Abstract
Taurine is the most abundant free amino acid in the body and is present at high concentrations during development and in the early milk. It is synthesized from cysteine via oxidation of cysteine to cysteinesulfinate by the enzyme cysteine dioxygenase (CDO), followed by the decarboxylation of cysteinesulfinate to hypotaurine, catalyzed by cysteine sulfinic acid decarboxylase (CSAD). To determine whether the taurine biosynthetic pathway is present in mammary gland and whether it is differentially expressed during pregnancy and lactation, and also to further explore the possible regulation of hepatic taurine synthesis during pregnancy and lactation, we measured mammary and hepatic CDO and CSAD mRNA and protein concentrations and tissue, plasma and milk taurine concentrations. CDO and CSAD mRNA and protein were expressed in mammary gland and liver regardless of physiological state. Immunohistochemistry demonstrated the expression of CDO in ductal cells of pregnant rats, but not in other mammary epithelial cells or in ductal cells of nonpregnant rats. CDO was also present in stromal adipocytes in mammary glands of both pregnant and nonpregnant rats. Our findings support an upregulation of taurine synthetic capacity in the mammary gland of pregnant rats, based on mammary taurine and hypotaurine concentrations and the intense immunohistochemical staining for CDO in ductal cells of pregnant rats. Hepatic taurine synthetic capacity, particularly CSAD, and taurine concentrations were highest in rats during the early stages of lactation, suggesting the liver may also play a role in the synthesis of taurine to support lactation or repletion of maternal reserves.
Introduction
Taurine is the most abundant free amino acid in the body. Hypotaurine is synthesized from cysteine by the sequential action of 2 enzymes, cysteine dioxygenase (CDO3; EC 1.13.11.20), which catalyzes the oxidation of cysteine to cysteinesulfinate, and cysteine sulfinic acid decarboxylase (CSAD; EC 4.1.1.29), which catalyzes the decarboxylation of cysteinesulfinate to hypotaurine. It has not yet been established whether the final oxidation of hypotaurine to taurine is enzymatic, but relatively low concentrations of hypotaurine are found in mammalian tissues and hypotaurine accumulates in plasma and tissues when excess cysteine is fed to rats (1). In liver, where the majority of whole body taurine biosynthesis occurs, changes in CDO activity have a much greater effect on the rate of taurine biosynthesis than do changes in CSAD activity (2-4).
Numerous studies have shown that CDO and CSAD activities directly correspond to the respective enzyme concentrations in tissues. Tissue CDO levels are regulated predominantly at the level of protein ubiquitination and proteasomal degradation in response to intracellular cysteine concentrations (4-6). CDO is degraded when cysteine is limiting and needs to be conserved, whereas CDO is stabilized when cysteine availability is high (5,7). Although CDO mRNA concentrations generally correlate well with CDO expression across tissues, CDO mRNA expression does not correspond well with actual CDO concentrations within the liver or in primary hepatocytes where an increase in dietary protein or sulfur amino acid supply results in <60% increase in CDO mRNA but an 11- to 45-fold increase in CDO concentrations (4-6). Although the generation of cysteinesulfinate from cysteine appears to be the major determinant of taurine biosynthesis, changes in CSAD concentrations can modulate the partitioning of cysteinesulfinate metabolism between decarboxylation to hypotaurine (which is further oxidized to taurine) and transamination to yield pyruvate and sulfite (which is further oxidized to sulfate) (8,9). Differences in the abundance of CSAD mRNA are closely paralleled by differences in CSAD protein abundance in liver, lung, and brain, but rat kidney contains very high concentrations of CSAD mRNA relative to CSAD protein, suggesting the possibility that CSAD expression in some tissues may not be regulated through changes in mRNA abundance (4).
Most of the work on CDO and CSAD has focused on liver, the primary site of cysteine catabolism and taurine synthesis. Recent work, however, has shown that these taurine synthetic enzymes are expressed in many extrahepatic tissues including high concentrations in adipose tissue and modest levels in kidney (4,10-13), suggesting that the taurine biosynthetic pathway may also play an important role in nonhepatic tissues. The mammary gland is of particular interest because of the nutritional importance of taurine during early postnatal development. However, there is essentially no available information about CDO and CSAD expression in mammary gland except for a single report of CSAD mRNA expression in rat mammary gland (11).
The importance of taurine in early development is consistent with the presence of relatively high concentrations of taurine in tissues of developing animals and in milk (14-16). In rat milk, the concentration of taurine is highest in the colostrum and then drops precipitously to a lower level that is maintained throughout the remainder of the lactation period. A deficiency in taurine during fetal life or during suckling, specifically from the early milk, manifests itself in fetal and postnatal growth deficits in the pup (14,17) and in a variety of developmental defects, most notably in the visual and central nervous systems (16,18). A specific role for taurine in tissue morphogenesis is suggested by studies of pancreatic development. Taurine supplementation ameliorated the negative effects of a low protein diet during gestation on the development of the fetal rat pancreas, including the normalization of vascularization, islet cell mass, and insulin secretion (19-22). Furthermore, taurine supplementation of pregnant nondiabetic obese mice throughout gestation or until weaning resulted in greater pancreatic islet mass, reduced insulitis, and delayed onset of diabetes in the offspring (23).
Thus, to determine whether the taurine biosynthetic pathway is expressed in mammary gland, where it might contribute to taurine biosynthesis for secretion in the milk, and whether its expression is altered in response to pregnancy or lactation, we examined the expression of CDO and CSAD and measured taurine concentrations in mammary gland of nonpregnant rats and in rats at several time points during the course of pregnancy and lactation. Because the cellular composition of the mammary gland changes with pregnancy, we also examined the cellular localization of CDO within the mammary gland of nonpregnant and pregnant rats. Finally, we measured CDO and CSAD expression and taurine concentrations in the liver and plasma of pregnant and lactating rats to determine whether peak hepatic taurine concentrations were associated with increased concentrations of taurine biosynthetic enzymes, because we previously reported a dramatic peak in hepatic taurine concentrations in rat dams near the end of pregnancy (d 19-21) that was rapidly dissipated following parturition (15).
