Abstract
Valid cueing has been shown to accelerate target identification and improve decision accuracy, however the precise nature and extent to which biasing influences the successive stages of target processing remain unclear. The present event-related potential (ERP) study used a “hybrid” task that combined features of standard cued-attention and task-switching paradigms in order to explore the effects of expectation on both identification and categorization of centrally-presented stimuli. Subjects made semantic judgments (living/nonliving) on word targets (“bunny”), and perceptual judgments (right/left) on arrow targets (“≪≪<”). Target expectancy was manipulated using cues that were valid (60% of trials), invalid (10%), or neutral (30%). Invalidly-cued targets required task-set switching before categorization could commence, and resulted in RT costs relative to validly- or neutrally-cued targets. Additionally, benefits from valid-cueing were observed for word targets. Invalid cueing of both arrow and word targets modulated early posterior visual potentials (P1/N1) and elicited a subsequent anterior P3a (270 ms). The temporal relationship of these effects suggests that the P3a indexed domain-general task-set switching processes recruited in response to the detection of unexpected perceptual information. Subsequent to the P3a and immediately preceding the behavioral response, validly-cued targets elicited enhanced stimulus-specific waveforms (arrows: parietal positivity [P290], words: inferior temporal negativity [late ITN: 400–600 ms]). The degree of neural enhancement relative to the invalid and neutral conditions mirrored the magnitude of corresponding RT benefits, suggesting that these waveforms indexed categorization and/or decision processes. Together, these results suggest that valid cueing increases the neural efficiency of initial stimulus identification, facilitating transmission of information to subsequent categorization stages, where increased neural activity leads to behavioral benefits.
Keywords: categorization, attention, P3a, N400, N1, P1
1. Introduction
Cueing tasks have often been used to study how expectancy and the violation of expectancy affect a variety of behavioral and neural measures (Posner et al., 1980; Posner et al., 1990; Downing, 1988; Hawkins et al., 1988; Henderson, 1991; Pestilli & Carrasco, 2005; Miniussi et al., 2005). For example, preparatory top-down orienting of attention to either the spatial location or physical properties (i.e., color, shape, or motion) of a visual stimulus has been shown to accelerate detection and categorization of the stimulus when it is presented (Mangun & Buck, 1998; Corbetta et al., 1990; Posner et al., 1980; Carrasco & McElres, 2001). These differences have been associated with changes in the magnitude of neural activity in brain regions that process information related to the selected location or attribute. Most event-related potential (ERPs) studies have found that activity in early perceptual processing regions (100–200 ms) is enhanced when the stimulus is expected compared to when it is unexpected (e.g., Mangun & Hillyard, 1991; Luck et al., 1994; see Soldan et.al., 2006 for a similar effect in repetition priming). Yet, numerous studies find that greater congruency between a concept and its preceding semantic context decreases the amplitude of the N400 component, findings taken to indicate that semantic expectancy reduces neural demands on retrieval of stored conceptual knowledge (e.g., Kutas & Federmeier, 2000). Taken together, these results suggest that expectancy can have differential effects on neural activity depending on the stage of stimulus processing biased by the preparatory information and the type of stimulus categorization required by the task. Thus far, however, few EEG/ERP studies have used cueing paradigms to examine the effects of top-down preparatory attention on multiple stages of visual information processing, from early perceptual identification through to later semantic classification (Tallon-Baudry et al, 2005; Miniussi et al., 2005).
In a study addressing this issue, Tallon-Baudry et al. (2005) demonstrated that preparatory attention differentially modulates activity along successive stages of the ventral visual processing stream. Intracranial EEG was recorded while patients viewed two types of visual stimuli, one that that they were prompted to attend to and encode for a delayed match-to-sample task, and another that was irrelevant to the memory task, and hence did not need to be attended. Attention had differential effects on gamma band activity (GBA) recorded from lateral occipital cortex (LO) and higher-level fusiform gyrus. During the anticipatory period there was an increase of GBA in LO for the attention relative to the no-attention task, but this was followed by a decrease in this area upon presentation of the stimulus. In contrast, although the fusiform gyrus exhibited no GBA effects of preparatory attention during the pre-stimulus period, attended stimuli elicited an increase in fusiform GBA upon stimulus presentation. The authors posit that preparatory attention leads to an increase of activity in early perceptual processing stages before the stimulus is presented, which results in more efficient processing through these earlier stages upon stimulus presentation (i.e., reduced activity) and increased activity at downstream processing stages. Because responses were not made to the unattended stimuli, however, their paradigm was not ideal for directly studying the relationship between the behavioral and neural effects of preparatory attention on stimulus identification and categorization (see also Kilner et al. 2005).
In the present study, we used ERPs to investigate the effects of valid and invalid preparatory attention on target processing in two cued-categorization tasks, one requiring categorization primarily on the basis of perceptual information and the other requiring categorization primarily on the basis of semantic information. Specifically, ERPs were recorded while subjects made perceptual (right/left) judgments on centrally-presented arrows (‘≫≫’), or semantic (living/non-living) judgments on concrete nouns (‘bunny’). Although both tasks required the initial detection of a visual stimulus, we hypothesized that subsequent categorization in the arrow task would rely primarily on processing within regions associated with object recognition, whereas categorization in the word task would necessarily engage semantic regions downstream of both object and lexical recognition. Thus, to the extent that categorization processes associated with these tasks would be expected to engage correspondingly distinct object and semantic processing regions in the brain, it may be possible to explore the effects of expectancy on target-specific processing activity even with the lower spatial resolution of ERP. We note that in contrast to Tallon-Baudry et al. (2005), all stimuli in the present task required a response, regardless of cue validity. In addition to providing behavioral data, we can also be confident that any systematic differences of neural modulation between conditions result from cueing specific processes rather than from general differences in attentional allocation.
Expectancy was manipulated using a standard cueing procedure in which we varied the validity of the cues. For the majority of trials (60%), arrow or word judgments were preceded by valid cues (‘< >’ or ‘W’) that accurately predicted the category (word or arrow) of the subsequent target and associated task set. On 10% of trials, however, stimuli were preceded by an invalid cue. Invalid cues result in preparatory attention being directed toward the inappropriate task-set (e.g., cue: ‘W’; target: arrows). Finally, to provide a baseline against which to directly compare the relative costs and benefits of valid and invalid cueing, 30% of trials were preceded by a neutral cue that provided no information about the upcoming target. We expect that valid cues would increase the speed with which the target stimulus is processed compared to those following neutral cues because they provide for optimal preparation of target-specific processing prior to stimulus onset. Inversely, invalid cues should result in longer RTs than either valid or neutral cues because they draw attention away from the appropriate target-specific processes. In addition, to redirect attention to the appropriate processes when the unexpected target arrives, subjects must disengage attention from the incorrect task-set and reorient attention to the appropriate task-set. Thus, we predict that the behavioral costs of invalid cues should be reflected not only in regions associated with task-specific processing of perceptual or semantic targets, but also in regions involved in processing conflict and task-set switching. Unlike the stimulus-specific modulation of target identification and categorization processes, however, task-set switching may involve more domain-general top-down attentional modulation (i.e., evidenced equally by both tasks).
