Abstract
Few studies have contrasted performance of typically developing boys and girls on standardized motor assessment. In the present study, developmental status of the motor system was assessed in 144 typically developing children (72 boys, 72 girls, ages 7–14), using the Physical and Neurological Examination for Subtle Signs (PANESS, Denckla, 1985). Four summary variables were examined: (1) Gaits and Stations, (2) Overflow, (3) Dysrythmia, and (4) Timed Movements. For most variables, gender differences were not significant; however significant gender effects were observed for some subtle signs (involuntary movements), gaits and stations, and timed patterned movements. In all instances, girls showed fewer subtle signs and were faster and more proficient than boys. Significant age-related changes were observed for some subtle signs (dysrythmia and overflow), and for timed movements. In contrast, by age 7, many of the skills assessed by the PANESS have reached “adult” level in typically developing children. Motor development appears to follow a different developmental course in girls than in boys; separate gender and age norms should be used in clinical assessment of motor function in children.
The assessment of motor function is critical to understanding the biological basis of neurodevelopmental disorders (Guz & Aygun, 2004; Hadders-Algra, 2001; Kroes et al., 2002; Mostofsky, Newschaffer, & Denckla, 2003). The motor capabilities and the underlying neuroanatomic structures show substantial growth, elaboration, and myelination during early childhood (Denckla, 1973, 1974). Examinations used in clinical practice should be sensitive to subtle developmental changes that occur and can have implications for central nervous system development.
Careful assessment of basic motor function in children can reveal subtle motor deficits. Such neurological subtle signs include overflow (also called “associated” or “extraneous”) movements, involuntary movements (i.e., limb tremor, odd posturing, choreiform) and dysrhythmia (described in detail below). These subtle signs can serve as markers for inefficiency in neighboring parallel brain systems important for control of cognition and behavior. While it is common to observe these subtle signs in typically developing younger children (Deuel & Robinson, 1987; Largo, Caflisch, Hug, Muggli, Molnar et al., 2001b), persistence of subtle signs into later childhood and adolescence suggests motor dysfunction and is associated with atypical neurological development (Mostofsky et al., 2003). While there is evidence that persistence of subtle signs into later childhood can be a marker for atypical neurological function, there is less evidence to support a direct link between neurodevelopmental disorders (e.g., ADHD, Dyslexia) and specific subtle signs. Subtle neurological signs can be variable and their presence alone should neither be considered diagnostic nor the sole basis for explaining complex behavioral and neurological disorders (Touwen, 1979, 1987).
Overflow is defined as co-movement of body parts not specifically needed to efficiently complete a task. As typically developing children mature, they manifest fewer overflow movements (Largo, Fischer, & Rousson, 2003). The presence of age-inappropriate overflow may reflect immaturity of cortical systems involved with automatic inhibition (Denckla & Rudel, 1978).
Among overflow movements, the most studied are mirror movements (also referred to as synkinesis). The presence of mirror overflow movements in adolescents and adults with disorders of both the motor cortex and the corpus callosum suggests that the ability to perform unilateral fine motor movements is dependent upon intact interhemispheric and corticospinal connections (Knyazeva et al., 1997; Meyer, Röricht, & Woiciechowsky, 1998; Nass, 1985). Using transmagnetic stimulation (TMS; Garvey et al., 2003; Heinen et al., 1998) investigators have demonstrated that transcallosal inhibition is absent in children under 6 years of age and that it gradually matures to adult levels by early adolescence. Thus, when intra-and inter-cortical inhibitory and excitatory systems are immature, overflow movements in children are at their peak; as these cortical systems mature, overflow movements are more difficult to elicit. The persistence of overflow into late childhood and adolescence, often seen in children with ADHD (Morris, Inscore, & Mahone, 2001; Mostofsky et al., 2003) and other developmental disabilities suggests a neurodevelopmental lag in systems supporting the inhibition of overflow.
Choreiform movements are characterized by involuntary random, jerking motions, most often in the extremities (Delgado & Albright, 2003), and often described as “dance-like” movements. Choreiform movements suggest lapses in postural control and implicate immaturity of the postural system. They can affect execution of motor tasks, contributing to dysgraphia and fatigue during writing (Denckla, 1997). Wolff and Hurwitz (1973) found choreiform movements to be more prevalent in children who were reported to be inattentive, disorganized and immature, positing that the presence of this subtle sign may implicate “minimal brain dysfunction.”
Dysrythmia is an abnormality in an otherwise normal pattern of movements; it can be seen as an improper rhythm or timing of the movement. Dysmetria is the failure to focus the trajectory of an intentional movement (extremity coordination) and whereas an intention tremor, produced by goal-directed motor movements, involves increased rhythmic oscillation at a right angle to the line of movement as the target is approached. While dysrythmia, dysmetria and intention tremor are not diagnostic, presence of these signs may implicate cerebellar dysfunction (Schmahmann, 2004).
Speed of repetitive (foot tap, hand pat, and finger tap) and patterned movements (heel-toe, hand pronation/supination, and finger sequence) of the fingers, hands and feet also appears to follow a developmental course. For example, in young school-aged children, Wolff et al. (1985) reported age-related improvement in the speed of repetitive and patterned foot, hand and finger tasks. Examining the same timed repetitive movements, Denckla (1973; 1974) found that speed of performance improves with age and begins to plateau between ages 8–10 years. Largo et al. (2003) also reported age-related improvement performance of repetitive and patterned hand and finger movements; however, their findings suggested that speed of hand movements does not plateau until puberty, and speed of sequenced finger movements continues to improve beyond 18 years of age. Thus, age-related improvement in speed and efficiency of movements may differ depending on the type of movement (i.e., repetitive vs. patterned).
Gender differences can also be observed in the trajectory of motor development, and appear to be related to the differing neurological maturation patterns of boys and girls. These gender differences are observed on a variety of measures of motor function, including both speed and subtle signs. For example, girls are more accurate than boys in maintaining a steady rhythm during finger tapping (Nolan, Grigorenko, & Thorstensson, 2005), are more proficient than boys in peg placing (Largo, Caflisch, Hug, Muggli, Molnar et al., 2001a), and perform timed repetitive and patterned movements faster than boys (Denckla, 1973, 1974). As a result, most published tests of motor function (all emphasizing speeded tasks) use gender specific norms. There are fewer studies delineating gender differences in assessment of subtle signs among typically developing children.
Over the past three decades, motor assessment has been used extensively in research and clinical practice (see Barnett & Peters, 2004, for an extensive list of exams). Of the available assessment tools, few offer data from large, typically developing samples with regard to both speed and presence of subtle signs to which performance of clinical groups can be directly compared. Using the Zurich Neuromotor Assessment (ZNA), a tool designed to assess balance, gaits, speed and mirror overflow (referred to as “associated movements,” AM), Largo and colleagues (Largo et al., 2003) examined motor function in a large sample of typically developing children. The authors found age-related improvements in both speed of movements and presence of overflow. Additionally, some gender differences were observed; girls were able to stand on one leg longer than boys and perform adaptive pegboard tasks more quickly than boys. Gender-based differences in AM (subtle signs) were also observed, with girls demonstrating fewer overflow movements than boys. While this study highlights the need for age- and gender-specific norms for motor assessment, there may be additional types of subtle signs that are not included in the ZNA. Assessment of these subtle signs are of clinical significance in differentiating early signs of minimal brain dysfunction and developmental disability, such as those that characterize ADHD (Denckla & Rudel, 1978; Morris et al., 2001; Mostofsky et al., 2003).