Materials and Methods
Animals, tissue collection, and preparation
Experimental procedures were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee. A total of 34 virgin female Sprague-Dawley rats (Harlan) were obtained and housed in a facility kept at constant temperature and humidity (∼22°C, ∼65% humidity) with controlled lighting ( 14-h light: 10-h dark cycle). All animals had access to a standard rodent nonpurified diet (Prolab RMH 1000, PMI Nutrition International 16.4% protein, 57.6% carbohydrate, 6.2% fat, 3.5% crude fiber) and water ad libitum. Estrus was determined by microscopic examination of vaginal smears, and females were mated with males in a breeding cage. The morning a copulation plug or sperm in the smear was detected was considered d 0.5. Pregnant females were randomly assigned to groups that were killed at d 13.5, 18.5, and 19.5 of pregnancy and d 2, 10, and 22 of lactation (birth = d 1 of lactation). Litter size was adjusted to 8 pups on d 2 after birth. Tissues from nonpregnant, nonlactating, nulliparous (NP) females were collected during estrus or diestrus and used as NP controls. Animals were anesthetized with sodium pentobarbital (50 mg/kg body weight, intraperitoneal). Lactating dams were given oxytocin (5 IU/kg body weight, intraperitoneal) and were then milked by manual stripping of the glands. Blood was collected by decapitation and immediately centrifuged to obtain plasma. Liver and the fourth inguinal mammary gland were removed and immediately frozen in liquid nitrogen.
Preparation of tissue lysates and Western immunoblotting
Tissue lysates were prepared and Western-blot analyses were conducted as previously described with minor modifications (5). Tissue samples were separated by SDS-PAGE and transferred to a PVDF membrane, and immunoreactive proteins were detected by chemiluminescence (Super-signal West Dura, Pierce) using an anti-CDO (1:5000 dilution) or anti-CSAD (1:10,000 dilution) and horseradish peroxidase-linked secondary antibody. Signals were detected by exposure to X-ray film and analyzed by NIH Image 1.63 software. The rabbit anti-CDO was raised against His6-tagged IMAC-purified rat CDO; the IgG fraction was purified from rabbit serum using a protein A column (BioRad Laboratories). The rabbit anti-rat CSAD was a generous gift from Dr. Marcel Tappaz [INSERM, France, (24)].
RNA isolation and analysis
Total RNA was isolated using Qiazol and RNeasy Mini kit (Qiagen). CDO and CSAD mRNA transcripts were measured by Northern blot analysis using radiolabeled cDNA probes corresponding to nucleotide 1- 600 of the coding region of rat CDO mRNA and nucleotide 1- 610 of the coding region of rat CSAD mRNA. Equality of loading and transfer was verified by 18S ribosomal RNA hybridization (25) and signals were quantified using a phosphorimager (Cyclone & OptiQuant Image Analysis, Perkin-Elmer).
Measurement of taurine
Frozen tissues were deproteinized by homogenization in 3 volumes of 5% (wt:v) sulfosalicylic acid, and plasma was deproteinized by mixing with 1 volume of 5% sulfosalicylic acid. The acid supernatant was obtained by centrifugation (18,000 × g for 15 min). Milk was defatted by centrifugation at 4°C, and defatted milk was deproteinized as described for plasma. The acid supernatants and standards were diluted 1:50 with a solution of 200 mmol/L borate buffer, pH 10.4, and analyzed for hypotaurine and taurine concentrations using a precolumn o-phthalaldehyde-2-mercaptoethanol derivatization method and separation by HPLC, as described in detail previously (5).
Immunohistochemistry
Mammary glands from a NP and a 16. 5-d pregnant rat were fixed in 4% (wt:v) paraformaldehyde, embedded in paraffin and sectioned into 4 μm sections. After deparaffinization, sections were immersed in 0.5% (v:v) hydrogen peroxide in methanol to block endogenous peroxidases and steamed in 0.01 mol/L citrate buffer, pH 6.0 for 15 min for antigen retrieval. Sections were blocked with 10% (v:v) nonimmune goat serum (Zymed, Invitrogen) in 2× casein (Vector Laboratories) prior to incubation, for 2 h at 37°C, with a 1:90 dilution of an affinity-purified rabbit- anti-rat CDO antibody (18) or an equivalent concentration of nonimmune rabbit IgG (IgG control) diluted in 1× casein in PBS. Application of biotinylated secondary antibody followed by streptavidin peroxidase and AEC chromagen substrate (Zymed) was performed for color development, and sections were counterstained with Gill’s #2 hematoxylin.
Statistical analysis
Quantitative data were analyzed by 1-way ANOVA followed by comparison of means with Tukey’s multiple comparisons test. Differences were accepted as significant at P ≤ 0.05. All quantitative data are expressed as means ± SE.
Results
CDO and CSAD are expressed in mammary gland
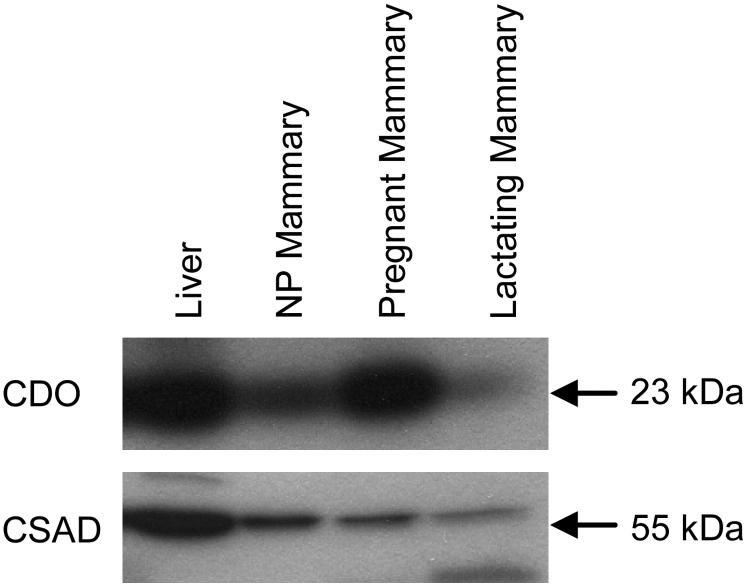
Both CDO and CSAD are expressed in rat mammary gland. Representative Western blots for CDO and CSAD are shown in Figure 1. Both proteins were clearly detectable in mammary gland of rats, although mammary CDO abundance appeared to vary with physiological state. In general, both CSAD and CDO were less abundant in mammary tissue than in liver, which expresses high levels of both enzymes.