We emphasize that unlike traditional cued spatial selective attention tasks, cues in the present task provide no explicit information about the correct response, only the physical nature of the upcoming stimulus, which itself signals only the type of task to be performed (i.e., word = semantic task; arrow = perceptual task). Subjects learn that a cue is “invalid” only after they perceptually identify the (unexpected) stimulus. They must then use this information to reorient attention to a new task set. Thus, this design represents a novel hybrid between the spatial selective attention designs commonly used to assess the effects of cued attention, and task-switching designs, in which there are generally no “invalid” conditions (for review see Monsell, 2003). To our knowledge the only study that bears some similarity to this design is that of Miniussi et al. (2005), who used ERPs to compare the effects of expectancy on a perceptual angle-orientation task and a lexical decision task. As we will detail below, results of that study provide a basis for predictions about what we might find in the present study. However, Miniussi and colleagues (2005) did not include neutral cues for comparison, making it more difficult to link neural modulation to behavioral costs and benefits. Addition of the neutral cue, as well as other design differences between our study and that of Miniussi et al. (2005), may also allow us to better isolate the effects of validity on target-specific activity from more domain-general task-switching processes. Unlike that study, however, we will not examine the effects of task-set preparation on cue period activity. Rather, by focusing our analyses to the target period activity, we will be able to explore not only the benefits of top-down expectation on processing of valid targets, but also the costs of task-switching initiated by identification of an unexpected target.
Event related potentials (ERPs) have been widely used in studying the time-course of the effects of spatial cueing on sites of early visual sensory and perceptual processes. Visual ERP components over posterior electrodes are typically characterized by an early positivity (P1) and subsequent negativity (N1). Studies of preparatory attention using spatial cueing paradigms generally find an enhancement in P1 amplitude for validly-cued stimuli relative to invalidly-cued stimuli in stimulus detection tasks, with the additional enhancement of the N1 when a perceptual choice is required (Mangun & Hillyard, 1991; Vogel & Luck, 2000). Although the stimuli in the present study were centrally-presented, these components have also demonstrated sensitivity to object-based selective attention (Valdes-Sosa, et al., 1998), as well as manipulation of central attentional resources to foveal stimuli (Mangels, Picton, & Craik, 2001). Thus, they may also be sensitive to the manipulation of expectancy in the present study. Nonetheless, Miniussi et al. (2005) did not observe cueing effects in the P1 or N1. Instead, they found effects of cueing only at a later posterior component (240–280 ms) that was larger in the invalid compared to the valid condition of the angle-orientation task, the functional correlates of which were unclear. Thus, we will re-examine the effects of cueing at the early perceptual P1 and N1, along with any later posterior components that appear to be modulated by the validity manipulation.
We predict that the word categorization task will also engage multiple processing stages, beginning with early perceptual processing of the word form and continuing onward to lexical and semantic processes necessary to make the living/nonliving judgment. Thus, we will examine the effects of cueing on language-related ERP components previously associated with lexical and semantic processing. These include a left anterior-temporal negativity peaking at about 320 ms (N3/N320/early ITN: Mangels et al., 2001; Butterfield & Mangels, 2003; Nobre & McCarthy, 1994), and a later N400 often observed over central scalp electrode sites (Kutas & Hillyard, 1980; Nobre & McCarthy, 1994; Nobre et al., 1994; see Van Petten & Luka, 2006 for review). Indeed, Miniussi et al. (2005) examined the effects of cue validity on a lexical decision task and found that valid cues enhanced the N3 and N400, which were both maximal over midline sites. They interpreted these modulations as evidence that valid cueing was successful in enhancing attention to language-related processing.
The finding that both the N3 and N400 were similarly modulated by expectation is surprising in light of other research suggesting that these components may index functionally distinct processes that could be differentially affected by cue validity. Specifically, Nobre & McCarthy (1994) found that semantic priming enhanced an early anterior-temporal negative peak at around 316 ms (N3), but attenuated the N400, consistent with studies indicating that the N400 indexes effort necessary to integrate the lexical-semantic content of an item with the surrounding context (Kutas & Hillyard, 1980; Nobre et al., 1994; Rossell et al., 2003). Priming can be viewed as a type of lexical-semantic preparation, however the cues in both the present study and in Miniussi et al. (2005) only specify that one should prepare the language system to process semantic information, hence facilitating retrieval of a wide range of semantic information. While semantically-primed words result in attenuated N400s, greater N400s are typically found for words that have a less constrained set of associations, such as words shown in isolation, low frequency words, concrete (versus abstract) words, and words that can easily be formed into other words by changing a letter (Van Petten & Luka, 2006). Such a difference between semantic priming and non-specific expectation of semantic information may explain why Miniussi and colleagues found that words elicited a larger N400 when they were expected (valid cues) than when they were unexpected (invalid cues).
In an attempt to replicate and extend the findings of Miniussi et al. (2005), we will examine the effects of cueing semantic categorization judgments on components at latencies corresponding to the N3 and N400. However, in addition to pursuing these effects at traditional midline sites, we will also analyze any observed modulations of temporal electrode sites, which are more proximal to the ventral stream regions putatively involved in the successive stages of word identification and semantic processing (Nobre, et al., 1994; see also Van Petten & Luka, 2006 for review). When an average reference is used, as in the present study, modulations associated with various aspects of conceptual processing are often observed at these sites (Mangels et al., 2001; Butterfield & Mangels, 2003; Stern & Mangels, 2006; Nessler et al., 2005). Although historically, use of an average reference in studying these effects has been less common that use of a mastoid reference, studies specifically comparing the N400 recorded with an average versus a mastoid reference have found considerable advantages to the average reference (Johnson & Hamm, 2000; Curran, et al., 1993). With an average reference, the distribution of the N400 shifts from a negativity over centro-parietal sites to a pronounced positivity over temporal electrodes (Johnson & Hamm, 2000; Curran, et al., 1993). A temporal distribution is more consistent with intracranial recordings (McCarthy et al., 2005) and fMRI studies (Rossell, et al., 2003; Van Petten & Luka, 2006) that locate one of the principle N400 generators to this lobe. Thus, the use of an average-reference may provide for a more accurate representation of the anatomical topography of semantic scalp-recorded ERPs.
