Abstract
Ethylene oxide is a multisite carcinogen in rodents and classified as a human carcinogen by the National Toxicology Program. In 2-year mouse studies, ethylene oxide (EO) induced lung, Harderian gland (HG), and uterine neoplasms. We evaluated representative EO-induced and equivalent spontaneous neoplasms for K-ras mutations in codons 12, 13, and 61. K-ras mutations were identified in 100% (23/23) of the EO-induced lung neoplasms and 25% (27/108) of the spontaneous lung neoplasms. Codon 12 G to T transversions were common in EO-induced lung neoplasms (21/23) but infrequent in spontaneous lung neoplasms (1/108). K-ras mutations were found in 86% (18/21) of the EO-induced HG neoplasms and 7% (2/27) of the spontaneous HG neoplasms. Codon 13 G to C and codon 12 G to T transversions were predominant in the EO-induced HG neoplasms but absent in spontaneous HG neoplasms (0/27). K-ras mutations occurred in 83% (5/6) of the EO-induced uterine carcinomas and all were codon 13 C to T transitions. These data show a strong predilection for development of K-ras mutations in EO-induced lung, Harderian gland, and uterine neoplasms. This suggests that EO specifically targets the K-ras gene in multiple tissue types and that this event is a critical component of EO-induced tumorigenesis.
Keywords: K-ras, ethylene oxide, mice, carcinogen, Harderian gland, lung, uterus, neoplasm
Introduction
Ethylene oxide (EO) is a major industrial chemical used primarily as an intermediate in the manufacture of several industrial chemicals. Exposure to EO is greatest in the health care industry where EO is used as a sterilizing agent and an estimated 75,000 workers are potentially exposed (NTP, 1987, 2004; IARC, 1994; Recio et al., 2004). In spite of its widespread application, EO is harmful to human health and is considered a human carcinogen (Steenland et al., 1991; Lerda and Rizzi, 1992; Shore et al., 1993; Hengstler et al., 1994; IARC, 1994; Nygren et al., 1994). In vitro and in vivo data in rodents support its role as a mutagen and carcinogen (Shore et al., 1993; Hengstler et al., 1994; Nygren et al., 1994; Lorenti Garcia et al., 2001). National Toxicology Program (NTP) studies revealed that EO is a multisite carcinogen in rodents capable of inducing neoplasia in the lung, Harderian gland (HG) and uterus (NTP, 1987). The primary objective of this study was to evaluate representative examples of these EO-induced neoplasms as well as equivalent spontaneous neoplasms for genetic alterations in the major cancer gene K-ras. The goal was to help define potential mechanisms underlying EO-induced tumorigenesis and to identify chemical specific mutations that might serve as biomarkers of occupational exposure with possible relevance to human health.
Materials and Methods
Lung, Harderian Gland, and Uterine Neoplasms
Male and female B6C3F1 mice were exposed to 0, 50, or 100 ppm ethylene oxide (50 animals each group) by inhalation 6 hours/day, 5 days/week for 2 years (NTP, 1987). Husbandry and experimental procedures were in compliance with the requirements set forth by the Public Health Service’s Guide for the Care and use of Laboratory Animals. At necropsy, tissues were fixed in 10% neutral-buffered formalin, routinely processed, embedded in paraffin, sectioned to a thickness of 5 μm and stained with hematoxylin and eosin. Subsequently, 5 unstained serial sections, 10 μm thick, were prepared from paraffin blocks containing alveolar/bronchiolar adenomas or carcinomas, Harderian gland adenomas, or carcinomas or uterine carcinomas. In order to isolate adequate amounts of DNA, neoplasms greater than 1 mm in diameter were identified for analysis. A total of 23 lung neoplasms (5 alveolar/bronchiolar adenomas and 18 alveolar/bronchiolar carcinomas) and 108 spontaneous lung neoplasms (8 were concurrent controls) were analyzed for K-ras mutations in exons 1 and 2 (codons 12, 13 and 61). Twenty-one ethylene oxide-induced HG neoplasms (20 cystadenomas and 1 cystadenocarcinoma) and 27 spontaneous HG neoplasms (2 were concurrent controls) were evaluated for K-ras mutations in exons 1 and 2 (codons 12, 13 and 61). Six EO-induced uterine carcinomas were examined for K-ras mutations in exon 1 (codons 12 and 13). There were no concurrent uterine carcinomas in control mice.