FIGURE 1.
Western blot of CDO and CSAD in liver and mammary gland of rats during pregnancy and lactation. A volume of tissue lysate containing an equal amount of total soluble protein was loaded per lane. NP, nonpregnant/nulliparous.
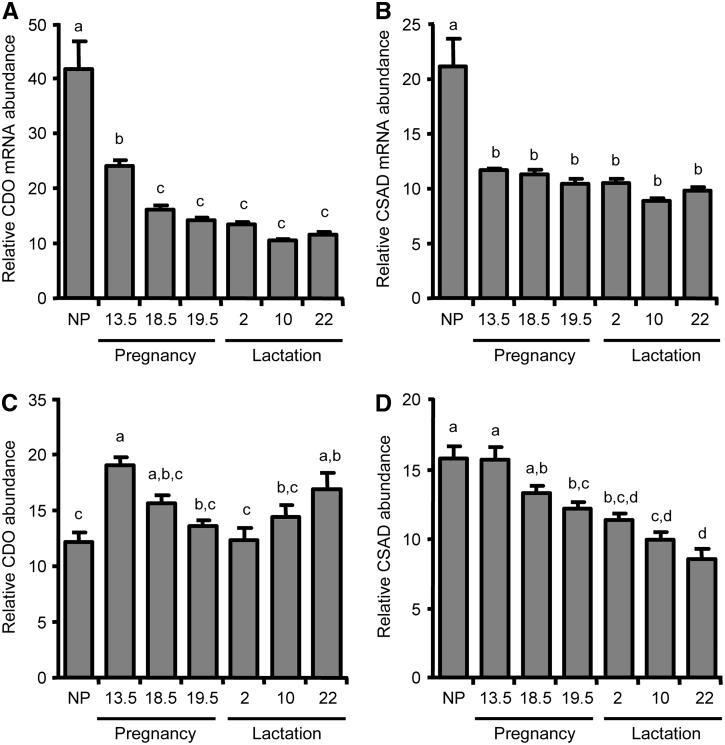
We further examined the expression of CDO and CSAD in mammary gland during pregnancy and lactation. Quantitative Northern blot analysis of CDO mRNA (Fig. 2A) and CSAD mRNA (Fig. 2B) showed decreases in the relative abundance of both CDO and CSAD mRNA in mammary gland during pregnancy to reach plateaus at ∼30 and ∼50% of the NP level, respectively. CDO and CSAD mRNA remained low throughout lactation. Quantitative Western blot analysis of CDO (Fig. 2C) and CSAD (Fig. 2D) showed less dramatic changes in relative protein abundance than in relative mRNA abundance over the course of pregnancy. Despite the much lower abundance of CDO mRNA, CDO protein abundance did not decrease over the course of pregnancy and lactation, and in fact, was higher (P ≤ 0.05) than the NP level at d 13.5 of pregnancy and d 22 of lactation. The disagreement in the expression of mammary gland CDO mRNA and protein concentrations is consistent with our previous observations for posttranscriptional regulation of CDO levels in liver, in which 11- to 45- fold changes in CDO concentration and CDO activity were obtained despite little or no change in CDO mRNA levels (4,9). In contrast to the abrupt decrease in mammary CSAD mRNA abundance within the first 13.5 d of pregnancy, CSAD abundance gradually decreased over the course of pregnancy and lactation, becoming lower (P ≤ 0.05) than the NP concentration by d 18.5 of pregnancy and remaining low throughout lactation. This slower decline of CSAD could be due to the longer half-life of CSAD protein compared with CSAD mRNA (26,27) or to regulation of CSAD expression at a posttranscriptional level in mammary gland, as suggested for rat kidney (4).
FIGURE 2.
Expression of CDO and CSAD mRNA and protein in rat mammary gland during pregnancy and lactation. Relative abundance of CDO mRNA (A) and CSAD mRNA (B) per unit of total RNA, as determined by Northern blot. Relative abundance of CDO (C) and of CSAD (D) per unit total soluble protein, as determined by Western blot. Each bar represents the mean ± SE (n = 4-5 rats). NP, nonpregnant/nulliparous. Bars not denoted by the same letter differ, P ≤ 0.05.