Finally, relevant to our second aim to locate domain-general components of top-down control, we will focus our analyses on the P3a components of the P300. This scalp potential has been associated with novelty of a variety of stimulus types, including words, pictures, tones, and tactile stimuli (Friedman et al., 2001). However, within the context of cueing tasks, a recent study found that it was also enhanced when subjects were signaled to switch to a new task set, even when the signal cue was presented well before the target (Rushworth et al., 2002; Barcelo et al., 2002; Kopp et al., 2006). This suggests that the P3a may be involved in attentional orienting processes associated with the initiation of task-set switching, and hence should be greater for invalidly cued stimuli. Minuissi et al., (2005) observed such a pattern on a P300 component extending across both frontal and parietal electrodes, but only during their angle-judgment task. It is unclear whether the absence of such an effect in the lexical decision task reflects a fundamental difference in cueing effects, or whether the large fronto-central N400 in this task may have obscured similar effects of validity on the overlapping P3a. Indeed, a desirable “side-effect” of the average reference’s redistribution of the N400 to temporal sites is that there is less chance of overlap between the language-related modulations and midline P300 components. Arguably, this should enhance our ability to directly compare midline P300 activity across the semantic and non-semantic tasks in the search for domain-general processes.
2. METHODS
2.1 Participants
Nineteen healthy right-handed, native English speakers (10 females; mean age = 23.9, SD = 3.8) were recruited from the Columbia University campus. All subjects gave informed consent and the study was approved by the Columbia University Institutional Review Board. Subjects were paid $10/hour for their participation.
2.2. Materials
Word stimuli were medium-frequency concrete nouns (mean SFI1 = 45.2, SD = 3.7) and 4–10 letters in length. Average word frequency and word length were equated across living and nonliving lists (mean SFI: living = 45.2, SD = 3.8; nonliving = 45.2, SD = 3.7). Words were randomly assigned to valid, invalid and neutral conditions. Arrow stimuli were composed of 4–10 ‘<’ or ‘>’ characters, all pointing in the same direction. All stimuli were presented foveally at the center of the screen. The horizontal visual angle subtended by word stimuli ranged from 1.9° to 5.3°, and for arrow stimuli ranged from 2.7° to 6.0°.
An equal number of word and arrow stimuli appeared in each block. For word stimuli, these items were equally divided between living (i.e. ‘bunny’) and nonliving (i.e. ‘pencil’), and for arrow stimuli, between right and left facing arrows (i.e. ‘≫≫’ or ‘≪≪’). In order to maximize expectancy, the order of cue presentations was pseudo-randomized such that the first 5 trials of each block were valid (in order to establish expectancy) and two invalid trials never occurred in a row (in order to preserve expectancy as much as possible).
2.3 Design and procedure
At the start of the experiment, subjects were seated in a sound-attenuated experimental chamber, 60 cm from the computer screen and within easy reach of a computer keyboard. They were told that they were going to make decisions about word and arrow stimuli. Specifically, for word stimuli, they were instructed make a decision about whether word represented a living or nonliving object (semantic decision); for arrow stimuli, they were instructed to make a decision about whether the arrows were pointing to the right or left (perceptual decision). The experimenter emphasized that they should make their responses as quickly and accurately as possible and that cues presented prior to each stimulus might help them to prepare for the decision and thus, optimize their decision speed and accuracy.
Figure 1 illustrates sample trials for each of the 3 cueing conditions (valid, invalid, and neutral) and each of the 2 target types (arrow, word). At the start of each trial, a cue was shown for 1500 ms. Cues were either informative in that they indicated that the upcoming stimulus would be a word (‘W’) or set of arrows (‘<>’) (Figures 1a–b), or were uninformative (neutral), and simply indicated that a target was going to appear (‘?’) (Figure 1c). In order to make the cues highly distinctive from each other and provide additional symbolic information about their meaning, cues were randomly assigned one of three possible colors (red, yellow, or blue) at the start of the experiment and presented in that color throughout.
Figure 1.
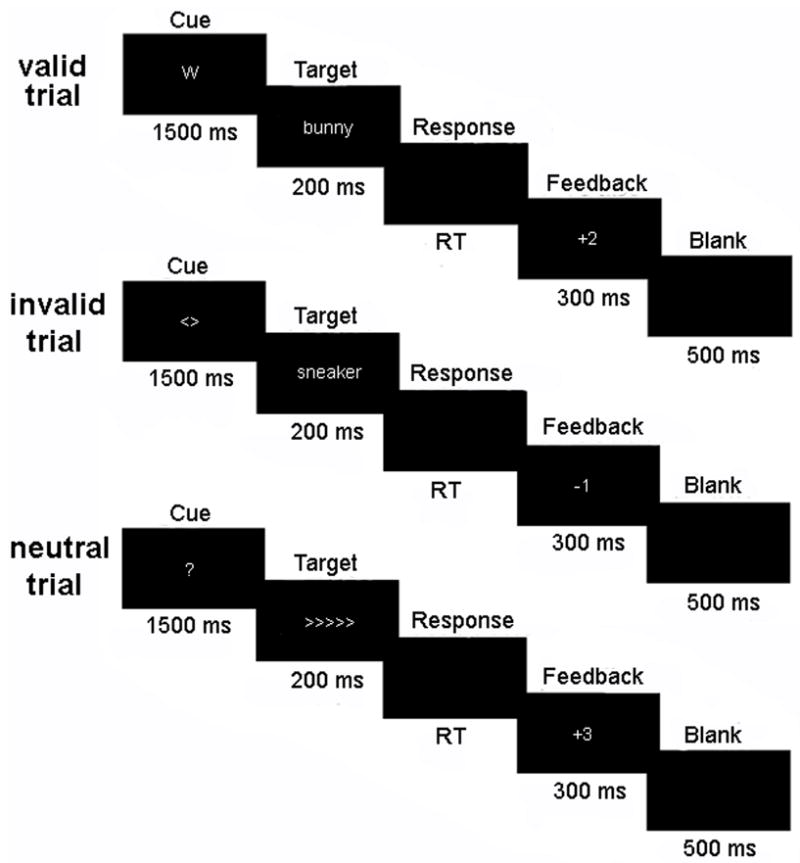
Subjects were first presented with one of three cues (‘W’, ‘>’, or ‘?’) that indicated the likelihood of the type of target (word or arrow) that will follow. A response was then made as quickly and accurately as possible and feedback was given based on the speed and accuracy of response. In the actual experiment, each cue was presented in a different color to make them highly distinctive from each other.