To better describe and quantify the range of subtle signs and speed-related skills that manifest in children, Denckla (1985) developed the Physical and Neurological Examination for Subtle Signs (PANESS). This assessment tool includes a laterality inventory and allows for detailed examination of subtle signs via gaits and stations, and timed basic motor function. In addition to those skills emphasized in the Zurich Neuromotor battery (Largo et al., 2003), the PANESS quantifies not only mirror, but also additional types of overflow (proximal, orofacial, feet-to-hand), and also delineates dysrythmia, dysmetria, and choreiform—movements that are often indicative of atypical neurodevelopment. The PANESS was developed to be sensitive to developmental changes, to minimize the need for equipment, to eliminate time-consuming and less reliable sensory tasks, and to be completed in 15–20 min (described in detail below).
The purpose of the present study was to contrast the development of basic motor functions in typically developing boys and girls, and to determine whether patterns of age-related changes in these skills differ between boys and girls. In particular, the present study emphasized both motor speed and presence of subtle signs (which, for the purpose of this paper, will include overflow, dysrythmia, dysmetria and choreiform movements). We hypothesized that observed gender differences in both speed and subtle signs would favor girls. We also hypothesized that motor speed would improve with age, and plateau by adolescence, and that anomalous subtle signs would decrease with age.
METHOD
Participants
A total of 144 typically developing children (72 boys, 72 girls), ages 7–14, participated in this study. Participants were recruited as part of research projects at the Kennedy Krieger Institute from 1992 to 2005. It was determined by either a structured telephone-screening interview (n = 52), or by a standardized psychiatric interview with parent (Diagnostic Interview for Children and Adolescents-IV; DICA-IV, Reich, 2000) (n = 92) that all participants were free of any psychiatric diagnosis, were not taking any psychotropic medication, and had no history of seizures or evidence of any other neurological disorder.
Procedure
Each child was administered both an IQ test and the PANESS as part of a battery of behavioral tasks. Given that the motor assessment data have been collected over a 13-year period, children were screened for intellectual functioning using the most current version of the Wechsler Intelligence Scale for Children (WISC) at the time of testing, WISC-Revised (Weschler, 1974) (n = 39), WISC-3rd edition (Weschler, 1991) (n = 70), or the WISC–4th edition (Weschler, 2003) (n = 35). Socioeconomic status (SES) was determined for each child in the sample using the Hollingshead Index (Hollingshead, 1975).
The study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and written assent was obtained from all participating children.
Motor assessment
Motor function was assessed using the revised PANESS (Denckla, 1985); an overview of the variables assessed is provided in Table 1. In the 1970s (Denckla, 1973, 1974) the PANESS was originally normed on 168, predominantly white, middle class, elementary age children with an average IQ. Since that time, the PANESS has been found to have adequate test-retest reliability (Holden, Tarnowski, & Prinz, 1982), inter-rater reliability, and internal consistency (Vitiello, Ricciuti, Stoff, Behar, & Denckla, 1989).
TABLE 1.
Variables of the PANESS
| Exam | Component | Scoring | Scoring Notes |
|---|---|---|---|
| Lateral preference | Eye
Foot Hand |
Right, left, mixed | ≥3 pantomimes performed with non-dominant hand, code as “mixed”, use left handed norms. |
| Gaits | Walking:
on heels on toes on sides of feet Forward tandem Backward tandem |
Errors: 0, 1, 2
Overflow: right, left or both |
Walking on sides of feet: Only code errors for children ≥9 years; children ≤8 years code 0
Backward tandem: Only code errors or children ≥10 years, children ≤ 9 years code 0 |
| Stations | Stand:
Tandem, one foot in front of the other Feet together, eyes closed, arms and fingers outstretched Feet comfortable, eyes closed, tongue protruding Finger-to-nose Stand on 1 foot (both right and left foot) Hop on 1 foot (both right and left foot) |
Time: 0 (20 sec.), 1 (10–19 sec.), 2 (<10 sec.)
Tongue/finger choreiform: 0, 1, 2 Finger-to-nose: 0 (normal), 1 (clumsy, mild dysmetria, mild limb tremor), 2 (intention tremor, past-pointing) |
Hops: maximum of 25 if ≤8 years and 50 if ≥ 9 years |
| Timed motor exam: repetitive movements | Foot tap
Hand pat Finger tap |
Time to do 20 touches (recorded in seconds) | Hand Pronation/Supination: code mirror overflow only for children ≥ 9 years |
| Timed motor exam: patterned movements | Heel-toe tap
Hand pronate/supinate Finger sequences Tongue wiggles |
Right and left overflow: 0, 1 (proximal, orofacial, mirror and jaw synkinesis)
Left, right, tongue dysrhythmia: 0, 1 |
Finger sequences: code mirror overflow only for children ≥ 13 years |
Note. PANESS = Physical and Neurological Examination of Subtle Signs; all tasks are given to all ages, however for some tasks age does play a factor when translating raw numbers into PANESS scores.
PANESS administration
Requiring only a stopwatch and record form, the PANESS measures salient components of motor function, including lateral preference, gaits, balance, motor persistence, coordination, overflow, dysrhythmia, and timed movements (repetitive and patterned). Lateral preference (hand, foot, eye) is assessed by asking the child to demonstrate a variety of lateralized tasks with the hand (show me how you: comb your hair, brush your teeth, cut with scissors, throw a ball, hit a ball with a bat, hit a ball with a racket, use a hammer, use a screwdriver, use a saw, flip a coin, and open a door with a key), the foot (show me how you: kick a soccer ball and stamp out a fire), and the eye (show me how you: look through the lens of a camera). Assessment of gaits includes asking the child to walk ten paces on heels, toes, and sides of feet, as well as walking ten paces in tandem (heel touching toe) both forwards and backwards. Balance is measured by having the child stand on one foot and then hop on one foot; both the right and left foot were tested. Motor persistence and involuntary movements are assessed with three “station” tasks: 1) standing tandem (one foot in front of the other) with eyes closed, 2) standing with feet together, arms outstretched with fingers spread and eyes closed, and 3) standing with eyes closed, mouth open and tongue protruding. Motor coordination is examined using a finger-to-nose task in which the child alternates placement of index finger from his/her nose to the examiner’s index finger (which is placed in four quadrants). The task is performed bilaterally.