Hypotaurine and taurine concentrations in mammary tissue and milk
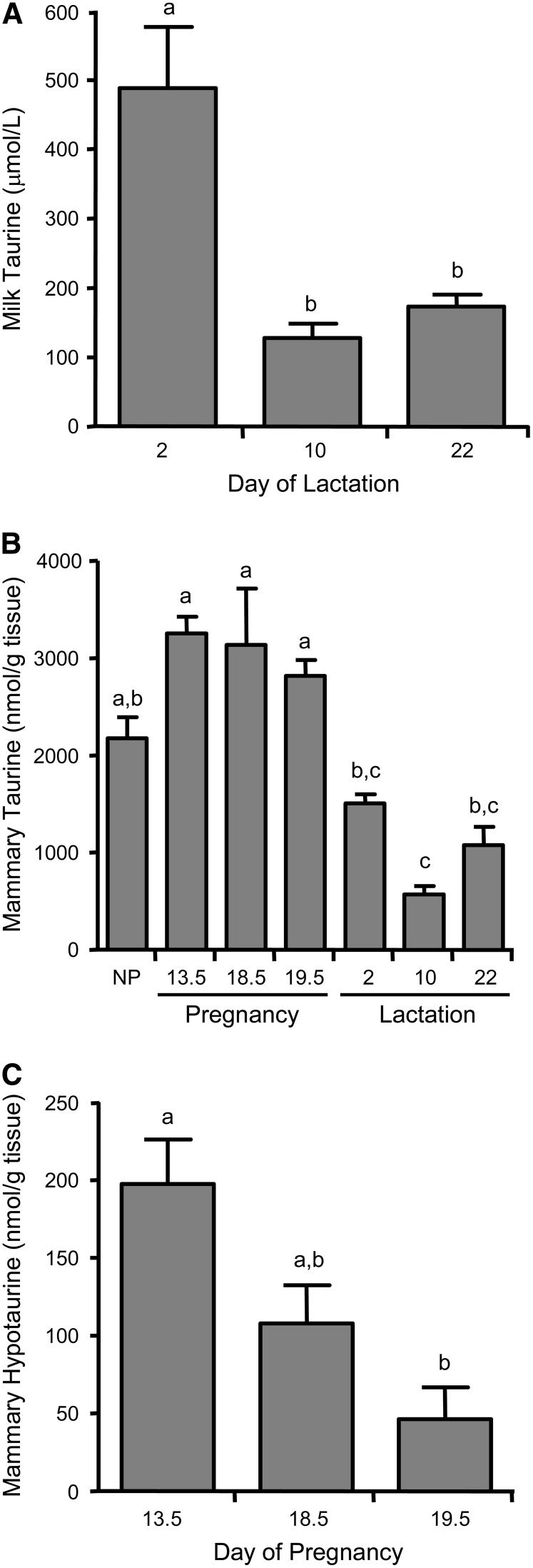
As previously reported (14,15), milk taurine concentration was highest in early lactation, just after birth (Fig. 3A), and then decreased by 75% and remained low at the end of the lactation period. Whereas milk taurine concentration declined dramatically shortly after the onset of lactation, the abundance of CDO in the mammary tissue increased and CSAD abundance declined only modestly over the course of lactation (Fig. 2C and D).
FIGURE 3.
Concentration of taurine in rat milk (A) and of taurine (B) and hypotaurine (C) in rat mammary gland during pregnancy and lactation. Each bar represents the mean ± SE (n = 4-5 rats). Hypotaurine was not detected in milk samples. NP, nonpregnant/nulliparous. Bars not denoted by the same letter differ, P ≤ 0.05.
Given that the enzymes required for the taurine biosynthetic pathway are present in the mammary gland, we investigated the concentrations of taurine and hypotaurine in mammary tissue. Mammary taurine concentrations were higher during pregnancy than during the lactation period (Fig. 3B). One possible explanation for lower taurine levels in mammary gland of lactating rats, compared with pregnant rats, could be the loss of taurine by secretion into the milk. We detected hypotaurine in mammary gland of pregnant rats but not in mammary gland of NP or lactating rats (Fig. 3C). The presence of hypotaurine, the immediate product of the sequential actions of CDO and CSAD, in tissues is generally considered to be a good indicator of a high rate of taurine biosynthesis in that tissue (1,5,7), suggesting mammary gland is actively synthesizing taurine during pregnancy. The significant 77% decrease in hypotaurine concentrations from d 13.5 to d 19.5 of pregnancy is consistent with the 29% decrease in CDO and the 23% decrease in CSAD abundance that occurred during this same time period. Taken together, these data indicate that rat mammary gland actively synthesizes taurine.
CDO and CSAD mRNA and protein concentrations in liver
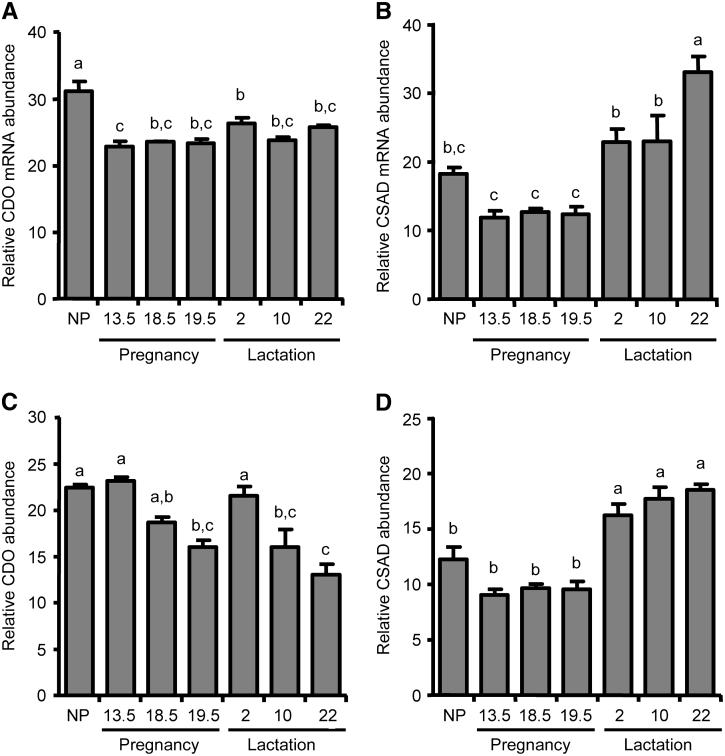
Hepatic CDO mRNA expression was highest in the NP rats, with abundance during pregnancy and lactation averaging 75% and 81%, respectively, of the NP level (Fig. 4A). The relative abundance of CSAD mRNA in liver during pregnancy did not differ from the NP level, but hepatic CSAD mRNA abundance was significantly higher during lactation than during pregnancy and reached 181% of the NP concentration by d 22 of lactation (Fig. 4B).
FIGURE 4.
Expression of CDO and CSAD mRNA and protein in rat liver during pregnancy and lactation. Relative abundance of CDO mRNA (A) and CSAD mRNA (B) per unit of total RNA, as determined by Northern blot. Relative abundance of CDO (C) and of CSAD (D) per unit of total soluble protein, as determined by Western blot. Each bar represents the mean ± SE (n = 4-5 rats). NP, nonpregnant/nulliparous. Bars not denoted by the same letter differ, P ≤ 0.05.