Immediately following the cue, the target appeared for 200 ms. Subjects indicated their response to the target by pressing a ‘1’ or ‘2’ on a keyboard with the index and middle fingers of their right hand respectively, where ‘1’ indicated ‘left’ or ‘living’, and ‘2’ indicated ‘right’ or ‘nonliving.’ Reaction times were computed from the onset of the stimulus to the time the subjects made a response. Although subjects had an indefinite amount of time to respond, only responses made between 200 ms and 2000 ms were included in the analysis. On 60% of all trials, the type of target (i.e., word or arrow) correctly predicted the upcoming stimulus (valid trials; Figure 1a), but on 10% of trials there was a mismatch between the cue and target (invalid trials; Figure 1b). The remaining 30% of all trials were preceded by the neutral cue (Figure 1c). Considering informative trials only, 86% were valid, and thus, the informative cues were highly predictive of the upcoming stimulus. We note, however, that even in the case of valid cues, the cue did not provide any information about the correct response.
Following response, visual feedback was presented for 300 ms. To motivate subjects, feedback was based on both speed and accuracy (ranging from +3 to −3 points), where fast correct responses received more points and fast incorrect responses resulted in the deduction of more points. Based on reaction times (RTs) from pilot data, for arrow stimuli, correct responses made faster than 325 ms were awarded ‘+3’ points, correct responses made between 325–475 ms were awarded ‘+2’ points, responses made between 475–600 ms were awarded ‘+1’ points, and correct responses slower than 600 ms were awarded no points. For words, correct responses faster than 525 ms were awarded ‘+3’, between 525–675 ms were awarded ‘+2’, between 675–800 ms were awarded ‘+1’, and slower than 800 ms resulted in no points. Incorrect responses falling within those time ranges resulted in a reduction of the same magnitude (i.e., incorrect response to word between 675–800 ms yielded ‘−1’ points). A number representing the percentage of points the subjects scored out of the possible maximum number of points was presented at the end of each block to inform subjects of their performance.
The experiment consisted of a 2-minute practice session followed by 4 blocks of 200 trials each. Trials were separated by a 500 ms ITI. Each block contained 20 invalidly-cued trials, 120 validly-cued trials, and 60 neutral trials. For each condition (invalid, valid, neutral), there was an equal distribution of word and arrow stimuli, and within each stimulus type, an equal distribution of possible responses (word: living, nonliving; arrow: left, right).
2.3 Electrophysiological recording and analysis
Continuous EEG was recorded from 64 Ag/AgCl electrodes using Neuroscan SYNAMP2 (Compumedics Inc.) at a sampling rate of 500 Hz (high pass filter = 0.1 Hz, low pass filter = 100 Hz, impedances kept below 11 KΩ). The majority of electrodes were embedded in an elastic cap and arranged according to the standard 10–20 system, with additional electrodes placed at TP9/TP10 and Cb1/Cb2. Cerebellar electrodes (Cb1/Cb2) were placed at 50% of the distance from Iz to TP9 and TP10. In addition, eye movements were recorded from electrodes on the left and right zygomatic arches (F9, F10), at the outer canthus of each eye (LO1, LO2), and on the infraorbital ridges directly below each eye (IO1, IO2). EEG recordings were initially referenced to Cz then converted to an average reference off-line. Eye movements, blinks, and other artifacts were removed using PCA in BESA 5.1 software (Electrical Geodesics Inc.).
Trials were averaged separately for each condition (valid, invalid, and neutral) of each stimulus category (word, arrow) between −100 ms to 1000 ms from stimulus presentation. The 100 ms pre-stimulus period served as baseline. A 35-Hz low-pass filter was applied before averaging. Only correct trials in which RT did not exceed 2000 ms and was greater than 200 ms were averaged for both behavioral and ERP analysis. The number of invalid trials remaining after removing trials with artifact and response time outliers was tallied to ensure that any significant differences across conditions would not be caused by averaging too few trials in this rare condition. For words, the average number of invalid trials across subjects was 29.9, and all subjects had more than 20 trials. For arrows, the average across subjects was 35.9, and all subjects had more than 30 trials.
We conducted our analysis of target-specific activity at electrodes where we expected to observe the greatest modulation based on the nature of the individual tasks and previous research using similar stimuli. For arrow stimuli, we predicted that cue validity effects would be maximal at posterior sites, whereas for word stimuli we predicted that effects would be maximal at temporal sites, particularly those over the more anterior portion. Indeed, visual inspection of the grand mean data at all electrodes confirmed that cue-related modulations were maximal at P3/P4 and P5/P6 for arrow stimuli and FT9/FT10 and T7/T8 for word stimuli.
As can be seen in the grand mean data for arrow stimuli (Figure 3) and word stimuli (Figure 4), the waveforms at electrodes in the parieto-occipital region, including P3/P4, P5/P6, and PO3/PO4, demonstrated a clear P1/N1 complex. For both words and arrows, this was followed by a positive deflection over lateral parietal sites. For arrows, this peak was relatively broad over the left hemisphere, but peaked at about 287 ms (P290) over the right hemisphere. A similar, but even more focal right-lateralized peak was observed around the same latency for words.
Figure 3.
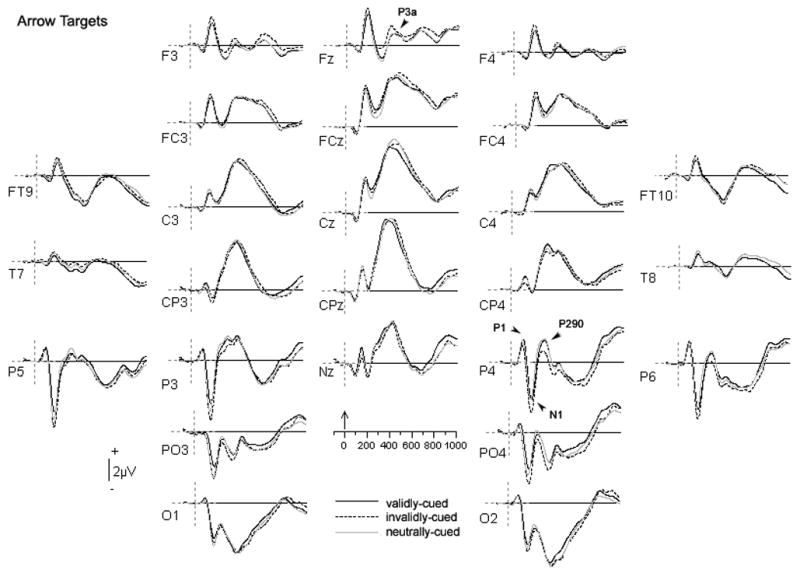
Grand average ERPs of the three experimental conditions for arrow targets. Waveforms were low-pass filtered at 15 Hz and positive is plotted up. The zero point in the timeline marks the onset of the arrow target.
