Abstract
Ocular infections with herpes simplex virus lead to corneal scarring and blindness, with herpes keratitis being the major infectious cause of blindness. There is currently no clinically approved vaccine and nearly all developmental vaccines are targeted against HSV-2 and genital herpes. We tested the ability of an HSV-2 replication-defective virus, a genital herpes vaccine candidate, to protect against HSV-1 corneal infection. Immunization with HSV-2 dl5-29 reduced viral replication in the cornea, prevented ocular disease and reduced latent infection by the HSV-1 strain. Therefore, this HSV-2 replication-defective mutant strain may have applications for prevention of herpes keratitis and genital herpes due to HSV-1 infection.
Keywords: Herpes simplex virus, herpetic stromal keratitis, vaccine, latency
Introduction
Herpes simplex virus (HSV) infects the mucosal epithelium at both orofacial and genital sites. Historically, HSV-1 has been the cause of orofacial infections while HSV-2 has been the agent responsible for genital infections. In a healthy individual, orofacial infections with HSV-1 are usually mild and free from serious complications, resulting in localized vesicular lesions that are resolved within a number of days. However, despite the apparent innocuous nature of these HSV-1 infections, several serious complications are also associated with this virus. HSV-1 infection of the brain results in herpes simplex encephalitis (HSE), while infection of the eye can produce herpetic stromal keratitis (HSK). Both are associated with severe morbidity. There are approximately 300,000 cases of ocular HSV-1 infection diagnosed annually in the US (Roizman, Knipe, and Whitley, 2007). The initial infection involves the corneal epithelium; however, repeated episodes of recurrent disease can lead to involvement of the underlying stroma. This results in HSK, and can eventually lead to blindness (Dawson and Togni, 1976). Generally thought to be a disease with a strong immunopathologic component (Doymaz and Rouse, 1992; Verjans et al., 1998), HSK is the leading cause of infectious corneal blindness in the developed world (Liesegang, 2001). A preventative therapy would be a major public health advance. However, the majority of vaccines currently in development to prevent HSV infection are directed against HSV-2. This is understandable given the view of HSV-2 as a sexually transmitted disease and the emerging evidence that genital herpes, i.e., HSV-2, plays an important role in the acquisition and progression of HIV infection (Corey et al., 2004; Freeman et al., 2006; Nagot et al., 2007; Wald et al., 2002). However, given that serious complications such as HSK and HSE are associated with HSV-1 infection, and the growing epidemiological evidence suggesting an increase in the incidence of genital herpes caused by HSV-1 (Malkin, 2004; Xu et al., 2006), a herpes vaccine should ideally protect against both HSV-1 and HSV-2. Such a vaccine would be capable of preventing the serious outcomes associated with HSV-1 infection, such as HSK, and would also be effective against genital herpes caused by either strain of HSV. With this is mind we tested the genital herpes vaccine candidate dl5-29, a replication-defective HSV-2 virus, for its ability to protect against ocular disease caused by HSV-1 infection. This virus has been previously shown to induce a protective immune response in both murine and guinea pig models of HSV-2 genital infection (Da Costa, Jones, and Knipe, 1999; Hoshino et al., 2005).
Results
To determine the ability of an HSV-2 replication-defective virus to protect against HSV-1 infection, we immunized mice with the HSV-2 dl5-29 replication-defective mutant virus and later challenged the mice with HSV-1 KOS strain virus via the corneal route, using a corneal infection model described previously (Leib et al., 1989). Mice were immunized subcutaneously on days 0 and 21 with HSV-2 dl5-29 using three different doses, 104 PFU, 105 PFU or 106 PFU. On day 35, these mice and mock-immunized control mice were challenged on the cornea with HSV-1 KOS following scarification. Mice were then monitored for facial lesions, facial swelling, and HSV-1 shedding in tear film.