Hepatic CDO concentration was similar to the NP level at d 13.5 of pregnancy and d 2 of lactation but decreased over the course of both pregnancy and lactation so that concentrations at d 19.5 of pregnancy and d 10 and 22 of lactation were lower (P ≤ 0.05) than the NP level (Fig. 4C). These findings for CDO concentration are consistent with earlier observations for hepatic CDO activity during pregnancy and lactation (28). However, as anticipated, changes in hepatic CDO levels significantly changed during pregnancy and lactation, whereas hepatic CDO mRNA abundance showed little change during late pregnancy through the end of lactation. In contrast, both the CSAD concentrations and transcript abundance were low during pregnancy and increased during lactation, suggesting that regulation of hepatic CSAD concentration occurs mainly at the level of mRNA (Fig. 4D). CSAD concentrations in liver of lactating rats were consistently higher than those in NP or pregnant rats; hepatic CSAD levels reached 151% of NP levels by d 22 of lactation.
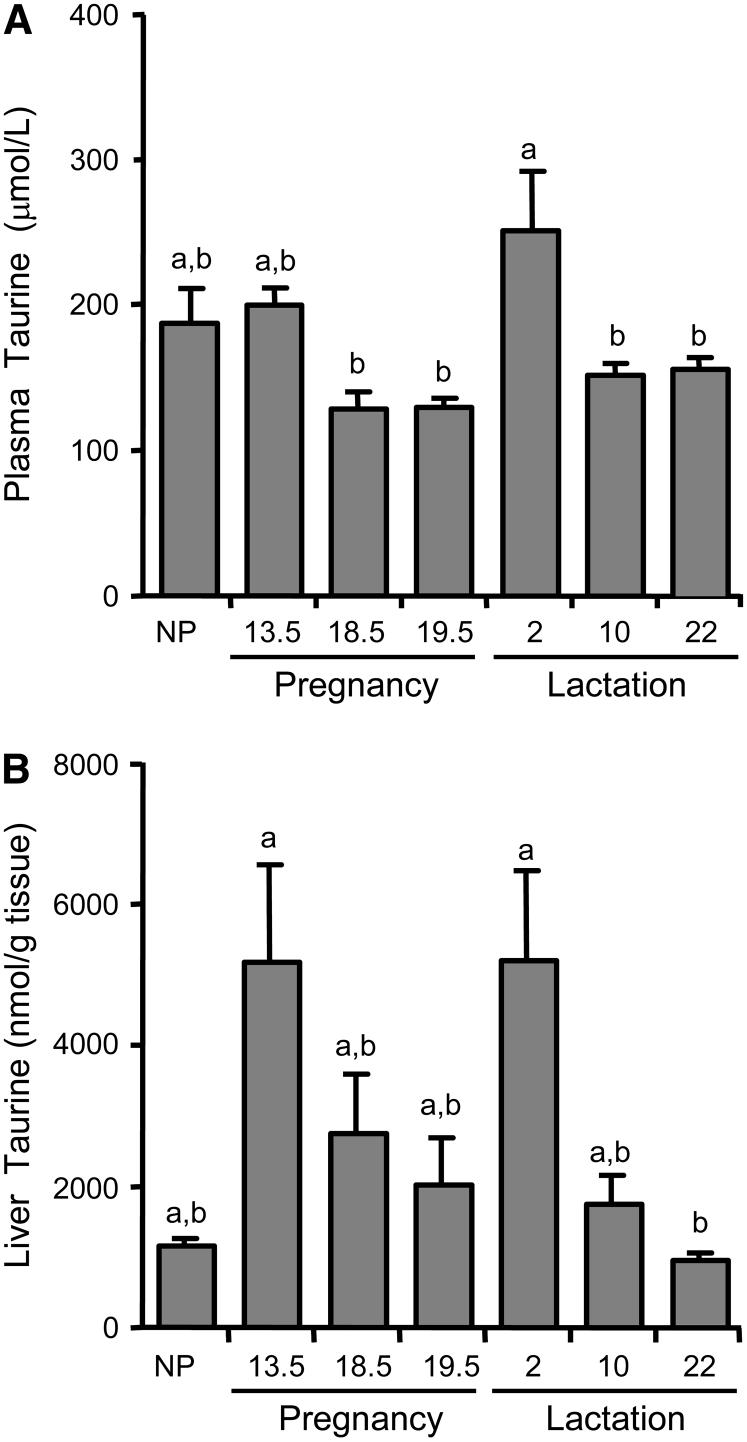
Taurine concentrations in plasma and liver
Neither the plasma nor the hepatic taurine concentration differed significantly from the NP level at any time point during pregnancy or lactation (Fig. 5A, 5B). However, plasma taurine was higher (P ≤ 0.05) at d 2 of lactation than at d 18.5 and 19.5 of pregnancy or d 10 and 22 of lactation. Also, the hepatic taurine concentration was greater (P ≤ 0.05) at d 13.5 of pregnancy and d 2 of lactation than at d 22 of lactation. Elevated taurine concentrations in liver and plasma were observed at d 2 of lactation when the concentration of taurine in the early milk was also high (Fig. 3A). In addition, hepatic levels of both CDO and CSAD at d 2 of lactation were greater (P ≤ 0.01) than at the end of pregnancy (d 19.5) (Fig. 4C and 4D). Furthermore, the changes in hepatic and plasma taurine concentrations over the course of lactation were similar to changes in the taurine concentration of the milk, although the fold changes were greater in liver than in plasma or milk. For example, liver taurine at the end of lactation (d 22) was only 18% of the d-2 level whereas plasma taurine was 62% and milk taurine was 35% of their d-2 levels. Overall, these results are consistent with a possible contribution of hepatic taurine synthesis to the pool of taurine secreted in the milk and suggest that increases in both CDO and CSAD may play a role in elevated taurine synthesis during early lactation.
FIGURE 5.
Concentration of taurine in rat plasma (A) and liver (B) during pregnancy and lactation. Each bar represents the mean ± SE (n = 4-5 rats). NP, nonpregnant/nulliparous. Bars not denoted by the same letter differ, P ≤ 0.05.