Figure 4.
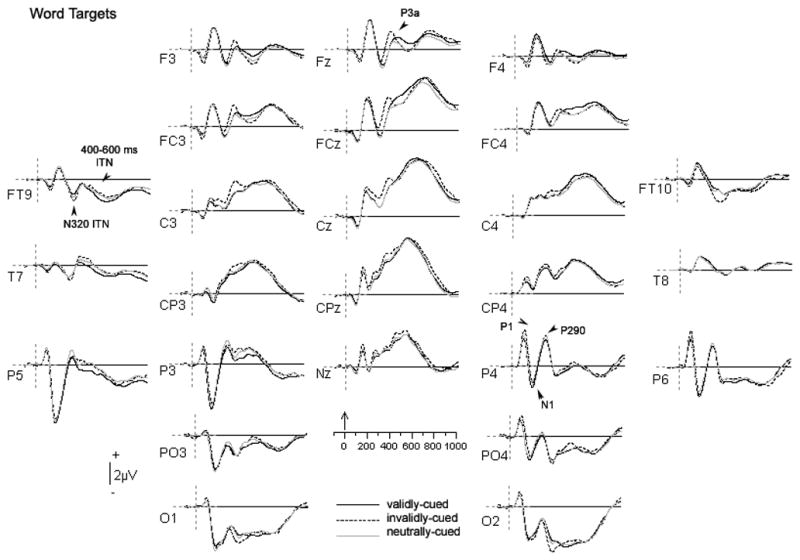
Grand average ERPs of the three experimental conditions for word targets. Waveforms were low-pass filtered at 15 Hz and positive is plotted up. The zero point in the timeline marks the onset of the word target.
At temporal electrodes (FT9/FT10, T7/T8), the waveforms associated with arrow and word stimuli appeared to exhibit a qualitatively different morphology. For arrow stimuli, only a single broad negativity was observed from 300–500 ms post-stimulus. For word stimuli, however, a negative going deflection was observed that peaked at approximately 320 ms (N320; early ITN), followed by a broader negative wave from 400–600 ms (late ITN). However, although a positive deflection was observed at ~400 ms at these anterior-temporal sites (in between the early and late ITN), we did not observe a negative deflection over centro-parietal sites as would be consistent with the classic N400. Rather, central activity from 300–500 ms was dominated by large positive-going waveforms. This positivity was followed by a late, broad negative-going waveform that spanned the same latency window as the negativity over anterior-temporal regions (400–600 ms).
Given differences in the morphology of the waveforms to word and arrow targets, we analyzed activity for each stimulus type separately, allowing us to choose time windows that best characterize the data for each. The P1 and N1 were compared using peak amplitude measurements taken at the point of their maximal deflection (P1: largest positive peak between 70–120 ms at P4; N1: largest negative peak between 140–210 ms at P3), but analyzed over a broader set of nearby electrodes (P3/P4, P5/P6, PO3/PO4). For arrow targets, the P1 peak latencies in the valid, invalid, and neutral conditions were 110 ms, 100 ms, and 107 ms, respectively; N1 latencies were 180 ms, 182 ms, 177 ms. For word stimuli, P1 latencies were 105 ms, 112 ms, and 106 ms in the valid, invalid, and neutral conditions respectively; N1 latencies were 171 ms, 176 ms, and 174 ms.2
For the P290, we also used the peak amplitude between 210 and 410 ms for words, given that this was a sharply defined peak (valid: 293 ms, invalid: 285 ms, neutral: 283 ms). However, the P290 for arrows was broader and hence was analyzed using the mean window between 210–410 ms. For each of these effects and for each stimulus type (word, arrow), we analyzed amplitudes recorded at electrodes P3/P4, P5/P6, PO3/PO4 in a 3 (condition: valid, invalid, neutral) x 2 (hemisphere) x 3 (electrode) repeated-measures ANOVA.
The inferior temporal negativities associated with word processing were analyzed using mean amplitude within specified windows (N320/early ITN [words]: 270–370 ms; Late ITN [words]: 400–600 ms). In addition, for the sake of comparison, we also analyzed the broad inferior frontal negativity from 300–500 ms that was observed for arrow stimuli. In each case, effects were analyzed at temporal sites (TP9/TP10, T7/T8) in a 3 (condition) 2 (stimulus) x 2 (hemisphere) x 2 (electrode) repeated-measures ANOVA.
Given that cue validity has also been found to influence the centrally-maximal N400 to verbal stimuli, we examined midline electrodes (Fz, FCz, Cz, CPz, Pz ) during the 400–600 ms window. A 3(condition: valid, invalid, neutral) x 2(stimulus: word, arrow) x 3(position: left, midline, right) x 5(electrode) repeated-measures ANOVA was preformed to identify condition-dependent as well as topographical differences of the P300.
Effects of electrode will only be discussed if they interact with other factors of interest. Greenhouse-Geisser corrections were applied when sphericity of variances between groups could not be assumed, with corrected degrees of freedom reported. Where appropriate, main effects and interactions were followed up by post-hoc comparisons using Tukey’s honestly significant difference (HSD) correction. The significant alpha level for all analyses was 0.05.
3. RESULTS
3.1. Costs and benefits of cueing on behavior
Percentage of errors was computed for each condition (valid, invalid, neutral) and for each type of stimulus (word, arrow). Subjects made more errors overall on the word task than the arrow task (word = 12.2%, arrow = 1.2%; F[1, 17] = 29.9, p < 0.001), but there were no differences in error rate as a function of cue validity.