The timed activities assessed in the PANESS include 3 sets of “repetitive” and three sets of “patterned” movements—all performed on the right and left while seated. Repetitive movements are simple flexion movements that are repeated as quickly as possible, including toe tapping (e.g., heel on floor lifting only toes), hand patting (e.g., wrist on thigh lifting hand), finger tapping (e.g., hand open, tapping index finger to thumb). Patterned movements are alternating patterns of more complex movements performed quickly as possible, including heel-toe tap (e.g., rock from heel to toe), hand pronate/supinate, and finger sequence (i.e., open hand, touch every finger to thumb, always in the following order: index, middle, ring, pinky, index, etc). For all timed movements, the child is instructed to “Do all of these movements as quickly as you can, and as best as you can,” the examiner then demonstrates the correct movement and allows the child to briefly practice. Once the child demonstrates a steady pace, the examiner begins timing. The “time to do 20 touches” is recorded for each movement, and includes 20 toe taps, 10 sets of heel-toe taps, 20 hand pats, 10 sets of hand pronate/supinate alternations, 20 finger taps, and 5 sets of finger sequences. Finally, tongue wagging is assessed by asking the child to move his/her tongue laterally back and forth while protruded, touching the corners of the mouth 20 times.
PANESS scoring
Detailed procedures for scoring are provided in Table 1. Hand preference is determined based on performance of the pantomimed tasks. The child is considered right- (left-) handed if he/she uses right (left) hand to perform 9 or more of the 11 pantomimed tasks. If the child uses his/her non-dominant hand to perform 3 or more of the 11 tasks, he/she is considered “mixed” handed, and left-handed norms are used for scoring. Gaits are scored by counting the number of errors (i.e., placement of foot flat on the ground for stressed gaits, foot misplacement or spacing errors on tandem gaits). Overflow movements are considered to represent inefficiency in performing a motor task, and can represent failure of inhibition of prepotent movement. Overflow is documented during both gaits and timed activities. For gaits, the examiner observes for “foot-to-hand overflow” which involves flexion of hand and wrist while the child is walking on heels, toes and sides of the feet. Awkward posturing of arms, hands or body, is also recorded during stressed gaits. Balance tasks are scored by counting the number of hops for each foot and the time standing on each foot. During tasks of motor persistence, the time the child stands and maintains closed eyes is recorded. In addition, choreiform movements of arms, fingers and tongue are recorded during performance of all station tasks. Errors observed during gait and station tasks are summed and reported as right, left, total “axial” scores. In the finger-to-nose motor coordination task, dysmetria, limb tremor, intention tremor, and past pointing are recorded. For timed movements, overflow is categorized by the proximity of the extraneous movement to the intended movement. Proximal overflow involves movement of a muscle group in close proximity to the intended movement, and also includes exaggerated movement of the intended body part (e.g., lifting at elbow rather than wrist during hand patting; movement of ring and pinkie finger when tapping index finger to thumb). Orofacial overflow involves movement of mouth, tongue, and facial muscles during hand and/or leg movements. Mirror overflow involves unintended contralateral movements of homologous muscles, often observed in distal limbs, which accompany voluntary movements (Mayston, Harrison, & Stephans, 1999). During timed movements, the time to complete 20 touches, dysrhythmia, and the presence of overflow are recorded. Based on initial findings during the development of the PANESS (Denckla, 1974, 1985), some tasks were scored (or not scored) as errors based on the age of the child. Some subtle signs are expected in younger children, but not older children (e.g., foot-to-hand overflow when walking on sides of feet is expected in children under 10-years-old, but not those 10 years and older). Thus, an 8-year-old showing overflow on that task would not be scored, whereas an 11-year-old with overflow would be scored.
Scores from each section of the PANESS are used to create four summary variables. For these four summary variables, the scores are expressed as either as mean time in seconds or as a sum of right- and left-sided errors. The summary variables include: (1) Total Gaits and Stations, which includes total axial (gait, station and balance tasks) performance errors and total involuntary movements (i.e., tremor, choreiform, abnormal posture); (2) Total Overflow, observed during stressed gaits and timed movements, (3) Total Dysrythmia, observed during timed movements; and, (4) Total Timed Movements, including all thirteen repetitive and patterned movements, and tongue wagging.
Statistical Analysis
Data were initially analyzed using a series of four 2 (gender) × 8 (whole year age bands) factorial ANOVAs, with the four summary scores as dependent measures.
RESULTS
Demographics and IQ
By design, there were equal numbers of boys and girls in the sample. The mean age for boys was 9 years 9 months and was 10 years 1 month for girls. The sample was predominantly Caucasian (85% Caucasian, 10% African-American, 5% Asian, Hispanic, and Native American), and right-handed (91%; with 6 left-handed boys and 3 left-handed girls). The average Full Scale IQ (FSIQ) for boys was 115.8 (range 87–138) and for girls was 112.1 (range 87–139). The SES of the group fell in the “medium” range, according to Hollingshead guidelines, with a mean score of 51 points (range 17–66). There were no significant differences between boys and girls in age, F(1, 143) = .40, p = .53, FSIQ, F(1, 141) = 3.4, p = .07), or SES, F(1, 143) = .76, p = .39. There were also no differences in the number of boys versus girls at each age level (7 through 14; χ2 = 3.3, p = .80) or within each IQ test given, χ2 = 3.3, p = .35. The breakdown of each age band is as follows: 7 years (n = 13, boys = 8, girls = 5); 8 years (n = 33, boys = 15, girls = 18); 9 years (n = 20, boys = 10, girls = 10): 10 years (n = 19, boys = 12, girls = 8); 11 years (n = 24, boys = 12, girls = 12); 12 years (n = 18, boys = 7, girls = 11); 13 years (n = 10, boys = 5, girls = 5); 14 years (n = 7, boys = 3, girls = 4).
Gender and Age Effects for PANESS Summary Variables
Gaits and stations
Gender- and age-specific performance scores for variables comprising the gaits and stations summary scores are listed in Table 2. There were significant effects for gender, F(1, 1) = 4.2, p < .05, on the total gaits and stations summary score, total involuntary movements, F(1, 1) = 6.0, p < .05, left-sided involuntary movements, F(1, 1 = 4.3), p < .05, but not right-sided involuntary movements, F(1, 1 = 1.9), p = .19. In all instances of gender differences, girls had better performance than boys (see Figure 1). There were no significant effects of age, and no significant age by gender interactions for any of the variables comprising the gaits and stations scores.
TABLE 2.