Disease
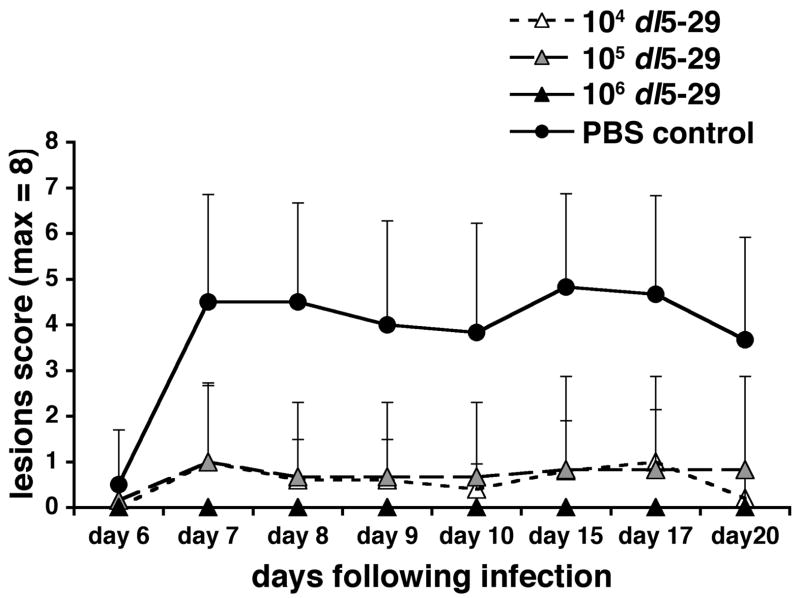
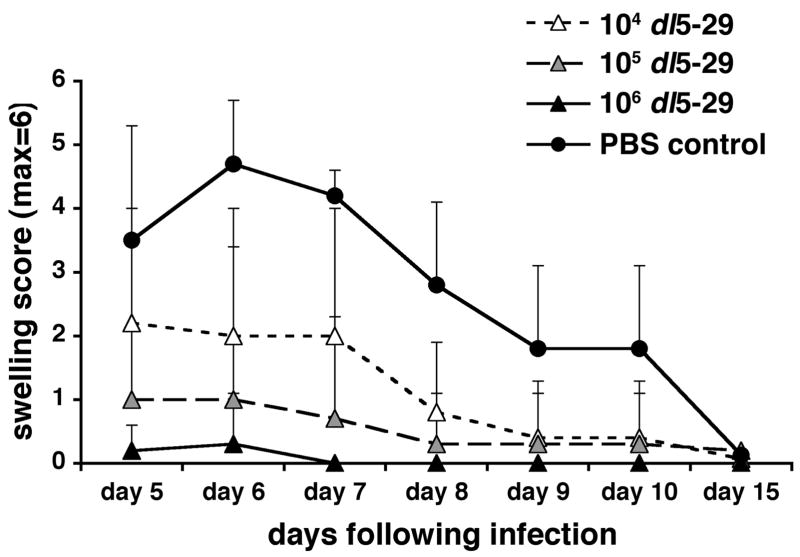
The control mice that had been mock-immunized with PBS developed both severe facial lesions and pronounced facial swelling following corneal challenge with HSV-1 (Figs. 1 and 2). When compared to these mice, all mice immunized with dl5-29 prior to challenge showed a significant reduction in both lesion severity and facial swelling, regardless of dl5-29 dose. The highest dose of dl5-29 (106 PFU) completely protected the mice from developing facial lesions (Fig. 1) and protected five out of six mice from developing facial swelling (Fig. 2). In the single mouse in this group where swelling was observed, it was mild and transient. The intermediate (105 PFU) and low (104 PFU) doses of dl5-29 provided partial protection against lesion development and facial swelling (Figs. 1 and 2). When compared to the control mice, the reductions in lesion severity seen in the three dl5-29 immunized groups were statistically significant from day 7 through to day 20 post-challenge, with the greatest reductions seen on day 15 post-challenge (p<0.005 for the 106 PFU group, p<0.01 for the 105 and 104 PFU groups on day 15). Reductions in facial swelling were statistically significant for the 106 and 105 PFU groups between days 5 and 10 post-challenge, and for the 104 PFU group between days 6 and 8 post-challenge. The greatest reductions in facial swelling were seen on days 6 and 7 post-challenge (p<0.001 for 106 PFU, p<0.01 for 105 PFU and p<0.05 for 104 PFU on days 6 and 7).
Figure 1. Immunization with a replication-defective HSV-2 virus prevents lesion development following an ocular challenge with HSV-1.
Groups of 6 to 8 CD-1 male mice were immunized with 104, 105 or 106 PFU of HSV-2 dl5-29 virus on days 0 and 21 via subcutaneous inoculation, and a group inoculated with PBS was included as a negative control. On day 35 all mice were challenged on the eye with HSV-1 KOS following corneal scarification. Mice were then monitored for lesion development for twenty days post-challenge.