Localization of CDO in mammary gland
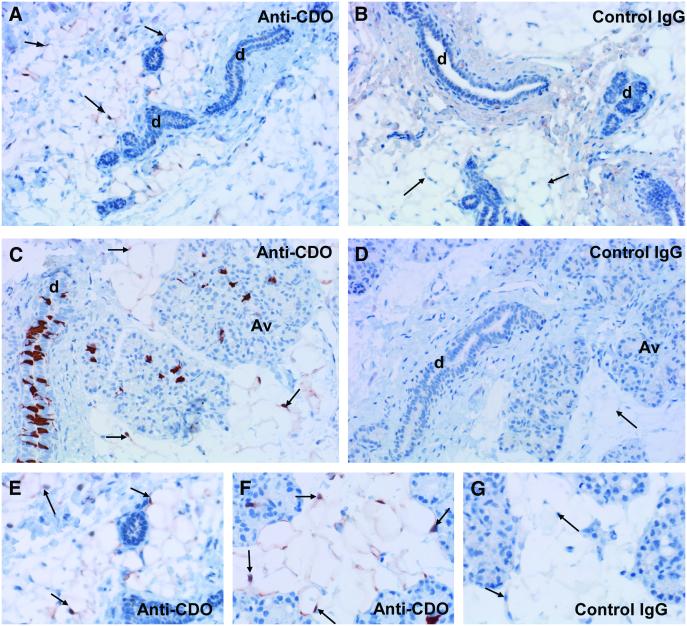
Immunohistochemistry demonstrated that localization of CDO differed between the NP and pregnant rat mammary gland (Fig. 6). The ductal epithelium did not show staining for CDO in the mammary of NP rats. In contrast, in the pregnant (d 16.5) rat, the ductal epithelial cells were intensely stained, and this staining was concentrated on the apical side of these cells. Little staining was observed in the milk secreting alveolar structures. We obtained evidence for the expression of CDO in mammary adipose cells for both NP and pregnant rats: the stromal adipoctyes showed staining for CDO, within the cytoplasm and some nuclei, located inside the plasma membrane and displaced by the fat droplet. In all cases, the IgG control sections showed no specific staining.
FIGURE 6.
Immunohistochemical localization of CDO. Mammary gland sections immunostained with anti-CDO (shown in red) and counterstained with Gill’s hematoxylin (shown in blue) are shown at 20× magnification. (A and B) Mammary gland of a nonpregnant rat. (A) Staining for CDO is detected in the cytoplasm and some nuclei of the adipocytes (indicated by arrows) in the stroma. No stain can be seen in the ducts. (B) The IgG control shows no specific staining in ductal epithelial cells (d) or adipocytes. (C and D) Mammary gland of a d 16.5 pregnant rat. (C) Staining for CDO is detected in the ductal epithelial cells with staining concentrated on the apical side of these cells. Little staining is observed in the alveolar structures (Av). Staining is found in the cytoplasm and some nuclei of the adipocytes. Note that the cytoplasm and nuclei of adipocytes are displaced toward the plasma membrane by the large central fat globule. (D) IgG control shows no specific staining in the ducts, alveolar structure or adipocytes. Representative sections of mammary adipoctyes from NP (E) or pregnant (F) rats stained for CDO or IgG control (G) from pregnant rats show staining in the cytoplasm and some nuclei of the adipocytes inside the plasma membrane, displaced by the fat droplet. The photos in panels E, F, and G were taken at 20× magnification, cropped and enlarged.
Discussion
Although both CDO and CSAD are highly expressed in liver where they play a primary role in the catabolism of cysteine and in taurine biosynthesis, it is now clear that CDO and CSAD are expressed in a number of nonhepatic tissues, including brown and white adipose tissues, kidney, lung, brain, and placenta (3,4,10,12,16,29,30). Expression of neither CDO nor CSAD has been detected in skeletal or cardiac muscle, testis, or spleen. Results presented here demonstrate that mammary gland is an additional tissue that expresses high concentrations of both CDO and CSAD.
In addition to demonstrating expression of CDO in mammary gland, we also observed both epithelial and adipocyte localization of CDO within the mammary gland. Expression of CDO in epithelial cells was specific for the ductal cells and depended upon physiological state. Ductal cells of pregnant rats showed intense staining for CDO, whereas no staining was observed in ductal cells of NP rats. This epithelial cell localization of CDO is consistent with CDO expression in the bron-chiolar epithelium in the lung and the convoluted tubular cells in the kidney (29) and with the localization reported for CSAD mRNA in the mammary epithelium in pregnant rats (11). In contrast, CDO was present in mammary adipocytes of both pregnant and NP rats. The expression of CDO within adipocytes in the stroma of the mammary gland is consistent with the observed expression of CDO in other adipose depots (12,30). Our immunohistochemical staining indicated that CDO exists in both the cytoplasm and the nucleus of the mammary adipocytes. This nuclear localization is consistent with the identification of a unique peptide fragment corresponding to the phosphorylation of a threonine 59 residue of CDO in a screen of nuclear phosphoproteins in HeLa cells (31).
In general, our results are consistent with a contribution of both mammary gland and liver to the biosynthesis of taurine. In particular, our findings provide evidence for significant taurine synthetic capacity in mammary gland of pregnant rats based on mammary taurine and hypotaurine concentrations and the intense immunohistochemical staining for CDO in ductal cells of pregnant rats. Hepatic taurine synthetic capacity, as judged by CDO and CSAD abundance, and taurine concentrations were highest in rats during the early stages of lactation. For both CDO and CSAD, enzyme activity and metabolic capacity are closely related to enzyme concentration (4,8,9). Nevertheless, given the complex changes in cell size and populations that occur during mammary development, including changes in adipoctye size and the relative proportion of adipoctyes to parenchymal epithelial cells, as well as the massive hyperplasia of both the ductal epithelial cells that express CDO and the nonstaining alveolar population, it is difficult to estimate total taurine synthetic capacity of mammary gland at various stages.