DNA Isolation, Amplification, and Cycle Sequencing
DNA was isolated and extracted from paraffin-embedded sections containing lung, HG, and uterine neoplasms and was amplified by the polymerase chain reaction (PCR). Details of the nested primers for the K-ras gene have been described previously (Devereux et al., 1991, 1993; Sills et al., 1995). A positive control for K-ras and a control without DNA (distilled water) were run with all sets of reactions. PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA). The purified samples were sequenced utilizing a cycle sequencing kit (U.S. Biochemical, Cleveland, OH), which incorporates α-33P dideoxynucleotide (ddNTP) terminators (A, C, G, T) into the sequencing products. Mutations were confirmed by repeated sequencing starting from amplification of the original DNA extract.
Results
Lung Neoplasms
Two years of inhalation exposure to EO caused lung neoplasms in B6C3F1 mice (Table 1). Twenty-three of these lung neoplasms from EO-exposed B6C3F1 mice and 108 lung neoplasms from untreated B6C3F1 controls (8 concurrent controls and 100 historical controls) were examined for mutations in exons 1 and 2 of the K-ras gene. A high frequency of K-ras mutations (23/23, 100%) was observed in the EO-induced lung neoplasms, as compared to spontaneous lung neoplasms from control animals (27/108, 25%) (Table 2). Mutations in spontaneous neoplasms included just 1 of 8 (13%) concurrent controls and 26 of 100 (26%) historical controls. Codon 12 mutations were the most common in both control and treated animals although the EO-induced neoplasms predominantly exhibited GGT to GTT mutations (21/23, 91%) as compared to the more common GGT to GAT mutations (11/27, 41%) in spontaneous neoplasms (Figure 1). Codon 12 GGT to GTT were infrequent in spontaneous lung neoplasms (1/108, 0.9%). Codon 61 mutations were more common in spontaneous lung neoplasms occurring in 7 of 108 (7%) controls as opposed to only 1 of 23 (4%) EO-induced neoplasms. A similar spectrum of K-ras mutations was detected in EO-induced neoplasms regardless of histo-logic subtype (adenoma or carcinoma) or dose group; however, there was a slight dose-related increase in incidence of K-ras mutations (Table 2). Two mice from the 100-ppm dose group exhibited double mutations. One had a GGT to GTT mutation at codon 12 and a GGC to AGC mutation at codon 13 and the other had a GGT to GAT mutation at codon 12 and a CAA to CGA mutation at codon 61.
Table 1.
Incidence of lung, Harderian gland, and uterine neoplasms in ethylene oxide-exposed B6C3F1 mice.a
| 0 ppm | 50 ppm | 100 ppm | |
|---|---|---|---|
| Lung | |||
| Male | |||
| Alveolar/bronchiolar adenoma | 5/50 (10%) | 11/50 (22%) | 11/50 (22%) |
| Alveolar/bronchiolar carcinoma | 6/50 (12%) | 10/50 (20%) | 16/50 (32%) |
| Female | |||
| Alveolar/bronchiolar adenoma | 2/49 (4%) | 4/48 (8%) | 17/49 (35%) |
| Alveolar/bronchiolar carcinoma | 0/49 (0%) | 1/48 (2%) | 7/49 (14%) |
| Harderian | |||
| Male | |||
| Papillary cystadenoma | 1/43 (2%) | 9/44 (20%) | 8/42 (19%) |
| Papillary cystadenocarcinoma | 0/43 (0%) | 0/44 (0%) | 1/42 (2%) |
| Female | |||
| Papillary cystadenoma | 1/46 (2%) | 6/46 (13%) | 8/47 (17%) |
| Papillary cystadenocarcinoma | 0/46 (0%) | 1/46 (2%) | 0/47 (0%) |
| Uterus | |||
| Female | |||
| Uterine carcinoma | 0/49 (0%) | 1/47 (2%) | 5/49 (10%) |
Male and female B6C3F1 mice were exposed to 0, 50 or 100 ppm ethylene oxide by inhalation for 6 hours/day, 5 days/week for 2 years.