Age and Gender Effects: PANESS Gaits and Stations
| 7
|
8
|
9
|
10
|
11
|
12
|
13
|
14
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Right gait overflow | M | 1.4 | 1.1 | 1.3 | 1.1 | 0.9 | 0.7 | 1.8 | 0.9 | 1.3 | 1.1 | 0.4 | 0.8 | 0.4 | 0.5 | 0.0 | 0.0 |
| F | 0.8 | 0.8 | 0.9 | 0.9 | 1.4 | 1.4 | 1.3 | 1.3 | 0.7 | 0.9 | 0.7 | 1.0 | 1.0 | 1.0 | 0.0 | 0.0 | |
| Left gait overflow | M | 1.0 | 1.2 | 1.3 | 1.2 | 1.0 | 1.1 | 1.9 | 1.1 | 0.8 | 1.2 | 0.6 | 0.8 | 0.6 | 0.9 | 0.0 | 0.0 |
| F | 0.8 | 0.5 | 0.6 | 0.6 | 1.2 | 1.2 | 1.3 | 1.0 | 0.4 | 0.7 | 0.8 | 1.0 | 1.0 | 1.0 | 0.5 | 1.0 | |
| Total gait overflow | M | 2.4 | 2.2 | 2.6 | 2.2 | 1.9 | 1.7 | 3.7 | 1.9 | 2.1 | 2.2 | 1.0 | 1.5 | 1.0 | 1.4 | 0.0 | 0.0 |
| F | 1.6 | 1.1 | 1.5 | 1.4 | 2.6 | 2.5 | 2.6 | 2.1 | 1.1 | 1.4 | 1.5 | 1.8 | 2.0 | 2.0 | 0.5 | 1.0 | |
| Right axial | M | 1.4 | 1.7 | 1.2 | 1.2 | 0.1 | 0.3 | 1.0 | 1.3 | 0.7 | 1.1 | 0.7 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| F | 1.2 | 1.3 | 0.6 | 1.0 | 0.9 | 1.6 | 0.0 | 0.0 | 0.8 | 1.5 | 0.8 | 1.8 | 0.0 | 0.0 | 0.3 | 0.5 | |
| Left axial | M | 0.9 | 1.2 | 0.9 | 1.1 | 0.2 | 0.6 | 0.3 | 0.7 | 0.8 | 1.3 | 1.4 | 1.4 | 0.8 | 1.3 | 0.0 | 0.0 |
| F | 1.0 | 1.4 | 0.2 | 0.4 | 0.6 | 1.1 | 0.1 | 0.4 | 0.4 | 1.4 | 0.8 | 1.8 | 0.0 | 0.0 | 0.3 | 0.5 | |
| Total axial | M | 2.6 | 3.4 | 3.1 | 2.8 | 1.0 | 1.2 | 3.3 | 3.7 | 2.3 | 2.4 | 3.9 | 3.3 | 1.6 | 1.8 | 0.7 | 1.2 |
| F | 4.0 | 3.9 | 1.6 | 1.8 | 2.2 | 2.8 | 0.7 | 0.8 | 2.6 | 3.7 | 2.7 | 4.6 | 0.2 | 0.5 | 1.3 | 1.3 | |
| Right involuntary movements | M | 0.5 | 0.8 | 0.3 | 0.6 | 0.3 | 0.5 | 0.2 | 0.4 | 0.3 | 0.5 | 0.1 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| F | 0.5 | 0.5 | 0.2 | 0.4 | 0.1 | 0.3 | 0.0 | 0.0 | 0.2 | 0.4 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Left involuntary movements+ | M | 0.5 | 0.8 | 0.3 | 0.7 | 0.5 | 0.7 | 0.1 | 0.3 | 0.3 | 0.5 | 0.1 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| F | 0.0 | 0.0 | 0.2 | 0.4 | 0.2 | 0.4 | 0.0 | 0.0 | 0.9 | 0.3 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Total involuntary movements+ | M | 1.4 | 1.8 | 0.9 | 1.6 | 0.9 | 1.3 | 0.6 | 0.8 | 0.8 | 1.2 | 0.4 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| F | 0.4 | 0.5 | 0.6 | 1.0 | 0.4 | 0.9 | 0.1 | 0.4 | 0.3 | 0.9 | 0.3 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Total gaits & stations+ | M | 6.4 | 6.3 | 0.7 | 4.9 | 0.4 | 2.7 | 4.5 | 5.1 | 5.1 | 4.8 | 5.9 | 3.5 | 2.6 | 2.4 | 0.7 | 1.2 |
| F | 6.0 | 4.6 | 0.4 | 3.1 | 5.2 | 4.4 | 3.4 | 2.9 | 3.9 | 5.0 | 4.6 | 4.6 | 2.2 | 2.3 | 1.8 | 1.5 | |
Note. PANESS = Physical and neurological examination of subtle signs;
Main effect for age, p < .05;
Main effect for gender, p < .05, in all cases boys > girls. All age by gender interactions were not significant.
FIGURE 1.
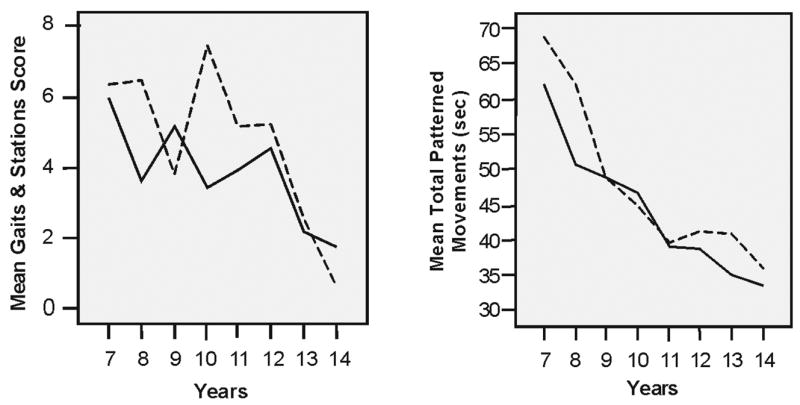
Gender differences on Gaits and Stations tasks and timed patterned movements. In all tasks in which there were gender–related differences, the girls performed better and quicker than the boys.
Overflow movements
Gender and age effects for variables comprising the total overflow score are listed in Table 3. There was a significant effect of age for right-sided overflow, F(7, 136) = 2.5, p < .05, left-sided overflow, F(7, 136) = 2.1, p < .05, and total overflow, F(7, 136) = 2.4, p < .05, in all cases, the frequency of overflow movement decreased with age. There were no significant gender differences, and no significant age by gender interactions for overflow movements.
TABLE 3.
Age and Gender Effects: PANESS Total Dysrhythmia and Total Gait and Timed Overflow
| 7
|
8
|
9
|
10
|
11
|
12
|
13
|
14
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Right dys.* | M | 2.6 | 1.2 | 2.1 | 1.3 | 1.4 | 0.7 | 1.1 | 0.7 | 1.0 | 1.0 | .07 | .08 | 1.0 | 1.0 | 0.7 | 0.6 |
| F | 4.0 | 0.7 | 1.8 | 1.4 | 2.0 | 1.1 | 0.9 | 0.9 | 1.4 | 1.2 | 1.2 | 1.0 | 1.6 | 1.1 | 1.3 | 1.3 | |
| Left dys.* | M | 2.8 | 1.6 | 2.1 | 1.5 | 1.5 | 0.9 | 1.8 | 1.4 | 1.3 | 1.0 | 0.3 | 0.5 | 1.0 | 1.0 | 1.0 | 1.0 |
| F | 4.8 | 0.8 | 1.3 | 1.2 | 2.0 | 1.3 | 1.4 | 1.5 | 1.8 | 1.6 | 1.4 | 1.1 | 1.8 | 2.0 | 1.5 | 1.7 | |
| Total dys.* | M | 5.6 | 2.7 | 4.5 | 2.5 | 3.1 | 1.0 | 3.1 | 2.0 | 2.3 | 1.7 | 1.0 | 1.2 | 2.2 | 2.3 | 2.0 | 2.0 |
| F | 9.2 | 1.3 | 3.3 | 2.6 | 4.2 | 2.3 | 2.4 | 2.4 | 3.4 | 2.6 | 2.3 | 1.7 | 3.6 | 3.1 | 3.0 | 2.8 | |
| Right overflow* | M | 3.0 | 2.4 | 2.5 | 1.8 | 2.2 | 1.4 | 3.3 | 2.1 | 2.3 | 1.9 | 1.1 | 0.9 | 0.8 | 1.1 | 1.0 | 0.0 |
| F | 1.4 | 1.7 | 1.9 | 2.0 | 2.9 | 2.9 | 2.9 | 2.1 | 1.5 | 1.2 | 1.5 | 1.6 | 1.4 | 1.3 | 0.5 | 1.0 | |
| Left overflow* | M | 2.4 | 2.5 | 2.7 | 1.9 | 2.1 | 1.7 | 3.3 | 2.2 | 2.2 | 2.4 | 0.7 | 1.0 | 0.8 | 1.3 | 1.7 | 0.6 |
| F | 2.0 | 1.6 | 1.7 | 2.2 | 2.5 | 2.2 | 2.1 | 1.5 | 1.1 | 1.3 | 1.3 | 1.1 | 1.4 | 1.7 | 0.8 | 1.0 | |
| Total overflow* | M | 5.9 | 4.5 | 5.5 | 3.6 | 4.7 | 2.9 | 6.9 | 4.2 | 4.6 | 4.4 | 2.3 | 1.2 | 2.4 | 2.2 | 3.0 | 0.0 |
| F | 3.8 | 3.3 | 4.1 | 4.2 | 5.8 | 5.2 | 5.4 | 3.6 | 2.8 | 2.2 | 3.2 | 2.7 | 3.2 | 2.8 | 1.5 | 1.7 | |
Note. PANESS = Physical and neurological examination of subtle signs;
Main effect for age, p < .05; all gender and age by gender interactions were not significant.