The precise function of the apparent regulation of CDO and of CSAD expression in the mammary gland during pregnancy and lactation is not obvious but could be related to mammary development and differentiation. The mammary gland is a dynamic organ that undergoes many cycles of proliferation, differentiation, and apoptosis during the reproductive cycle (32,33). The mammary gland is composed primarily of an adipose stroma, composed mainly of adipocytes and fibroblasts, within which the parenchyma, composed of a system of branching ducts and alveoli, can grow. Growth and development of the mammary gland in the first half of pregnancy involves a significant increase in proliferation of the epithelial parenchymal cells, with the formation of branching ducts and alveolar buds, as well as increased vascularization of the tissue. The basement membrane and ductal structures form in vitro only when mammary epithelial cells are cocultured with adipocytes or preadipocytes (34), suggesting that stromal cells may be necessary for mammary growth and morphogenesis. During the second half of pregnancy, the majority of differentiation of the gland occurs, especially near the end of pregnancy. During this lobuloalveolar phase, the proportion of ductal and alveolar epithelial cells constituting the parenchymal compartment increases dramatically, such that the relative proportion of parenchymal to stromal cells decreases markedly (33). It is possible that the differentiating mammary gland requires taurine for normal development and, in particular, for cell proliferation for ductal branching. An alternative possibility is that CDO is necessary to regulate cellular thiol concentration and redox state in cells undergoing proliferation and differentiation (35). Furthermore, CDO expression in the mammary gland could be subject to regulation by hormones, as has been shown in a few tissues by microarray analysis. For example, the combination of estrogen and progesterone, but not either hormone alone, increased CDO expression in the lacrimal gland of ovariectomized female mice (36), and growth hormone treatment suppressed CDO expression in liver of male rats (37,38). Clearly, further investigation of the hormonal regulation of the taurine synthetic enzymes is necessary.
The periparturient period and the onset of lactation mark rapid and dynamic changes in physiological state and nutrient demands on the dam for milk synthesis. Taurine synthesis may be important in providing taurine for secretion in the milk or in repletion of maternal reserves depleted during pregnancy or lactation. Mammary taurine levels appeared to become depleted during lactation, which might be due to further depletion of the stromal adipose tissue, to a decline in the CDO-expressing ductal cells, or to active secretion of taurine in the milk. Hepatic taurine levels also decreased over the course of lactation, as has been reported previously (15). Hepatic partitioning of cysteinesulfinate to taurine may increase in liver of lactating rats because increases in CSAD expression were observed in liver of lactating rats; this increase in CSAD expression could, in fact, be an adaptation to the low hepatic taurine concentration. The initial burst of CDO observed in liver during early lactation, possibly related to increased food intake or cysteine availability, might also play a role in increasing taurine availability to the mammary gland via the plasma in the periparturient period. However, additional studies would be necessary to assess the actual contribution of either hepatic or mammary taurine synthesis to the pool of taurine secreted during lactation.
In summary, we have clearly demonstrated the substantial expression of both CDO and CSAD in mammary gland. Furthermore, the unexpected specific localization of CDO to the ductal epithelium of pregnant rats, as well as the localization of CDO in the adipocytes within the mammary gland, raises new questions about the roles of these enzymes in nonhepatic tissues. The function of the robust expression of CDO and CSAD, and presumably the capacity for taurine synthesis, in mammary ductal epithelial cells and mammary adipocytes warrants further investigation.
Acknowledgments
We thank Dr. Marcel Tappaz (Institut National de la Santé et de la Recherche Médicale, Lyon, France) for providing us with the cysteine sulfinic acid decarboxylase antiserum. We would also like to thank Mr. Lawrence Hirschberger for his technical assistance with measurements of taurine and hypotaurine.
Footnotes
Supported by NIH grant DK056649.
Author disclosures: I. Ueki and M. H. Stipanuk, no conflicts of interest.
- CDO
- cysteine dioxygenase
- CSAD
- cysteine sulfinic acid decarboxylase
- NP
- nonpregnant
Literature Cited
- 1.Coloso RM, Hirschberger LL, Dominy JE, Jr., Lee JI, Stipanuk MH. Cysteamine dioxygenase: Evidence for the physiological conversion of cysteamine to hypotaurine in rat and mouse tissues. In: Oja SS, Saransaari P, editors. Taurine 6. Springer; New York: 2006. pp. 25–36. [DOI] [PubMed] [Google Scholar]
- 2.Garcia RA, Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr. 1992;122:1693–701. doi: 10.1093/jn/122.8.1693. [DOI] [PubMed] [Google Scholar]
- 3.Stipanuk MH. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem Res. 2004;29:105–10. doi: 10.1023/b:nere.0000010438.40376.c9. [DOI] [PubMed] [Google Scholar]
- 4.Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002;132:3369–78. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- 5.Dominy JE, Jr., Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. Biochem J. 2006;394:267–73. doi: 10.1042/BJ20051510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab. 2004;286:E439–48. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- 7.Stipanuk MH, Dominy JE, Jr., Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr. 2006;136:1652S–9S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 8.Bella DL, Hahn C, Stipanuk MH. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol. 1999;277:E144–53. doi: 10.1152/ajpendo.1999.277.1.E144. [DOI] [PubMed] [Google Scholar]