Table 2.
K-ras mutations in lung neoplasms from ethylene oxide-exposed B6C3F1 mice.
| Codon 12 (GGT)
|
Codon 13 (GGC)
|
Codon 61 (CAA)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | K-ras mutations (%) | GAT | TGT | GTT | CGT | AGC | CGC | CGA | CAT | CAC |
| Controla | 27/108 (25) | 11b | 5 | 1 | 0 | 0 | 3 | 2 | 4 | 1 |
| Ethylene oxidec | 23/23 (100)d | 2 | 0 | 21 | 0 | 1 | 0 | 1 | 0 | 0 |
| A/B adenoma | 5/5 (100) | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| A/B carcinoma | 18/18 (100) | 2 | 0 | 16 | 0 | 1 | 0 | 1 | 0 | 0 |
| 50 ppm | 8/8 (100) | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 ppm | 15/15 (100) | 2 | 0 | 13 | 0 | 1 | 0 | 1 | 0 | 0 |
Concurrent controls (8) combined with historical spontaneous lung neoplasms (100) from control B6C3F1 mice.
One mutation was from a concurrent control.
Male and female B6C3F1 mice were exposed to 0, 50, or 100 ppm ethylene oxide by inhalation 6 hours/day, 5 days/week for 2 years.
Two neoplasms had 2 mutations.
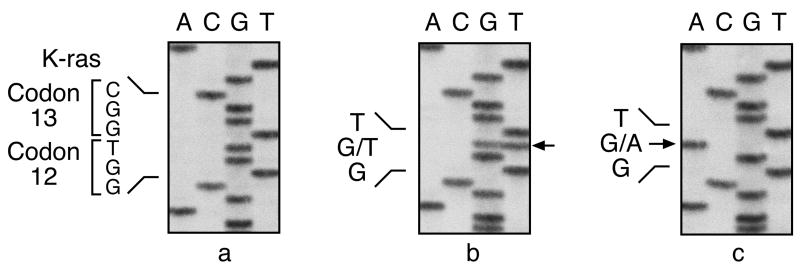
Figure 1.
Identification of K-ras mutations at codon 12 in ethylene oxide-induced lung neoplasms from B6C3F1 mice by cycle sequencing analysis. A, C, G, and T represent the 4 nucleotides in DNA, adenine, cytosine, guanine, and thymine, respectively. Sequencing panels are from left to right: (a) normal K-ras codon 12 sequence (GGT) from control B6C3F1 lung. (b–c) mutated sequences from lung neoplasms. Arrows point to mutant bands in each sequence. The wild-type allele is invisible in panel (c).
Harderian Gland Neoplasms
The incidence of EO-induced Harderian gland (HG) neoplasms is summarized in Table 1. Twenty-one HG neoplasms from EO exposed B6C3F1 mice and 27 spontaneous HG neoplasms from control B6C3F1 mice (2 concurrent controls and 25 historical controls) were examined for mutations in exons 1 and 2 of the K-ras gene. A high frequency (18/21, 86%) of K-ras mutations was detected in EO-induced HG neoplasms as compared to spontaneous HG neoplasms (2/27, 7%). Mutations in spontaneous neoplasms included just 2 of the 25 historical controls and none of the 2 concurrent controls. The predominant mutations in EO-induced HG neoplasms consisted of GGC to CGC transversions at codon 13 (15/18, 83%) and GGT to TGT transversions at codon 12 (8/18, 44%). Neither of these mutations was found in spontaneous HG neoplasms (0/27) (Table 3; Figure 2). A similar incidence and spectrum of K-ras mutations were detected in the 50-ppm and 100-ppm dose groups (Table 3). Point mutations were not detected in DNA isolated from 4 nontumor regions of the Harderian gland from control or EO-exposed mice. Eight of 11 mice (73%) in the 50-ppm dose group and 4 of 10 mice (40%) in the 100-ppm dose group had 2 or more mutations per neoplasm.