Figure 2. Immunization with a replication-defective HSV-2 virus reduces facial swelling following an ocular challenge with HSV-1.
Groups of 6 to 8 CD-1 male mice were immunized with 104, 105 or 106 PFU of HSV-2 dl5-29 virus on days 0 and 21 via subcutaneous inoculation, and a group inoculated with PBS was included as a negative control. On day 35 all mice were challenged on the eye with HSV-1 KOS following corneal scarification. Mice were then monitored for facial swelling for fifteen days post-challenge.
Infection
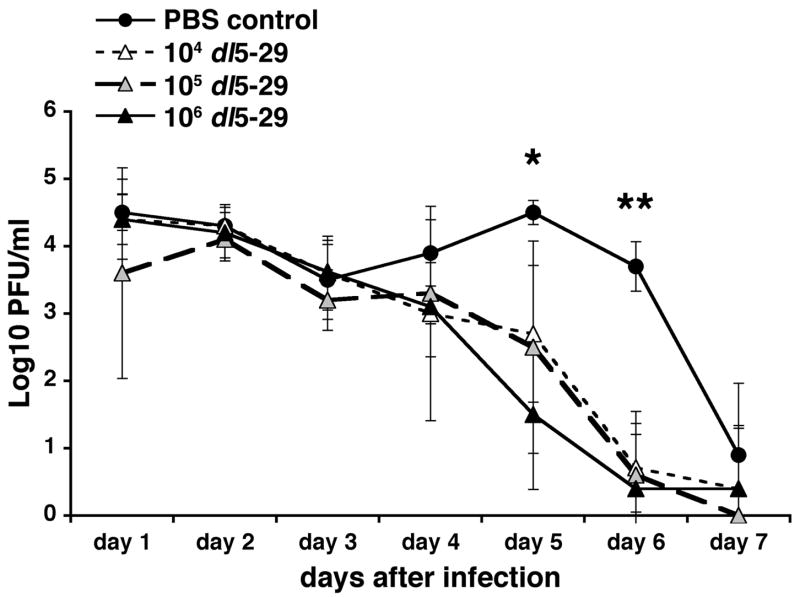
Following ocular challenge with HSV-1, virus shedding from the eye was measured in eye swabs taken on days one to seven post-challenge. The control group showed high levels of virus shedding from days one to six post-challenge, after which virus began to clear (Fig. 3). The three groups of mice immunized with dl5-29 showed a pattern of virus shedding similar to the controls for the first three days post-challenge. However, from day 4 post-challenge onwards, in correlation with the lesion and facial swelling observations, all three immunized groups began to show enhanced clearance of infectious virus as compared to the control group (Fig. 3). The reduction in virus shedding seen in these groups was such that by days 5 and 6 post-challenge all three groups of mice immunized with dl5-29 showed a statistically significant reduction in virus shedding as compared to controls. The level of reduction seen on day 5 post-challenge was greatest in those mice immunized with 106 PFU dl5-29. However, by day 6 post-challenge all three groups showed a similar level of reduction as compared to controls. By day 7 post-challenge, 50% of the control mice were still shedding detectable levels of virus, whereas 80% of mice immunized with 104 PFU dl5-29, 100% of mice immunized with 105 PFU dl5-29, and 83% of mice immunized with 106 PFU dl5-29 had cleared infectious virus to below detectable levels (data not shown).
Figure 3. Immunization with a replication-defective HSV-2 virus reduces viral shedding following ocular challenge with HSV-1.
Groups of 6 to 8 CD-1 male mice were immunized with varying doses of dl5-29 on days 0 and 21 via subcutaneous injection, and a group inoculated with PBS was included as a negative control. On day 35 all mice were challenged on the eye with HSV-1 KOS following corneal scarification. Eyeswabs were collected daily from days 1 to 7 post-challenge, and viral titers were determined using standard PFU assays. * p<0.01 for PBS vs. 104 dl5-29; p<0.05 for PBS vs. 105 dl5-29; p<0.005 for PBS vs. 106 dl5-29. ** p<0.005 for PBS vs. each of the three immunized groups.