- 9.Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol. 1999;276:E326–35. doi: 10.1152/ajpendo.1999.276.2.E326. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberger LL, Daval S, Stover PJ, Stipanuk MH. Murine cysteine dioxygenase gene: structural organization, tissue-specific expression and promoter identification. Gene. 2001;277:153–61. doi: 10.1016/s0378-1119(01)00691-6. [DOI] [PubMed] [Google Scholar]
- 11.Hu JM, Ikemura R, Chang KT, Suzuki M, Nishihara M, Takahashi M. Expression of cysteine sulfinate decarboxylase mRNA in rat mammary gland. J Vet Med Sci. 2000;62:829–34. doi: 10.1292/jvms.62.829. [DOI] [PubMed] [Google Scholar]
- 12.Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism. 2002;51:1191–7. doi: 10.1053/meta.2002.34036. [DOI] [PubMed] [Google Scholar]
- 13.Stipanuk MH, Londono M, Hirschberger LL, Hickey C, Thiel DJ, Wang L. Evidence for expression of a single distinct form of mammalian cysteine dioxygenase. Amino Acids. 2004;26:99–106. doi: 10.1007/s00726-003-0001-4. [DOI] [PubMed] [Google Scholar]
- 14.Hu JM, Rho JY, Suzuki M, Nishihara M, Takahashi M. Effect of taurine in rat milk on the growth of offspring. J Vet Med Sci. 2000;62:693–8. doi: 10.1292/jvms.62.693. [DOI] [PubMed] [Google Scholar]
- 15.Stipanuk MH, Kuo SM, Hirschberger LL. Changes in maternal taurine levels in response to pregnancy and lactation. Life Sci. 1984;35:1149–55. doi: 10.1016/0024-3205(84)90185-1. [DOI] [PubMed] [Google Scholar]
- 16.Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–47. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- 17.Ejiri K, Akahori S, Kudo K, Sekiba K, Ubuka T. Effect of guanidinoethyl sulfonate on taurine concentrations and fetal growth in pregnant rats. Biol Neonate. 1987;51:234–40. doi: 10.1159/000242658. [DOI] [PubMed] [Google Scholar]
- 18.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Boujendar S, Arany E, Hill D, Remacle C, Reusens B. Taurine supplementation of a low protein diet fed to rat dams normalizes the vascularization of the fetal endocrine pancreas. J Nutr. 2003;133:2820–5. doi: 10.1093/jn/133.9.2820. [DOI] [PubMed] [Google Scholar]
- 20.Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia. 2002;45:856–66. doi: 10.1007/s00125-002-0833-6. [DOI] [PubMed] [Google Scholar]
- 21.Cherif H, Reusens B, Ahn MT, Hoet JJ, Remacle C. Effects of taurine on the insulin secretion of rat fetal islets from dams fed a low-protein diet. J Endocrinol. 1998;159:341–8. doi: 10.1677/joe.0.1590341. [DOI] [PubMed] [Google Scholar]
- 22.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861–73. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 23.Arany E, Strutt B, Romanus P, Remacle C, Reusens B, Hill DJ. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia. 2004;47:1831–7. doi: 10.1007/s00125-004-1535-z. [DOI] [PubMed] [Google Scholar]
- 24.Reymond I, Bitoun M, Levillain O, Tappaz M. Regional expression and histological localization of cysteine sulfinate decarboxylase mRNA in the rat kidney. J Histochem Cytochem. 2000;48:1461–8. doi: 10.1177/002215540004801103. [DOI] [PubMed] [Google Scholar]
- 25.Deindl E. 18S ribosomal RNA detection on Northern blot employing a specific oligonucleotide. Biotechniques. 2001 Dec;31:1250–2. doi: 10.2144/01316bm04. [DOI] [PubMed] [Google Scholar]
- 26.Jerkins AA, Jones DD, Kohlhepp EA. Cysteine sulfinic acid decarboxylase mRNA abundance decreases in rats fed a high-protein diet. J Nutr. 1998;128:1890–5. doi: 10.1093/jn/128.11.1890. [DOI] [PubMed] [Google Scholar]
- 27.Jerkins AA, Steele RD. Dietary sulfur amino acid modulation of cysteine sulfinic acid decarboxylase. Am J Physiol. 1991;261:E551–5. doi: 10.1152/ajpendo.1991.261.5.E551. [DOI] [PubMed] [Google Scholar]
- 28.Kuo SM, Stipanuk MH. Changes in cysteine dioxygenase and cysteinesulfinate decarboxylase activities and taurine levels in tissues of pregnant or lactating rat dams and their fetuses or pups. Biol Neonate. 1984;46:237–48. doi: 10.1159/000242071. [DOI] [PubMed] [Google Scholar]
- 29.Shimada M, Koide T, Kuroda E, Tsuboyama N, Hosokawa Y, Watanabe M. Expression and localization of cysteine dioxygenase mRNA in the liver, lung, and kidney of the rat. Amino Acids. 1998;15:143–50. doi: 10.1007/BF01345287. [DOI] [PubMed] [Google Scholar]
- 30.Tsuboyama-Kasaoka N, Hosokawa Y, Kodama H, Matsumoto A, Oka J, Totani M. Human cysteine dioxygenase gene: structural organization, tissue-specific expression and downregulation by phorbol 12-myristate 13-acetate. Biosci Biotechnol Biochem. 1999;63:1017–24. doi: 10.1271/bbb.63.1017. [DOI] [PubMed] [Google Scholar]
- 31.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA. 2004;101:12130–5. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masso-Welch PA, Darcy KM, Stangle-Castor NC, Ip MM. A developmental atlas of rat mammary gland histology. J Mammary Gland Biol Neoplasia. 2000;5:165–85. doi: 10.1023/a:1026491221687. [DOI] [PubMed] [Google Scholar]
- 33.Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–41. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- 34.Wiens D, Park CS, Stockdale FE. Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Dev Biol. 1987;120:245–58. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]
- 35.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Schirra F, Richards SM, Treister NS, Lombardi MJ, Rowley P, Jensen RV, Sullivan DA. Estrogen’s and progesterone’s impact on gene expression in the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2006;47:158–68. doi: 10.1167/iovs.05-1003. [DOI] [PubMed] [Google Scholar]
- 37.Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic Acid microarray analysis. Mol Endocrinol. 2004;18:747–60. doi: 10.1210/me.2003-0138. [DOI] [PubMed] [Google Scholar]
- 38.Ono M, Chia DJ, Merino-Martinez R, Flores-Morales A, Unterman TG, Rotwein P. Stat5b-mediated inhibition of IGF binding protein-1 gene transcription: a mechanism for repression of gene expression by growth hormone. Mol Endocrinol. 2007 Apr;:10. doi: 10.1210/me.2006-0543. [DOI] [PubMed] [Google Scholar]