Table 3.
K-ras mutations in Harderian gland neoplasms from ethylene oxide-exposed B6C3F1 mice.
| Codon 12 (GGT)
|
Codon 13 (GGC)
|
Codon 61 (CAA)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | K-ras mutations (%) | GAT | TGT | CGC | AGC | CTA | CGA | CAG |
| Controla | 2/27 (7) | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Ethylene oxideb | 18/21 (86)c | 1 | 8 | 15 | 1 | 1 | 3 | 1 |
| Cystadenoma | 18/20 (90) | 1 | 8 | 15 | 1 | 1 | 3 | 1 |
| Cystadenocarcinoma | 0/1 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 ppm | 8/11 (73) | 0 | 5 | 7 | 1 | 1 | 2 | 0 |
| 100 ppm | 10/10 (100) | 1 | 3 | 8 | 0 | 0 | 1 | 1 |
Concurrent controls (2, with no mutations detected) combined with historical spontaneous Harderian gland neoplasms (25) from control B6C3F1 mice.
Male and female B6C3F1 mice were exposed to 0, 50, or 100 ppm ethylene oxide by inhalation 6 hours/day, 5 days/week for 2 years.
Several neoplasms had 2 or 3 mutations.
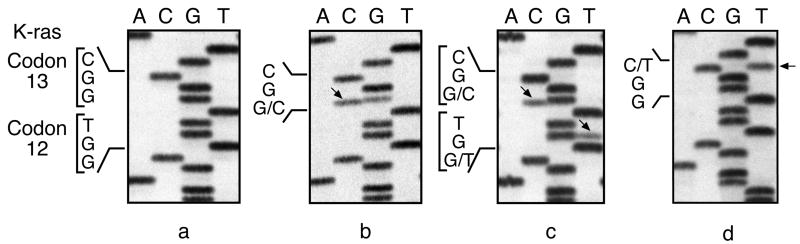
Figure 2.
Identification of K-ras codon 12 and 13 mutations in ethylene oxide-induced Harderian gland neoplasms and uterine carcinomas from B6C3F1 mice by cycle sequencing of amplified exon 1. Sequencing panels are from left to right: (a) normal K-ras codon 12 sequence (GGT), and codon 13 sequence (GGC). (b–c) mutated sequences from Harderian gland neoplasms, and (d) mutated sequence from uterine carcinoma. Arrows point to mutant bands in each sequence. The wild-type allele is also visible.
Uterine Neoplasms
The incidence of EO-induced uterine carcinomas is summarized in Table 1. All 6 uterine carcinomas from EO-exposed B6C3F1 mice were examined for mutations in exon 1 of the K-ras gene. The predominant mutation was a GGC to GGT transition in codon 13 (5/6, 83%) (Table 4 and Figure 2).
Table 4.
K-ras mutations in uterine carcinomas from ethylene oxide-exposed B6C3F1 mice.
| Treatment | K-ras mutations (%) | Codon 12 (GGT) | Codon 13 (GGC → GGT) | Codon 61 (CAA) |
|---|---|---|---|---|
| Ethylene oxidea | ||||
| Uterine carcinoma | 5/6 (83) | 0 | 5 | ND |
| 50 ppm | 1/1 (100) | 0 | 1 | ND |
| 100 ppm | 4/5 (80) | 0 | 4 | ND |
Male and female B6C3F1 mice were exposed to 0, 50, or 100 ppm ethylene oxide by inhalation 6 hours/day, 5 days/week for 2 years.
ND—not done.