Mean reaction time (RT) for correct responses was computed for each condition (valid, invalid, neutral) and type of stimulus3 (word, arrow), as shown in Figure 2. As expected, subjects responded faster to arrow than word stimuli overall, F(1,18) = 283.2, p < 0.001. There was also a significant main effect of cue validity, F(2, 36) = 15.2, p < 0.001, however this overall effect was qualified by a stimulus x condition interaction, F(2, 36) = 5.5, p < 0.01. Specifically, in the word task, RT in the valid condition (620 ms) was faster than in the neutral condition (664 ms; p < 0.01), which in turn was faster than the invalid condition (641 ms; p < 0.05). Thus, for the word task, there were significant cueing costs and benefits. For the arrow task, however, we only found a significant cost to having an invalid cue (invalid > neutral: p < 0.01). Although valid arrow cues resulted in numerically faster RTs than the neutral condition, this comparison did not reach significance, perhaps because RTs were already close to their asymptote. RTs for arrows were 396, 414, and 402 ms for the valid, invalid, and neutral conditions respectively.
Figure 2.
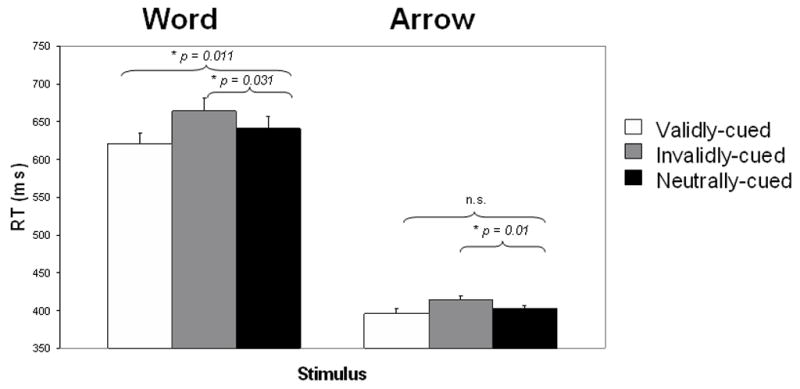
Mean reaction times for each condition in response to word (left) and arrow (right) targets. For each task, the p-values of comparisons between the neutral condition and each of the other two conditions are provided.
3.2. Target-specific activity
3.2.1 Effects of cue validity on arrow targets
Figure 3 shows the waveforms observed for arrow stimuli.
3.2.1.1 Posterior potentials
Cue validity did not influence the amplitude of the P1 over parietal-occipital electrodes (P3/P4, P5/P6, and PO3/PO4), where it was generally larger over the right hemisphere, F(1, 18) = 14.9, p = 0.001. However, there was a significant effect of condition on the N1 at these electrodes, F(2, 36) = 3.9, p < 0.05, as well as a significant hemisphere x condition interaction, F(2, 36) = 3.7, p < 0.05. Post-hoc Tukey’s HSD comparisons revealed that invalidly-cued arrows elicited a more negative-going N1 than validly-cued arrows over both hemispheres. Additionally, over the right hemisphere, the N1 to invalidly-cued arrows was significantly more negative than the N1 to arrows preceded by neutral cues.
The parieto-occipital P290 was also sensitive to the effects of cue validity, F(2, 36) = 10.3, p < 0.001. Yet, unlike the effects observed at the N1, here arrows preceded by invalid cues appeared to elicit a smaller P290 relative to those in either the valid or neutral conditions, which did not differ from each other. These effects mirrored the behavioral RT differences in that they reflected the cost of having an invalid cue, but no benefit to having a valid cue. Condition effects were larger at certain electrodes than others, however, as indicated by a significant hemisphere x electrode x condition interaction, F(4, 72) = 2.7, p = 0.05, which also subsumed a significant hemisphere x condition interaction, F(1.5, 26.7) = 16.1, p < 0.001. Post-hoc comparisons revealed that validity modulated activity more reliably over the right hemisphere, where invalidly cued arrows consistently elicited smaller P290s than either validly- or neutrally- cued arrows at all right hemisphere electrodes included in the analysis (P4, P6, PO4). In contrast, over the left hemisphere, the parietal electrodes (P3, P5) demonstrated significant differences between invalidly-cued and neutrally-cued targets only, while there were no significant condition differences over the parietal-occipital site (PO3).
3.2.1.2. Inferior temporal negativity
We also analyzed activity at temporal electrodes (FT9/FT10, T7/T8) between 300–500 ms for arrow targets, where significant differences were predicted to occur primarily for words. Somewhat surprisingly, we observed a hemisphere x condition interaction, F(2, 36) = 6.9, p < 0.005. Post-hoc comparisons of conditions within the right hemisphere revealed no significant differences as a function of validity. However in the left hemisphere, validly-cued arrows elicited significantly more negative-going activity than invalidly-cued arrows. No other comparisons were significant. Given the latency and location of this component, we cannot rule out the possibility that it reflects verbal processing of the feedback, which would have generally occurred earlier for validly-cued targets.
3.2.2. Effects of cue validity on word targets
Figure 4 shows the waveforms observed for word stimuli.
3.2.2.1 Posterior potentials
In contrast to the findings for arrow targets, the P1 to word targets was modulated by cue validity, F(2, 36) = 11.3, p < 0.001, whereas the N1 was not. Specifically, post-hoc comparisons showed that invalidly-cued words elicited a significantly larger P1 than words preceded by valid or neutral cues. As with the arrow stimuli, the P1 to words was larger over the right hemisphere, F(1, 18) = 16.2, p = 0.001. Additionally, as with the arrow stimuli, the P290 was larger in the right hemisphere, F(1, 18) = 12.0, p < 0.01, but did not differ as a function of condition for word targets.
3.2.2.2 Inferior temporal negativities
Word targets elicited two distinct negative-going peaks at temporal sites (T7/8 and TP9/10) during the target epoch. The first deflection peaked at an average of 320 ms (N320/early ITN) post-stimulus and was analyzed over a window between 270–370 ms. The second prominent waveform in this region was broader and analyzed from 400–600 ms post-stimulus (late ITN).
The earlier waveform (N320/early ITN) exhibited a main effect of condition, F(1.3, 23.1) = 6.0, p < 0.05, but no effect of hemisphere (p = 0.2). Post-hoc comparisons showed that invalidly cued words elicited a greater negativity relative to both validly- and neutrally- cued words, which did not differ from each other. At the late ITN (400–600 ms), we observed a hemisphere x condition interaction, F(2, 36) = 3.2, p = 0.05. Post-hoc comparisons revealed that in the left hemisphere, validly-cued words produced a larger negativity than both invalidly- and neutrally-cued words. No other significant condition effects were observed.