Reduction in the level of latent virus by immunization with HSV-2 dl5-29
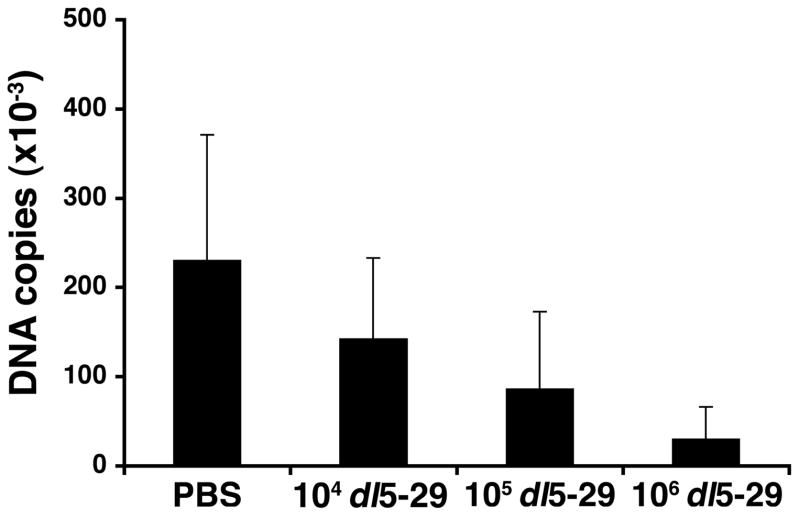
To determine the ability of dl5-29 virus immunization to protect against establishment of latent infection by HSV-1, we immunized mice with dl5-29, challenged on the eye with HSV-1 and then allowed thirty days to pass to enable the establishment of a latent infection in the trigeminal ganglion (TG). At this point the mice were sacrificed, TGs were recovered, and numbers of HSV-1 viral genome copies were determined using quantitative PCR. While all mice were found to harbor latent virus within their TGs, we observed a statistically significant (p<0.0005) 10-fold reduction in the number of viral genomes in the TGs of mice immunized with 106 PFU of dl5-29 as compared to mock-immunized mice (Fig. 4). As with previous results, mice that had received the intermediate and low dose of dl5-29 showed a reduction in latent viral copies as compared to controls, but the reduction was not as great as seen in those mice that had been immunized with the highest dose of dl5-29, indicating a fairly consistent dose-response.
Figure 4. Levels of latent virus in the trigeminal ganglion are reduced in mice immunized with a replication-defective HSV-2 virus prior to ocular HSV-1 challenge.
Groups of 6 to 8 CD-1 male mice were immunized with varying doses of dl5-29 on days 0 and 21 via subcutaneous injection, and a group inoculated with PBS was included as a negative control. On day 35 all mice were challenged with HSV-1 KOS on the eye following corneal scarification. At one month post-challenge the mice were sacrificed, and the trigeminal ganglia were removed. Individual TGs were digested and the number of viral genome copies in each sample was determined using real-time PCR.
Discussion
In this study we observed protection against HSV-1 ocular disease using a systemically administered vaccine directed against HSV-2, to our knowledge the first to do so. An earlier study in a rabbit model of ocular infection used the suboptimal gB2/gD2 subunit vaccine in MF59 emulsion and a live HSV-1 virus vaccine to demonstrate the requirement for periocular vaccination when protecting against HSV-1 ocular disease (Nesburn et al., 1998). The fact that dl5-29 was able to provide such robust protection against ocular disease following systemic administration further supports the evidence that this virus vaccine induces a strong, protective immune response (Da Costa, Jones, and Knipe, 1999; Hoshino et al., 2005), and provides evidence for the first time that this response is effective against HSV-1. While we did not test the ability of dl5-29 to protect against HSV-1 genital disease, a recent study observed that systemic administration of the HSV-2 subunit vaccine gD2/AS04 could prevent HSV-1 genital disease in a guinea pig model (Bourne et al., 2003). This finding strengthens the idea that a vaccine capable of protecting against genital HSV-2 disease, such as dl5-29 (Da Costa, Jones, and Knipe, 1999; Hoshino et al., 2005), would also be capable of preventing HSV-1 genital disease.