Discussion
To our knowledge, this is the first study to evaluate EO-induced neoplasms for genetic alterations in K-ras. Here we show that K-ras mutations are particularly common in EO-induced lung, Harderian gland, and uterine neoplasms suggesting that this is a critical event underlying the mechanism behind EO-induced tumorigenesis.
EO is a direct-acting carcinogen that reacts with nucleophilic molecules to form a variety of different adducts involving DNA, RNA and protein (Brown et al., 1996). It is a powerful mutagen and clastogen at all phylogenetic levels capable of inducing an increased frequency of lacI and/or Hprt mutations in mice, rats, and humans (Segerback, 1990; Walker et al., 1992, 2000; IARC, 1994; Sisk et al., 1997; Tates et al., 1999; Wu et al., 1999; van Sittert et al., 2000; Melnick, 2002). In one study, human EO exposure correlated with elevated ras and p53 expression (Ember et al., 1998). Genetic damage and mutations are thought to play a critical role in the induction of cancer by alkylating agents, such as EO (Miller and Miller, 1966). N 7-(2-hydroxyethyl) guanine (HEG) is the major EO-induced adduct formed in rodents and humans (Tates et al., 1991; Li et al., 1992; Walker et al., 1992). The frequent targeting of guanine in the lung and Harderian gland neoplasms in this study suggests that formation of guanine adducts is a mechanism involved in the induction of these neoplasms.
Point mutations in ras are most common at codons 12, 13, and 61, which abolish GTPase activity rendering constitutively activated ras signalling (Mammas et al., 2005). Ras mutations are common in several human neoplasms including lung tumors in which K-ras mutations occur in ~30% of all non-small cell lung cancers (Sagawa et al., 1998). K-ras mutations also occur in human uterine carcinomas although the incidence tends to be more variable ranging from 0 to 64% (Mammas et al., 2005). As in this study, K-ras mutations in human lung and uterine neoplasms most commonly occur in codon 12 (Sagawa et al. 1998; Mammas et al., 2005). Codon 61 K-ras mutations are reportedly rare in human uterine carcinomas (Semczuk et al., 2001).
The incidence and spectrum of K-ras mutations in spontaneous lung and HG neoplasms from this study were comparable to that previously reported in B6C3F1 mice (Sills et al., 1999; Hayashi et al., 2001). Interestingly, the profile of K-ras mutations in the EO-induced neoplasms from this study was different from that described for many other chemically induced lung and HG neoplasms. This suggests that EO induces a chemical-specific signature. Previous studies have identified K-ras mutations in lung and HG neoplasms induced by chloroprene-, isoprene- and 1,3-butadiene, however, these mutations were predominantly localized to codon 61 rather than codons 12 and 13 as was seen in this study (Goodrow et al., 1994; Hong et al., 1997; Sills et al., 1999). Additional studies looking at K-ras mutations in 1-Amino-2, 4-dibromoanthraquinone- and urethane-induced lung neoplasms in B6C3F1 mice also revealed a predominance of codon 61 mutations (Nuzum et al., 1990; Kawano et al., 1995; Hayashi et al., 2001). The incidence of K-ras mutations in mouse uterine neoplasms is poorly characterized, although one study reported that ras mutations were rare in N-methyl-N-nitrosurea and 17β-estradiol-induced uterine adenocarcinomas in mice (Murase et al., 1995).
In summary, we found a high incidence of K-ras mutations in EO-induced lung, HG and uterine neoplasms in B6C3F1 mice. The prominent targeting of guanine bases in the lung and HG neoplasms suggests that direct DNA damage due to the formation of EO induced N -guanine adducts is a likely mechanism of action. In addition, this is to our knowledge, the first study in mice to show an increased incidence of K-ras mutations in uterine carcinomas. Overall, our data suggests that EO can specifically target the K-ras gene in multiple tissue types and that this event is a critical component of EO-induced tumorigenesis in B6C3F1 mice and has potential relevance to equivalent neoplasms in humans.