To capture possible effects of cue validity on the classic N400 for word stimuli, an analysis of the 400–600 ms window was also performed across the midline electrodes (Fz, FCz, Cz, CPz, and Pz). This analysis revealed a significant electrode x condition interaction, F(2.8, 49.9) = 3.6, p < 0.05. Post-hoc comparisons confirm significant differences between conditions where validly cued words elicited a greater positivity over the 400–600 ms window than both invalidly- and neutrally-cued words.
3.3 Domain-general task-set switching activity
To assess the possibility that the anterior P3a represents a more domain-general process associated with task-set switching (see Figures 3 and 4), we included stimulus (word, arrow) as a factor in our analyses of midline activity (Fz, FCz, Cz, CPz, and Pz) rather than analyzing stimulus separately. These analyses revealed an overall effect of condition, F(1.4, 26.0) = 5.8, p < 0.05, which was qualified by a significant electrode x condition interaction, F(3.0, 53.3) = 3.1, p < 0.05. Post-hoc comparisons confirmed the visual impression that the effects of cue validity were largest over anterior electrode sites, where invalidly-cued stimuli elicited a greater positivity than those in the valid and neutral conditions at electrodes Fz and FCz. At Cz, invalid-cued stimuli differed only from validly-cued stimuli. At posterior electrodes (CPz, Pz), no differences between conditions emerged (see Figures 3 and 4).
The electrode x condition interaction did not interact further with stimulus (p = 0.41), supporting the view that invalidly cued words and arrows both elicited an anterior P3a sensitive to the invalid condition. Yet, although both tasks appeared to elicit condition effects at Fz and FCz, these effects in the word task appeared to extend to posterior sites as well (Figs. 3–5). We opted to investigate this impression further with separate ANOVAs evaluating the effects of condition for each stimulus at each electrode. At Fz, there was a significant effect of condition for both words, F(2, 40) = 7.7, p = 0.001, and arrows, F(2, 40) = 6.4, p < 0.005, which in both cases was driven by invalidly-cued targets eliciting a larger positivity than either validly- or neutrally-cued targets. No significant condition differences were observed for arrow targets at any other electrode. For word targets, significant validity effects were observed at FCz, F(2,40) = 7.2, p < 0.005, where post-hoc comparisons revealed a significant difference between invalidly- and neutrally-cued words. An overall effect of condition also was found at CPz for words, F(2, 36) < 0.05, however none of the individual comparisons survived post-hoc correction. Overall, these findings support the view that the P3a condition effect was more widespread for words (see Figure 5), despite the lack of significant 3-way interaction between electrode x condition x stimulus. Indeed, this greater spatial extent appeared to be driving a trend for the condition effects to be larger overall in the word than arrow task, as indicated by a marginal stimulus x condition interaction F(2, 36) = 2.9, p = 0.07.
Figure 5.
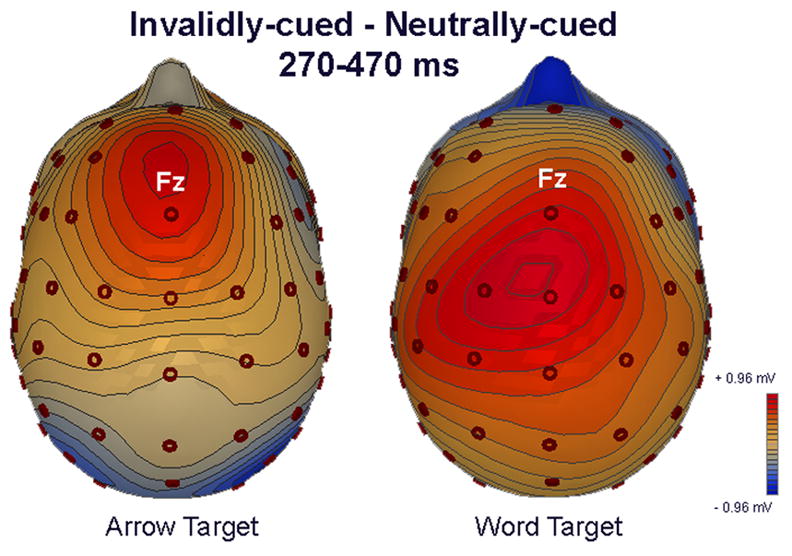
Scalp maps of difference waves between invalidly-cued and neutrally-cued stimuli for both word (left) and arrow (right) targets averaged across the interval 270–470 ms.
A further indication of stimulus-related differences along the midline was a significant stimulus x electrode interaction, F(2.0, 35.2) = 14.3, p < 0.001. Post-hoc comparisons indicated that arrow stimuli elicited more positive-going activity overall during this period than word stimuli at central and posterior sites, most likely as a result of the earlier peak of the posterior P3b for arrows relative to words regardless of condition (see Figure 3). Nonetheless, in support of the view that effects at Fz were more domain-general, we found no effect of stimulus at this electrode.
We take these findings to illustrate that for both target types, validity exerted robust effects on P3a activity at anterior sites, with invalidly-cued trials eliciting a greater positivity than either neutral- or validly-cued trials. However, for word targets, this activity could also be observed at more posterior midline sites. It is not possible to determine whether the more widespread distribution for word targets results from quantitatively greater activity in anterior regions and/or from the presence of additional neural generators (see Urbach & Kutas, 2005 for further discussion of difficulties in inferring generator distribution from either scaled or unscaled scalp topography).
4. Discussion
The present study integrated aspects of cued attention and task-switching paradigms in order to evaluate the effects of cueing on processes including and extending beyond stimulus detection and identification. Using tasks that required subjects to make higher-level categorical decisions regarding either object form (arrow task) or word meaning (word task), we found strong similarities in the overall pattern of cueing effects on ERP waveforms across tasks, even though the specific components that were modulated were largely task-specific. Valid cueing appeared to positively prime early components related to the stimulus identification as evidenced by relative decreases in target-specific activity relative to neutral- and invalidly-cued targets. This apparent reduction in processing effort at early stimulus processing stages (P1/N1; early ITN/N3) may facilitate transmission of information to subsequent stages involved with categorization and response selection, as evidenced by relative increases in target-specific activity for validly-cued targets just preceding the average RT (Tallon-Baudry, 2005; see also Kilner et al., 2005). For the arrow task, categorization-related validity effects were maximal over posterior parietal sites (P290), generally consistent with processing of visuospatial information (although a smaller, but significant validity effect was also observed at left inferior temporal sites from 300–500 ms). For the word task, categorization-relevant validity effects were maximal over left temporal sites (late ITN), generally consistent with processing of language-relevant information. In addition, regardless of the task, invalidly cued targets elicited a frontal P3a (maximal at Fz) that may provide an index of general task-switching and reorienting processes. Taken together, these results suggest that the manner in which top-down attention modulates stimulus identification and categorization follows a similar overall pattern regardless of the nature of the task. However, the specific level of processing (i.e., perceptual vs. semantic), and corresponding ERP components exhibiting these modulations will depend on task demands.