We have also shown here that dl5-29 is able to reduce the latent viral load in the trigeminal ganglion of immunized mice following HSV-1 challenge. Mice immunized with the highest does of dl5-29 in this study showed a 10-fold reduction in latent viral load as compared to controls, equivalent to reductions in HSV-2 latent viral load seen with dl5-29 in a guinea pig model of HSV-2 genital infection (Hoshino et al., 2005), further indicating the potential of this virus vaccine against both HSV-1 and 2. Reductions in latent virus may be of particular clinical significance to HSK given the relationship between latent viral loads and reactivation frequencies. Work by Sawtell and others has demonstrated that a reduction in the level of HSV-1 latency correlates with a reduction in the frequency of reactivation (Sawtell, 1998; Sawtell et al., 2001). Given that HSK develops over a series of recrudescent infections, a reduction in reactivation may significantly reduce the development of HSK in immunized individuals. In addition, any reduction in reactivation of either HSV-1 or HSV-2 reduces the opportunities for the virus to spread to susceptible individuals.
A vaccine capable of protecting against HSV-2 infection is obviously highly desirable, given the virus’ historical position as the major cause of genital HSV infection and the growing evidence for a link between genital HSV and an increased risk of HIV acquisition, transmission and disease progression (Corey et al., 2004; Freeman et al., 2006; Nagot et al., 2007; Wald et al., 2002). However, a vaccine capable of protecting against both types of HSV would be of even greater public benefit. HSV-1 is the agent responsible for HSK, the leading cause of infectious corneal blindness, and is the most common cause of herpes encephalitis. There is also increasing epidemiological evidence that HSV-1 is becoming a significant cause of genital herpes (Malkin, 2004; Xu et al., 2006), which is likely to lead to an increase in the incidence of neonatal infections caused by HSV-1. The replication-defective virus tested in this study has previously been shown to be effective in preventing genital disease and reducing levels of latent virus in murine and guinea pig models of HSV-2 genital infection. It has also been shown to induce the rapid trafficking of HSV-specific CD8+ T cells to sites of infection following ocular HSV-2 infection in mice (Hoshino et al., 2005). The evidence presented in our current study shows that dl5-29 is able to prevent disease and reduce levels of latency in a mouse model of HSV-1 ocular infection. Combined, these data indicate that dl5-29 could potentially be used as a vaccine to protect against both HSV-1 and HSV-2 infections.
The most obvious caveat to this conclusion is the fact that previous HSV candidate vaccines, most notably the HSV-2 glycoprotein D subunit vaccines, also showed promise in small animal studies while failing to show efficacy in later human trials. However, the subunit vaccines contained only one or two viral glycoproteins and were designed to elicit CD4+ T cell and neutralizing antibody responses (Koelle and Corey, 2003). The replication-defective virus dl5-29 not only provides the immune system with a much more extensive array of viral epitopes but also induces a much broader immune response including CD4, CD8 and antibody responses (Hoshino et al., 2005). Clinical trials are needed to determine how dl5-29 will perform in humans.
Materials and Methods
Viruses
The HSV-2 replication defective virus, dl5-29, and wildtype HSV-1 KOS were propagated and assayed for titers on V5-29 (Da Costa, Jones, and Knipe, 1999) and Vero cells, respectively. The HSV-2 dl5-29 replication-defective mutant virus contains deletions in the UL5 and UL29 genes (Da Costa, Jones, and Knipe, 1999; Da Costa et al., 2000).
Mice
CD-1 outbred male mice were obtained from Charles River Laboratories and housed under SPF conditions. All animal protocols were approved by the Institutional Animal Use and Care Committee.
Immunizations and infections
Mice were immunized with varying doses of dl5-29 in PBS or with PBS alone via subcutaneous injection in a total volume of 100 μl. Mice were immunized on days 0 and 21 and then infected on the cornea on day 35. For the corneal infection (Leib et al., 1989), mice were anesthetized with 13 μl/g ketamine (80 mg/ml)/xylazil (12 mg/ml) (Sigma) via intraperitoneal injection. A 30-gauge needle was used to scratch the eye 20 times, 10 times in a vertical direction and 10 times in a horizontal direction. A 5 μl aliquot of HSV-1 KOS containing 2×106 PFU was placed on the eye and allowed to adsorb before the mice regained consciousness.