Dysrhythmia
Gender and age effects for variables comprising the total dysrhythmia score are also listed in Table 3. There were significant effects for age in right-sided dysrhythmia, F(7, 7) = 6.8, p < .01, left-sided dysrhythmia, F(7, 7) = 4.5, p < .01, and total dysrhythmia, F(7, 7) = 6.7, p < .01, with the amount of dysrhythmia decreasing with age. There were no significant gender differences, and no significant age by gender interactions for dysrhythmia scores.
Total timed movements
Gender and age effects for the variables comprising the total timed movements score are listed in Table 4 (repetitive movements) and Table 5 (patterned movements). There was a significant effect for age, F(1, 7) = 19.6, p < .01, but not gender, F(1, 7) = 3.0, p = .08, for the total timed movements summary score; and for all individual timed movements on both right and left sides (all p<.01). In all instances times decreased with age. There were also significant gender differences on three of the six patterned movements: right heel-toe, F(1, 1) = 4.6, p < .05, left heel-toe, F(1, 1) = 5.3, p < .05, and left finger sequencing, F(1, 1) = 6.1, p < .05, with girls faster than boys in all cases. There were no significant gender differences for any of the repetitive timed movements. Given that earlier studies (Denckla, 1973, 1974; Largo, Caflisch, Hug, Muggli, Molnaletal., 2001a; Largoetal., 2003; Wolffet al., 1985) have identified different developmental trajectories of patterned versus repetitive movements, we examined gender differences separately for total repetitive movements (i.e., toe tap, hand pat, and finger tap) and total patterned movements (i.e., heel-toe, hand pronate/supinate, and finger sequence), and found gender effects (favoring girls) only on the total-patterned movements, F(1, 1) = 4.7, p < .03 (see Figure 1). There were no significant age-by-gender interactions for any of the individual timed activities or total repetitive and patterned variables.
Table 4.
Age and Gender Effects: PANESS Motor Exam: Repetitive Movements
| 7
|
8
|
9
|
10
|
11
|
12
|
13
|
14
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| R-toe tap * | M | 8.4 | 4.6 | 6.7 | 1.8 | 6.2 | 1.3 | 5.9 | 2.4 | 4.9 | 0.8 | 5.7 | 1.3 | 6.3 | 1.0 | 5.3 | 1.9 |
| F | 6.5 | 0.6 | 7.1 | 2.3 | 6.5 | 1.7 | 5.4 | 0.7 | 5.1 | 1.0 | 5.6 | 1.0 | 5.2 | 1.0 | 4.5 | 0.8 | |
| L-toe tap* | M | 9.8 | 2.1 | 7.1 | 2.1 | 6.5 | 1.7 | 6.0 | 2.0 | 4.8 | 1.0 | 6.0 | 0.9 | 6.0 | 1.4 | 4.9 | 2.0 |
| F | 7.9 | 1.6 | 6.6 | 1.8 | 6.8 | 2.1 | 5.4 | 0.5 | 6.0 | 1.5 | 5.5 | 1.1 | 5.7 | 0.8 | 5.2 | 0.9 | |
| R-hand pat* | M | 5.5 | 1.5 | 4.6 | 0.9 | 4.5 | 0.1 | 4.9 | 1.6 | 3.7 | 0.6 | 3.7 | 0.5 | 4.0 | 0.7 | 4.0 | 1.4 |
| F | 5.7 | 1.4 | 4.5 | 0.9 | 4.8 | 1.0 | 4.3 | 0.4 | 4.0 | 0.9 | 4.0 | 0.6 | 3.9 | 0.5 | 3.8 | 0.8 | |
| L-hand pat* | M | 5.1 | 0.9 | 5.2 | 1.0 | 4.7 | 0.6 | 5.3 | 1.1 | 3.9 | 0.7 | 4.1 | 0.6 | 4.1 | 0.6 | 3.9 | 1.4 |
| F | 5.9 | 1.5 | 5.0 | 1.1 | 5.3 | 1.0 | 4.3 | 0.6 | 4.3 | 1.0 | 4.2 | 0.6 | 4.6 | 1.1 | 4.3 | 0.9 | |
| R-finger tap* | M | 7.1 | 0.8 | 5.9 | 0.9 | 5.9 | 0.8 | 5.6 | 1.0 | 5.2 | 0.7 | 5.3 | 0.4 | 5.9 | 0.8 | 4.8 | 1.3 |
| F | 6.9 | 0.7 | 5.9 | 0.8 | 6.3 | 1.0 | 5.7 | 0.9 | 5.3 | 1.1 | 5.9 | 0.8 | 5.1 | 0.4 | 4.4 | 0.9 | |
| L-finger tap* | M | 7.1 | 0.7 | 6.8 | 0.9 | 6.6 | 0.8 | 6.0 | 1.1 | 6.0 | 1.2 | 5.5 | 1.0 | 5.9 | 0.9 | 5.0 | 1.3 |
| F | 7.8 | 1.0 | 6.6 | 1.1 | 6.6 | 1.0 | 5.9 | 1.0 | 5.7 | 1.2 | 6.1 | 1.0 | 5.4 | 0.7 | 5.4 | 0.7 | |
Note. PANESS = Physical and neurological examination of subtle signs;
Main effect for age, p < .001; all gender and age by gender interactions were not significant
TABLE 5.