Acknowledgments
The authors thank Dr. Gordon Flake and Dr. Nobuko Wakamatsu for their critical reviews of the manuscript. We also thank the National Toxicology Program and all those from the many labs that contributed to these studies. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Abbreviations
- EO
ethylene oxide
- PCR
polymerase chain reaction
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- A, C, G, T
adenine, cytosine, guanine, thymine
- NTP
National Toxicology Program
- HG
Harderian gland
- ddNTP
dideoxynucleotide
- HEG
N 7-(2-hydroxyethyl) guanine
References
- Brown CD, Wong BA, Fennell TR. In vivo and in vitro kinetics of ethylene oxide metabolism in rats and mice. Toxicol Appl Pharmacol. 1996;136:8–19. doi: 10.1006/taap.1996.0002. [DOI] [PubMed] [Google Scholar]
- Devereux TR, Anderson MW, Belinsky SA. Role of ras protooncogene activation in the formation of spontaneous and nitrosamine-induced lung tumors in the resistant C3H mouse. Carcinogenesis. 1991;12:299–303. doi: 10.1093/carcin/12.2.299. [DOI] [PubMed] [Google Scholar]
- Devereux TR, Foley JF, Maronpot RR, Kari F, Anderson MW. Ras proto-oncogene activation in liver and lung tumors from B6C3F1 mice exposed chronically to methylene chloride. Carcinogenesis. 1993;14:795–801. doi: 10.1093/carcin/14.5.795. [DOI] [PubMed] [Google Scholar]
- Ember I, Kiss I, Malovics I. Oncogene and tumour suppressor gene expression changes in persons exposed to ethylene oxide. Eur J Cancer Prev. 1998;7:167–8. [PubMed] [Google Scholar]
- Goodrow TL, Nichols WW, Storer RD, Anderson MW, Maronpot RR. Activation of H-ras is prevalent in 1,3-butadiene-induced and spontaneously occurring murine Harderian gland tumors. Carcinogenesis. 1994;15:2665–7. doi: 10.1093/carcin/15.11.2665. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Hong HH, Toyoda K, Ton TV, Devereux TR, Maronpot RR, Huff J, Sills RC. High frequency of ras mutations in forestomach and lung tumors of B6C3F1 mice exposed to 1-amino-2,4-dibromoanthraquinone for 2 years. Toxicol Pathol. 2001;29:422–9. doi: 10.1080/01926230152499908. [DOI] [PubMed] [Google Scholar]
- Hengstler JG, Fuchs J, Gebhard S, Oesch F. Glycolaldehyde causes DNA-protein crosslinks: a new aspect of ethylene oxide genotoxicity. Mutat Res. 1994;304:229–34. doi: 10.1016/0027-5107(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Hong HL, Devereux TR, Melnick RL, Eldridge SR, Greenwell A, Haseman J, Boorman GA, Sills RC. Both K-ras and H-ras protooncogene mutations are associated with Harderian gland tumorigenesis in B6C3F1 mice exposed to isoprene for 26 weeks. Carcinogenesis. 1997;18:783–9. doi: 10.1093/carcin/18.4.783. [DOI] [PubMed] [Google Scholar]
- IARC. IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon, 15–22 February 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;60:1–560. [Google Scholar]
- Kawano R, Nishisaka T, Takeshima Y, Yonehara S, Inai K. Role of point mutation of the K-ras gene in tumorigenesis of B6C3F1 mouse lung lesions induced by urethane. Jpn J Cancer Res. 1995;86:802–10. doi: 10.1111/j.1349-7006.1995.tb03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerda D, Rizzi R. Cytogenetic study of persons occupationally exposed to ethylene oxide. Mutat Res. 1992;281:31–7. doi: 10.1016/0165-7992(92)90033-e. [DOI] [PubMed] [Google Scholar]