Viral Shedding
On days 1 to 7 post-challenge mice were anesthetized briefly with Isoflurane (Abbott Labs), sterile cotton applicators (Puritan Medical Products) soaked in Dulbecco’s minimum essential medium (DMEM) (Invitrogen) were used to collect the fluid on each eye. For each mouse the samples from both eyes were placed together in a 3 ml glass vial containing 1 ml sterile DMEM and stored at −80°C until needed. To determine viral shedding, plaque-forming assays were performed whereby serial dilutions of the medium in each vial were plated onto confluent Vero cell monolayers (Morrison and Knipe, 1994). Statistical analysis was performed using the unpaired student’s t-test.
Facial lesions and swelling
Between days 5 and 15 post-challenge mice were monitored for facial swelling. Facial swelling scores were based on the presence of swelling in 3 different regions of the left and right sides of the face. Swelling in each individual area earned a score of 1, with a maximum score of 6 possible. Mice were monitored for facial lesions between day 6, when lesions first appeared, and day 20 post-challenge. Lesions were scored based on the severity of lesions on the left and right sides of the face. Each side was scored from 0 to 4, with 0 indicating no lesions and 4 indicating severe lesions. The scores from either side were then added together, giving a maximum possible score of 8.
Quantitative PCR
On day 30 post-ocular challenge, the TGs were removed from each mouse and immediately placed on dry ice before being transferred to −80°C until required. Each TG was digested overnight at 56°C in proteinase K digestion buffer (200 μg/ml proteinase K, 25 mM KCl, 5 mM Tris, pH 9, 500 μMgCl2). The exact volume of each sample was then determined, and 1 μl of each sample was used in a real time PCR reaction. Real-time PCR amplification and detection was performed using an ABI 770 Sequence Detector, with HSV-1 thymidine kinase gene specific primers used to detect HSV-1 KOS viral DNA. Forward primer: ACCCGCTTAACAGCGTCAACA, Reverse primer: CCAAAGAGGTGCGGGAGTTT (Katz, Bodin, and Coen, 1990; McKnight, 1980). Reactions were performed in 10 μl volumes containing SYBRGreen PCR mix and primers at a final concentration of 500 nM. PCR reactions were amplified at 50°C for 2 minutes, 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
In each reaction a standard curve was included. Total genomic DNA was extracted from HSV-1 infected Vero cells using the QIAGEN DNeasy tissue kit. Viral DNA was obtained by running the total genomic DNA on a 0.9% Low Melt agarose gel and extracting the viral band. The viral DNA was then quantified, using absorbance and comparison to mass standards on a gel, to determine the number of viral genomes/μl. To create a standard curve, the purified HSV-1 KOS DNA was serially diluted such that each reaction contained 10-fold dilutions of HSV-1 DNA from 2×107 to 2×100 molecules.
Acknowledgments
This research was supported by NIH grant AI57552.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003;187:542–9. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J Acquired Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- Da Costa XJ, Jones CA, Knipe DM. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc Natl Acad Sci USA. 1999;96:6994–6998. doi: 10.1073/pnas.96.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa XJ, Kramer MF, Zhu J, Brockman MA, Knipe DM. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol. 2000;74:7963–7971. doi: 10.1128/jvi.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson CR, Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Survey of Ophthalmology. 1976;21:121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- Doymaz MZ, Rouse BT. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest Ophthal & Vis Sci. 1992;33:2165–2173. [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, Cohen JI, Straus SE. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005;79:410–8. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JP, Bodin ET, Coen DM. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Micro Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Malkin JE. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes. 2004;11:2A–23A. [PubMed] [Google Scholar]
- McKnight SL. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LA, Knipe DM. Immunization with replication-defective mutants of herpes simplex virus type 1: Sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB, Becquart P, Segondy M, Vallo R, Sawadogo A, Van de Perre P, Mayaud P. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. NEJM. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol. 1998;72:7715–21. doi: 10.1128/jvi.72.10.7715-7721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ. Herpes simplex virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 2501–2602. [Google Scholar]
- Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NM, Thompson RL, Stanberry LR, Bernstein DI. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis. 2001;184:964–71. doi: 10.1086/323551. [DOI] [PubMed] [Google Scholar]
- Verjans GM, Remeijer L, van Binnendijk RS, Cornelissen JG, Volker-Dieben HJ, Baarsma SG, Osterhaus AD. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis. 1998;177:484–8. doi: 10.1086/517382. [DOI] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Warren T, Ashley R, Corey L. Genital shedding of herpes simplex virus among men. J Infect Dis. 2002;186:S34–S39. doi: 10.1086/342969. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]