Age and Gender Effects: PANESS Motor Exam: Patterned Movements and Tongue Wags
| 7
|
8
|
9
|
10
|
11
|
12
|
13
|
14
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| R-heel toe* | M | 10.7 | 1.5 | 11.3 | 6.4 | 7.3 | 2.0 | 7.3 | 2.2 | 5.9 | 1.2 | 7.4 | 1.9 | 7.9 | 1.8 | 5.7 | 2.8 |
| F | 8.7 | 2.9 | 7.9 | 2.3 | 7.6 | 2.3 | 4.5 | 2.3 | 6.2 | 1.8 | 6.4 | 1.9 | 6.0 | 1.1 | 5.4 | 1.5 | |
| L-heel toe* | M | 10.5 | 3.9 | 10.7 | 6.0 | 7.9 | 2.0 | 7.7 | 2.4 | 6.8 | 1.4 | 7.4 | 1.8 | 7.9 | 2.2 | 6.6 | 3.1 |
| F | 10.1 | 2.8 | 4.6 | 2.3 | 7.7 | 2.4 | 7.6 | 2.0 | 6.8 | 2.3 | 6.2 | 2.2 | 6.0 | 0.8 | 5.3 | 1.0 | |
| R-hand P/S* | M | 8.8 | 2.0 | 7.3 | 1.9 | 6.4 | 0.8 | 6.3 | 1.0 | 5.7 | 0.8 | 5.3 | 1.1 | 5.9 | 1.0 | 5.3 | 0.5 |
| F | 8.3 | 2.4 | 6.8 | 1.4 | 7.2 | 1.3 | 6.2 | 1.4 | 6.1 | 1.1 | 5.9 | 0.7 | 5.6 | 0.4 | 5.6 | 0.8 | |
| L-hand P/S* | M | 8.8 | 1.8 | 7.8 | 1.9 | 7.0 | 1.6 | 6.7 | 1.4 | 6.1 | 1.1 | 5.6 | 1.3 | 6.2 | 1.0 | 5.5 | 0.2 |
| F | 8.4 | 1.9 | 7.0 | 1.4 | 7.4 | 1.1 | 6.6 | 1.6 | 6.2 | 1.0 | 6.4 | 1.0 | 5.9 | 0.9 | 5.8 | 0.4 | |
| R-finger sequence* | M | 15.2 | 3.7 | 12.3 | 4.5 | 9.7 | 3.0 | 8.1 | 1.5 | 7.5 | 1.8 | 7.9 | 1.1 | 6.3 | 1.1 | 6.1 | 0.7 |
| F | 12.4 | 3.5 | 10.1 | 3.5 | 9.2 | 3.1 | 9.4 | 2.8 | 6.9 | 1.6 | 6.9 | 1.5 | 5.8 | 0.8 | 5.6 | 0.6 | |
| L-finger sequence* | M | 15.9 | 5.5 | 12.6 | 3.6 | 10.5 | 3.1 | 8.8 | 1.1 | 7.7 | 3.2 | 7.6 | 1.2 | 6.4 | 0.9 | 6.9 | 0.8 |
| F | 14.0 | 1.5 | 10.4 | 2.7 | 9.8 | 3.0 | 9.4 | 1.7 | 6.8 | 1.1 | 6.9 | 1.5 | 5.8 | 1.2 | 5.7 | 0.5 | |
| Tongue wag score* | M | 1.1 | 1.0 | .80 | 1.0 | .40 | 0.8 | 1.7 | 0.7 | 1.3 | 1.0 | 2.0 | 0.0 | 2.0 | 0.0 | 1.3 | 1.2 |
| F | 1.6 | 0.9 | 1.0 | 1.0 | 1.0 | 1.0 | 1.7 | 0.8 | 1.5 | 0.9 | 2.0 | 0.0 | 1.2 | 1.1 | 2.0 | 0.0 | |
Note. PANESS = Physical and neurological examination of subtle signs; Tongue wag score is not reported in seconds, but rather in the slow for age (SFA) score which is determined by the number of SD; Main effect for age, p < .001;
Main effect for gender where ANOVA (1,1) df, p < .05, in all cases boys were slower than girls. All age by gender interactions were not significant.
DISCUSSION
As hypothesized, performance on most of the timed and untimed motor tasks improved between the ages of 7 and 14 years. This is consistent with those from earlier investigations revealing age-related changes in motor speed and efficiency of function (Denckla, 1973, 1974; Largo, Caflisch, Hug, Muggli, Molnal et al., 2001a; Largo et al., 2003; Wolff et al., 1985). Age-related changes were not, however, observed for all tasks. By design, many of the tasks administered as part of the PANESS were mastered by age 7. Given that performance on the gaits and stations tasks had already reached the ceiling in our youngest age group (7 years), we observed no age-related improvements in the any of the tasks comprising gaits and stations (i.e., hopping, balancing, dysmetria, stressed gaits, choreiform,). This finding suggests that by age 7 these motor functions (and the neural systems supporting these functions) have reached an “adult” level of maturity in typically developing children. In contrast, significant age-related changes in subtle signs were clearly observed after age 7. Among untimed aspects of PANESS, overflow and dysrhythmia appear the most affected by age, with continued improvement (i.e., reduction in rate of overflow and dysrhythmia, shown by a decrease in mean across age groups) observed throughout the 7–14 year age range.
The gradual disappearance overflow movements from age 7–14 years, suggests an underlying maturational process of the cortex and corticospinal tracts. Specifically relevant to age-related decreases in mirror movements, magnetic resonance imaging (MRI) findings (Paus et al., 2001) reveal that growth of the corpus callosum continues into adulthood; when compared to young adults (20–29 years), adolescents ages 11–19 years showed a ten percent increase in corpus callosum growth over a two-year period. Furthermore, the corticospinal and thalamocortical tracts that support motor functions also demonstrate age-related maturation (Paus et al., 1999), suggesting that as motor systems mature, behavioral inhibition increases.
Previous studies comparing gender differences in motor development have reported that girls are faster and better coordinated than boys early in the elementary school years, but these differences may disappear by adolescence (Denckla, 1973, 1974). Moreover, girls tend to display fewer and less pronounced overflow movements throughout childhood (Largo, Caflisch, Hug, Muggli, Molnar et al., 2001b). Our findings provide partial support for the assumption that girls “mature” earlier than boys in motor proficiency and speed. In our sample of typically developing children in which boys and girls were closely matched on all salient attributes (i.e., age, handedness, IQ, SES), girls and boys performed equally well on most measures of speed and motor proficiency; however, for those timed tasks (right-and-left heel-toe, left finger sequencing), and for all subtle signs with respect to which significant gender differences were observed (total gaits/stations, total involuntary movements), girls performed better than boys. Furthermore, between the ages of 7 and 14, the boys did not appear to “catch up” to girls on these tasks, although our data cannot resolve the issue that these differences may diminish after age 14. The consistency of girls’ motor superiority in this age range is even more striking because girls in this sample showed a trend for lower IQ than boys (p = .07). Previous research has indicated a significant relationship between Verbal IQ and performance on timed tasks on the PANESS (Mahone, Prahme, Koth, Morris, & Denckla, 2004).
The pattern observed is supported by findings from anatomical MRI studies showing that systems underlying motor development (i.e., frontal and parietal gray matter) reach maximum size one year earlier in girls thaninboys (Gieddetal., 1999). Furthermore, both girls and boys show increase in white matter and corpus callosum volume from 6–17 years of age; however, girls show these developmental changes gradually over this age range, while boys show a dramatic increase over a shorter time period, with greater volumetric changes than the girls between ages 10–14 years (De Bellis et al., 2001).