- Li F, Segal A, Solomon JJ. In vitro reaction of ethylene oxide with DNA and characterization of DNA adducts. Chem Biol Interact. 1992;83:35–54. doi: 10.1016/0009-2797(92)90090-8. [DOI] [PubMed] [Google Scholar]
- Lorenti Garcia C, Darroudi F, Tates AD, Natarajan AT. Induction and persistence of micronuclei, sister-chromatid exchanges and chromosomal aberrations in splenocytes and bone-marrow cells of rats exposed to ethylene oxide. Mutat Res. 2001;492:59–67. doi: 10.1016/s1383-5718(01)00149-8. [DOI] [PubMed] [Google Scholar]
- Mammas IN, Zafiropoulos A, Spandidos DA. Involvement of the ras genes in female genital tract cancer. Int J Oncol. 2005;26:1241–55. [PubMed] [Google Scholar]
- Melnick RL. Carcinogenicity and mechanistic insights on the behavior of epoxides and epoxide-forming chemicals. Ann N Y Acad Sci. 2002;982:177–89. doi: 10.1111/j.1749-6632.2002.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Miller EC, Miller JA. Mechanisms of chemical carcinogenesis: nature of proximate carcinogens and interactions with macromolecules. Pharmacol Rev. 1966;18:805–38. [PubMed] [Google Scholar]
- Murase T, Niwa K, Morishita S, Itoh N, Mori H, Tanaka T, Tamaya T. Rare occurrence of p53 and ras gene mutations in preneoplastic and neoplastic mouse endometrial lesions induced by N-methyl-N-nitrosourea and 17 beta-estradiol. Cancer Lett. 1995;92:223–7. doi: 10.1016/0304-3835(95)03782-r. [DOI] [PubMed] [Google Scholar]
- NTP. Toxicology and Carcinogenesis Studies of Ethylene Oxide (CAS No. 75-21-8) in B6C3F1 Mice (inhalation studies) U.S. Department of Health and Human Services, National Institutes of Health; Research Triangle Park, NC: 1987. [Google Scholar]
- NTP. Report on Carcinogens. 11. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; Research Triangle Park, NC: 2004. [Google Scholar]
- Nuzum EO, Malkinson AM, Beer DG. Specific Kiras codon 61 mutations may determine the development of urethan-induced mouse lung adenomas or adenocarcinomas. Mol Carcinog. 1990;3:287–95. doi: 10.1002/mc.2940030509. [DOI] [PubMed] [Google Scholar]
- Nygren J, Cedervall B, Eriksson S, Dusinska M, Kolman A. Induction of DNA strand breaks by ethylene oxide in human diploid fi-broblasts. Environ Mol Mutagen. 1994;24:161–7. doi: 10.1002/em.2850240304. [DOI] [PubMed] [Google Scholar]
- Recio L, Donner M, Abernethy D, Pluta L, Steen AM, Wong BA, James A, Preston RJ. In vivo mutagenicity and mutation spectrum in the bone marrow and testes of B6C3F1 lacI transgenic mice following inhalation exposure to ethylene oxide. Mutagenesis. 2004;19:215–22. doi: 10.1093/mutage/geh017. [DOI] [PubMed] [Google Scholar]
- Sagawa M, Saito Y, Fujimura S, Linnoila RI. K-ras point mutation occurs in the early stage of carcinogenesis in lung cancer. Br J Cancer. 1998;77:720–3. doi: 10.1038/bjc.1998.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerback D. Reaction products in hemoglobin and DNA after in vitro treatment with ethylene oxide and N-(2-hydroxyethyl)-N-nitrosourea. Carcinogenesis. 1990;11:307–12. doi: 10.1093/carcin/11.2.307. [DOI] [PubMed] [Google Scholar]
- Semczuk A, Schneider-Stock R, Berbec H, Marzec B, Jakowicki JA, Roessner A. K-ras exon 2 point mutations in human endometrial cancer. Cancer Lett. 2001;164:207–12. doi: 10.1016/s0304-3835(01)00380-9. [DOI] [PubMed] [Google Scholar]
