine dependent women.
Acknowledgments
We thank Ritu Mehta for providing excellent technical assistance. This research was supported by National Institute on Drug Abuse grants DA016511 (RES), DA07288 (MWF), and NIH grant C06 RR015455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Larson EB, Carroll ME. Effects of progesterone and estrogen on the reinstatement of cocaine-seeking in female rats. 68th Annual Meeting of the College on Problems of Drug Dependence.2006. [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brebner K, Childress AR, Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Demaria JE, Nagy GM, Lerant AA, Fekete MI, Levenson CW, Freeman ME. Dopamine transporters participate in the physiological regulation of prolactin. Endocrinology. 2000;141:366–374. doi: 10.1210/endo.141.1.7281. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Ramos JA. Time-course of the effects of ovarian steroids on the activity of limbic and striatal dopaminergic neurons in female rat brain. Pharmacol Biochem Behav. 1990;36:603–606. doi: 10.1016/0091-3057(90)90262-g. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz JJ, Hernandez ML, de Miguel R, Ramos JA. Nigrostriatal and mesolimbic dopaminergic activities were modified throughout the ovarian cycle of female rats. Journal of neural transmission. 1991;85:223–229. doi: 10.1007/BF01244947. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, Javors MA, Schenken RS. Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharmacol Exp Ther. 1993;264:29–34. [PubMed] [Google Scholar]
- King TS, Schenken RS, Kang IS, Javors MA, Riehl RM. Cocaine disrupts estrous cyclicity and alters the reproductive neuroendocrine axis in the rat. Neuroendocrinology. 1990;51:15–22. doi: 10.1159/000125310. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28:1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- Li CS, Kemp K, Milivojevic V, Sinha R. Neuroimaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2005;2:174–182. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kelly M, Diaz-Migoyo N, Sholar JW. The effects of chronic cocaine self-administration on the menstrual cycle in rhesus monkeys. J Pharmacol Exp Ther. 1997;281:70–83. [PubMed] [Google Scholar]
- Mendelson JH, Teoh SK, Lange U, Mello NK, Weiss R, Skupny A, Ellingboe J. Anterior pituitary, adrenal, and gonadal hormones during cocaine withdrawal. Am J Psychiatry. 1988;145:1094–1098. doi: 10.1176/ajp.145.9.1094. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Russo SJ, Festa ED, Akhavan A, Jenab S, Quinones-Jenab V. Progesterone inhibits behavioral responses and estrogen increases corticosterone levels after acute cocaine administration. Pharmacol Biochem Behav. 2005;80:603–610. doi: 10.1016/j.pbb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Perrotti LI, Ho A, Jenab S, Schlussman SD, Franck J, Kreek MJ. Cocaine affects progesterone plasma levels in female rats. Pharmacol Biochem Behav. 2000;66:449–453. doi: 10.1016/s0091-3057(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Strahle U, Boshart M, Klock G, Stewart F, Schutz G. Glucocorticoid- and progesterone-specific effects are determined by differential expression of the respective hormone receptors. Nature. 1989;339:629–632. doi: 10.1038/339629a0. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend. 1997;44:35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]