Interestingly, we observed gender differences for timed patterned movements, but not for timed repetitive movements, suggesting that the neural pathways and motor systems that underlie patterned movement may mature differently in girls than in boys. Using TMS, Chen et al. (1997) concluded that the left hemisphere is more involved in the timing of complex sequences than the right hemisphere. Similar findings were reported by Grafton et al. (2002) using positron emission tomography (PET). Several investigators have asserted that there is greater recruitment of the left hemisphere in execution sequenced/patterned tasks regardless of which hand executed the movement (Grafton et al., 2002; Haaland, Elsinger, Mayer, Durgerian, & Rao, 2004; Harrington & Haaland, 1991). Thus, our findings are consistent with the observation that cortical maturation of the left hemisphere may occur earlier in girls than in boys.
Few studies have examined data from large, typically developing samples with regard to both speed and presence of subtle signs. Largo and colleagues (2001a, 2001b, 2003) recently reported age and gender differences in neuromotor development on a large sample using the ZNA. In many respects, the current findings are similar to the Largo et al. (2001a) findings—especially with regard to improvements in timed motor performance with age. Largo reported that all motor tasks steadily improve through age 18 years. Our findings suggest that this is true through age 14 years (our oldest age band) for patterned movements, but not for repetitive movements, which plateau by age 11. Also consistent with Largo et al. (2003), we found that girls were faster than boys at left finger sequencing. In addition, we also found that girls performed all patterned movements more rapidly than boys. While girls had significantly fewer involuntary movements (i.e., choreiform and dysmetria) than boys in this age range, we found no significant gender differences in presences of overflow or dysrhythmia across the age span, which is in contrast to the Largo (2001b) findings.
Several limitations should be noted. While this study examines data from a large typically developing sample (n = 144), caution should be used in conceptualizing the group as a representative “normative” sample. First, the age range is wide (7–14 years), thus limiting the number of children at each age level. With greater numbers of children at each age level, more discrete age-related changes might be identified, and better comparisons to performance of clinical groups could be made for all variable sate a chage level. Secondly, our sample was recruited from a single large metropolitan center, and was not a nationally representative sample. The demographics of our sample are roughly representative of SES and racial distribution in the U.S.; however, geographic differences could not be accounted for. Third, the mean IQ of the sample was in the high average range, which may have influenced performance on the motor tasks. The IQ range of our sample may have been elevated because we screened out commonly observed psychiatric diagnoses (i.e., depression, conduct and oppositional defiant disorders, obsessive compulsive disorder, learning disorders). By creating a diagnostically “pure” control group, we eliminated many of the conditions that may have served to reduce the mean IQ of the sample. Lastly, although all examiners were properly trained in a consistent manner, the inability to confirm inter-rater reliability, given the extensive period over which the data were collected, should be noted as a limitation. Thus, future research should emphasize: (1) measuring the developmental course of subtle signs early childhood (preschool and early elementary school years) using smaller age bands with more a diverse range of FSIQ, (2) collecting motor data on older adolescents and young adults to examine whether age related changes persist after age 14, (3) use of overflow movements and dysrhythmia as diagnostic predictors, (4) developmental course of subtle signs (e.g., overflow) in clinical groups, (5) the use of PANESS to discriminate different clinical groups from one another, and (6) reliability of the PANESS.
Historically, motor assessment with children has been used to delineate behavioral development patterns that can distinguish clinical groups, and to examine the effects of treatment (especially medication). In addition to ADHD, subtle signs on motor examination can be linked, to several other disorders, including, but not limited to, learning disabilities, obsessive compulsive disorder (OCD), depression, and conduct disorder (Adams, Kocsis, & Estes, 1974; Denckla, 1985; Denckla & Rudel, 1978; Guz & Aygun, 2004; Largo et al., 2003; Mostofsky et al., 2003; Schuerholz, Cutting, Mazzocco, Singer, & Denckla, 1997). As motor examinations have become more sophisticated, they are being used for co-localization of function. Subsequently, as imaging technologies have progressed, investigators have begun to establish some validity of their assumptions in relation to neuro-imaging methods. Thus, the current findings extend previous research and provide more explicit and comprehensive descriptions of typical development, and how this development differs between boys and girls. Using this restructured data as a standard by which to assess “abnormal” motor function among clinical groups, clinicians can test a priori hypotheses about the neurobiology underlying the specific disorders (e.g., autism, ADHD). Recommendations can then be made using a more “brain-based” understanding of the child’s difficulties, including accommodations for handwriting (abnormal movements, choreiform), extra time (speeded activities), strength and conditioning.
Acknowledgments
The research for this article was supported by NS-25806, Neurodevelopmental Pathways to Learning Disabilities; Mental Retardation and Developmental Disabilities Research Center, HD-24061; U.S. Congressionally Directed Materiel and Medical Command (DAMD17-00-1-0548); K08 NS 02039, K01 MH 01824, MH 52432R29, P01 HD 35468, K02 NS04485, 1R01 NS047781, and the Rita Rudel Foundation. The authors also wish to thank Rebecca Landa, Ph.D., for her assistance in obtaining data.
Contributor Information
Jennifer C. Gidley Larson, Department of Developmental Cognitive Neurology, Kennedy Krieger Institute, Baltimore, MD
Stewart H. Mostofsky, Department of Developmental Cognitive Neurology, Kennedy Krieger Institute, Departments of Neurology and Pediatrics Johns Hopkins University School of Medicine
Melissa C. Goldberg, Department of Psychiatry, Johns Hopkins University School of Medicine
Laurie E. Cutting, Department of Developmental Cognitive Neurology, Kennedy Krieger Institute, Departments of Neurology and Education Johns Hopkins University School of Medicine
Martha B. Denckla, Department of Developmental Cognitive Neurology, Kennedy Krieger Institute, Departments of Neurology, Pediatrics, and Psychiatry Johns Hopkins University School of Medicine
E. Mark Mahone, Department of Neurology, Kennedy Krieger Institute, Department of Psychiatry Johns Hopkins University School of Medicine.