- Shore RE, Gardner MJ, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br J Ind Med. 1993;50:971–97. doi: 10.1136/oem.50.11.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills RC, Hong HL, Greenwell A, Herbert RA, Boorman GA, De-vereux TR. Increased frequency of K-ras mutations in lung neoplasms from female B6C3F1 mice exposed to ozone for 24 or 30 months. Carcinogenesis. 1995;16:1623–8. doi: 10.1093/carcin/16.7.1623. [DOI] [PubMed] [Google Scholar]
- Sills RC, Hong HL, Melnick RL, Boorman GA, Devereux TR. High frequency of codon 61 K-ras A → T transversions in lung and Harderian gland neoplasms of B6C3F1 mice exposed to chloroprene (2-chloro-1,3-butadiene) for 2 years, and comparisons with the structurally related chemicals isoprene and 1,3-butadiene. Carcinogenesis. 1999;20:657–62. doi: 10.1093/carcin/20.4.657. [DOI] [PubMed] [Google Scholar]
- Sisk SC, Pluta LJ, Meyer KG, Wong BC, Recio L. Assessment of the in vivo mutagenicity of ethylene oxide in the tissues of B6C3F1 lacI transgenic mice following inhalation exposure. Mutat Res. 1997;391:153–64. doi: 10.1016/s1383-5718(97)00063-6. [DOI] [PubMed] [Google Scholar]
- Steenland K, Stayner L, Greife A, Halperin W, Hayes R, Hornung R, Nowlin S. Mortality among workers exposed to ethylene oxide. N Engl J Med. 1991;324:1402–7. doi: 10.1056/NEJM199105163242004. [DOI] [PubMed] [Google Scholar]
- Tates AD, Grummt T, Tornqvist M, Farmer PB, van Dam FJ, van Mossel H, Schoemaker HM, Osterman-Golkar S, Uebel C, Tang YS, Zwinderman AH, Natarajan AT, Ehrenberg L. Biological and chemical monitoring of occupational exposure to ethylene oxide. Mutat Res. 1991;250:483–97. doi: 10.1016/0027-5107(91)90205-3. [DOI] [PubMed] [Google Scholar]
- Tates AD, van Dam FJ, Natarajan AT, van Teylingen CM, de Zwart FA, Zwinderman AH, van Sittert NJ, Nilsen A, Nilsen OG, Zahlsen K, Magnusson AL, Tornqvist M. Measurement of HPRT mutations in splenic lymphocytes and haemoglobin adducts in erythrocytes of Lewis rats exposed to ethylene oxide. Mutat Res. 1999;431:397–415. doi: 10.1016/s0027-5107(99)00182-7. [DOI] [PubMed] [Google Scholar]
- van Sittert NJ, Boogaard PJ, Natarajan AT, Tates AD, Ehrenberg LG, Tornqvist MA. Formation of DNA adducts and induction of mutagenic effects in rats following 4 weeks inhalation exposure to ethylene oxide as a basis for cancer risk assessment. Mutat Res. 2000;447:27–48. doi: 10.1016/s0027-5107(99)00208-0. [DOI] [PubMed] [Google Scholar]
- Walker VE, Fennell TR, Upton PB, Skopek TR, Prevost V, Shuker DE, Swenberg JA. Molecular dosimetry of ethylene oxide: formation and persistence of 7-(2-hydroxyethyl)guanine in DNA following repeated exposures of rats and mice. Cancer Res. 1992;52:4328–34. [PubMed] [Google Scholar]
- Walker VE, Wu KY, Upton PB, Ranasinghe A, Scheller N, Cho MH, Vergnes JS, Skopek TR, Swenberg JA. Biomarkers of exposure and effect as indicators of potential carcinogenic risk arising from in vivo metabolism of ethylene to ethylene oxide. Carcinogenesis. 2000;21:1661–9. doi: 10.1093/carcin/21.9.1661. [DOI] [PubMed] [Google Scholar]
- Wu KY, Ranasinghe A, Upton PB, Walker VE, Swenberg JA. Molecular dosimetry of endogenous and ethylene oxide-induced N7-(2-hydroxyethyl) guanine formation in tissues of rodents. Carcinogenesis. 1999;20:1787–92. doi: 10.1093/carcin/20.9.1787. [DOI] [PubMed] [Google Scholar]