References
- Adams R, Kocsis J, Estes R. Soft neurological signs in learning-disabled children and controls. American Journal of Diseases of Children. 1974;128:614–618. doi: 10.1001/archpedi.1974.02110300024004. [DOI] [PubMed] [Google Scholar]
- Annett M. The growth of manual preference and speed. British Journal of Psychology. 1970;61:545–558. doi: 10.1111/j.2044-8295.1970.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Barnett AL, Peters J. Motor proficiency assessment batteries. In: Dewey D, Tupper D, editors. Developmental motor disorders: A neuropsychological prospective. New York: The Guilford Press; 2004. pp. 66–83. [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen L. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Annals of Neurology. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- De Bellis M, Keshavan MS, Beers S, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Delgado M, Albright A. Movement disorders in children: Definitions, classifications, and grading systems. Journal of Child Neurology. 2003;18:S1–8. doi: 10.1177/0883073803018001S0301. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Developmental Medicine and Child Neurology. 1973;15:635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of motor co-ordination in normal children. Developmental Medicine and Child Neurology. 1974;16:729–741. doi: 10.1111/j.1469-8749.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21:773–800. [PubMed] [Google Scholar]
- Denckla MB. The neurobehavioral examination in children. In: Feinberg T, Farrah M, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill; 1997. pp. 721–728. [Google Scholar]
- Denckla MB, Rudel R. Anomalies of motor development in hyperactive boys. Annals of Neurology. 1978;3:231–233. doi: 10.1002/ana.410030308. [DOI] [PubMed] [Google Scholar]
- Deuel R, Robinson D. Developmental motor signs. In: Tupper D, editor. Soft neurological signs. Orlando, FL: Grune & Stratton; 1987. pp. 95–129. [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Bartko J, Denckla MB, Barker C, Wassermann E. Cortical correlates of neuromotor development in healthy children. Clinical Neurophysiology. 2003;114:1662–1670. doi: 10.1016/s1388-2457(03)00130-5. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries N, Castellanos F, Liu H, Zijdenbos A, et al. Brain development during childhood andadolescents: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grafton S, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand: A PET functional imaging study. Experimental Brain Research. 2002;146:369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Guz H, Aygun D. Neurological soft signs in obsessive-compulsive disorder. Neurology India. 2004;52:72–75. [PubMed] [Google Scholar]
- Haaland KY, Elsinger C, Mayer A, Durgerian S, Rao S. Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. Journal of Cognitive Neuroscience. 2004;16:621–636. doi: 10.1162/089892904323057344. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M. Evaluation of motor function in young infants by means of the assessment of general movements: A Review. Pediatric Physical Therapy. 2001;13:27–36. [PubMed] [Google Scholar]
- Hadders-Algra M, Mavinkurve-Groothuis A, Groen S, Stremmelaar E, Martijn A, Butcher P. Quality of general movements and the development of minor neurological dysfunction at toddler and school age. Clinical Rehabilitation. 2004;18:287–299. doi: 10.1191/0269215504cr730oa. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland K. Hemispheric specialization for motor sequencing: Abnormalities in levels of programming. Neuropsychologia. 1991;29:147–163. doi: 10.1016/0028-3932(91)90017-3. [DOI] [PubMed] [Google Scholar]
- Heinen F, Glocker F, Fietzek U, Meyer B, Lucking C, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Annals of Neurology. 1998;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- Holden E, Tarnowski K, Prinz R. Reliability of neurological soft signs in children: Reevaluation of the PANESS. Journal of Abnormal Child Psychology. 1982;10:163–172. doi: 10.1007/BF00915938. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Knyazeva M, Koeda T, Njiokiktjien C, Jonkman E, Kurganskaya M, deSonneville L, et al. EEG coherence changes during finger tapping in acallosal and normal children: A study of inter- and intrahemispheric connectivity. Behavioural Brain Research. 1997;89:243–258. doi: 10.1016/s0166-4328(97)00070-3. [DOI] [PubMed] [Google Scholar]
- Kroes M, Kessels A, Kalff A, Feron F, Vissers Y, Jolles J, et al. Quality of movement as predictor of ADHD: Results from a prospective population study in 5- and 6-year-old children. Developmental Medicine and Child Neurology. 2002;44:753–760. doi: 10.1017/s0012162201002882. [DOI] [PubMed] [Google Scholar]
- Largo R, Caflisch J, Hug F, Muggli K, Molnar A, Molinari L, et al. Neuromotor development from 5 to 18 years. Part 1: Timed performance. Developmental Medicine and Child Neurology. 2001a;43:436–443. doi: 10.1017/s0012162201000810. [DOI] [PubMed] [Google Scholar]
- Largo R, Caflisch J, Hug F, Muggli K, Molnar A, Molinari L. Neuromotor development from 5 to 18 years. Part 2: Associated movements. Developmental Medicine & Child Neurology. 2001b;43:444–453. doi: 10.1017/s0012162201000822. [DOI] [PubMed] [Google Scholar]
- Largo R, Fischer J, Rousson V. Neuromotor development from kindergarten age to adolescence: developmental course and variability. Swiss Medical Weekly. 2003;133:193–199. doi: 10.4414/smw.2003.09883. [DOI] [PubMed] [Google Scholar]
- Mahone E, Prahme C, Koth C, Morris M, Denckla MB. Effects of age and verbal IQ on timed motor performance in children [Abstract] Journal of International Neuropsychological Society. 2004;10:181. [Google Scholar]
- Mayston M, Harrison L, Stephans J. A neurophysiological study of mirror movements in adults and children. Annals of Neurology. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Meyer B, Röricht S, Woiciechowsky C. Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Annals of Neurology. 1998;43:360–369. doi: 10.1002/ana.410430314. [DOI] [PubMed] [Google Scholar]
- Morris M, Inscore A, Mahone E. Overflow movement on motor examination in children with ADHD [Abstract] Archives of Clinical Neuropsychology. 2001;16:782. [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and Motor Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Nass R. Mirror movement asymmetries in congenital hemiparesis: the inhibition hypothesis revisited. Neurology. 1985;35:1059–1062. doi: 10.1212/wnl.35.7.1059. [DOI] [PubMed] [Google Scholar]
- Nolan L, Grigorenko A, Thorstensson A. Balance control: sex and age difference in 9- to 16-year-olds. Developmental Medicine and Child Neurology. 2005;47:449–454. doi: 10.1017/s0012162205000873. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins D, Evans A, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins D, Blumenthal J, Giedd J, et al. Structural maturation of neural pathways in children and adolescents. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Schmahmann J. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neuroscience. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schuerholz L, Cutting L, Mazzocco M, Singer H, Denckla MB. Neuromotor functioning in children with Tourette syndrome with and without attention deficit hyperactivity disorder. Journal of Child Neurology. 1997;12:438–442. doi: 10.1177/088307389701200705. [DOI] [PubMed] [Google Scholar]
- Touwen B, Sporrel T. Soft Signs and MBD. Developmental Medicine and Child Neurology. 1979;21:528–538. [PubMed] [Google Scholar]
- Touwen B. The meaning and value of soft signs in neurology. In: Tupper D, editor. Soft neurological signs. New York: Grune & Stratton, Inc; 1987. pp. 281–295. [Google Scholar]
- Vitiello B, Ricciuti A, Stoff D, Behar D, Denckla MB. Reliability of subtle (soft) neurological signs in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:749–753. doi: 10.1097/00004583-198909000-00017. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children—revised. New York: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wolff P, Gunnoe C, Cohen C. Neuromotor maturation and psychological performance: A developmental study. Developmental Medicine and Child Neurology. 1985;27:344–354. doi: 10.1111/j.1469-8749.1985.tb04546.x. [DOI] [PubMed] [Google Scholar]
- Wolff P, Hurwitz I. Functional implications of the minimal brain damage syndrome. Seminars in Psychiatry. 1973;5:105–115. [PubMed] [Google Scholar]
- Wolff P, Hurwitz I. Sex differences in finger tapping: A developmental study. Neuropsychologia. 1976;14:33–41. doi: 10.1016/0028-3932(76)90005-1. [DOI] [PubMed] [Google Scholar]