4.1 Target-specific activity: arrows
Arrow stimuli demonstrated reliable cue-related modulations over regions of posterior parietal cortex. Consistent with Miniussi, et al. (2005), we found no effects of validity on the P1 in our arrow task. Furthermore, unlike that study, we did observe an enhancement of the N1 for invalidly-cued arrows. At first this may seem to conflict with previous studies of spatial attention, which generally observe an enhanced N1 to validly-cued targets when a perceptual decision is required (Mangun & Hillyard, 1991). In studies of spatial attention, however, the enhancement associated with valid cueing is thought to reflect the benefit of attention being already present at that location (Hillyard & Anllo-Vento, 1998; Mangun & Buck, 1998; Johannes et al., 1995; Henderson, 1991; Hawkins et al., 1988). Given that all of the stimuli in the present study were centrally presented, and therefore, spatial attention was held constant across conditions, we suggest that the paradoxical enhancement of the N1 for invalidly-cued arrows may have resulted from enhanced attention to visual input that arrives in a different configuration than expected (i.e., ≪< or ≫> vs. letter string). Indeed, object-based selective attention studies have demonstrated the sensitivity of the P1 and N1 to perceptual grouping processes (Valdes-Sosa et al, 1998). It could be argued that because our arrow stimuli exhibit stronger perceptual grouping than the typical word, failure to anticipate this type of grouping prior to the onset of the stimulus would result in greater attention to this stimulus when it was ultimately presented. Providing indirect support for this view, we note that arrow stimuli elicited a larger N1 component than word stimuli overall, F(1,18) = 20.2, p < 0.001.
A prominent parieto-occipital P290 was also observed for arrow stimuli, which was smaller for invalidly-cued arrows. Although less is known about the functional significance of this later posterior component (see Mangels, Picton, & Craik, 2001), a waveform with similar morphology, latency, and sensitivity to validity was observed in the perceptual categorization task used by Miniussi et al. (2005). We argue that the spatiotemporal characteristics of this component make it a good candidate for the final categorization of the direction/form of the arrow stimulus. First, the time course of our P290 spanned 200–400 ms, and thus, it occurred immediately before the mean RT for arrows (404 ms). The latency and parietal-occipital location of this component are also consistent with other waveforms associated with object-form decision processes such as those found in perceptual closure experiments (Doniger, et al., 2001; Sehatpour et al., 2006) and when viewing meaningful versus meaningless stimuli (Tallon-Baudry, et al., 1997). Finally, the finding that the P290 differed only for the invalid condition (relative to valid and neutral conditions) mirrors our behavioral results, where costs were observed for the invalid condition with no additional benefit of the valid condition over the neutral condition.
Taken together, the N1 enhancement and P290 attenuation for invalidly-cued trials may indicate two distinct stages of visual-form processing that are differentially modulated by top-down expectancies regarding the type of categorization to be made. It is well known that processing of visual stimuli progresses along a hierarchy, from early perceptual processes that are generally stimulus-driven to those that are more category-specific (Grill-Spector, et al., 1998; Grill-Spector, 2003). There is also evidence that top-down modulation can occur at any of these stages depending on which feature a particular task requires subjects to respond to. For example, a study using both fMRI and ERPs (Gazzaley, et al., 2005) showed that when subjects were instructed to remember and attend to face stimuli, activity in the fusiform face area and the face-related N170 were enhanced relative to a free-viewing condition. Tallon-Baudry, et al. (2005) went on to observe that such pre-orientation of attention enhances EEG synchronization at relevant locations of processing specific to the degree in which stimuli were attended, while earlier stages were found to be attenuated. They suggest that reduced effort at earlier stages due to cueing may lead to the faciliation of processing at later stages (see also Kilner et al., 2005). A similar effect may be occurring at our N1 and P290, where invalidly-cued trials elicited a larger N1, followed by a smaller P290.
We argue that invalid cueing results in more attentional effort at early stages at the expense of processing at later stages, however one issue here is that there could have been more variability in latency at which categorization processes onset in the invalid condition, leading to a broader and smaller waveform at this subsequent stage. Arguing against this possibility, we found no difference in the variability of behavioral performance (RT) across conditions, suggesting that the variability in the onset of corresponding categorization and response selection processes was reasonably matched (p = 0.1). Another issue of interpretation is that the increased negativity of the N1 for invalid trials could have produced an overall negative-going shift that made it appear as though there was attenuation of subsequent positive-going waveforms. We believe this is unlikely to be the case given that the N1 also produced observable effects at other waveforms in the occipital region (i.e., O1) where effects on subsequent positive waveforms were less apparent.
4.2 Target-specific activity: words
Word stimuli showed early condition-related modulations over posterior sites, as well as later modulations at anterior temporal sites that have been implicated in the conceptual processing of words (Mangels, et al., 2001; Stern & Mangels, 2006; Nessler, et al., 2005; Nobre & McCarthy 1994; Hauk et al., 2006). Although word targets elicited P1, N1, and P290 components, similar to the arrow targets, cueing effects were found only at the P1. Specifically, invalidly-cued words elicited a larger P1 than either validly- or neutrally-cued words. Similar to our explanation of the enhanced N1 for invalidly-cued arrows, we suggest that the P1 was paradoxically larger for invalidly-cued targets because of the greater attention that must be allocated toward perceptual processing of information that differs from expectation. For words, however, this attentional modulation may have been apparent at the P1 because the visual features of word stimuli generally invite processing at a more local level than arrows (Shihui, et al., 2000). This hypothesis receives some support from the greater P1 observed for word stimuli compared to arrow stimuli overall, F(1,18) = 38.6, p < 0.001.
At anterior-temporal electrodes, two negative-going components showed significant condition dependent effects, an N320/early ITN and a broader, later ITN between 400–600 ms. Different effects of validity were observed at these sequential components. Although invalidly-cued words elicited an enhanced N320 (bilaterally) relative to validly cued words, validly-cued words elicited an enhanced later left-lateralized ITN relative to invalid and neutrally cued words. This latter pattern was also observed in a late positivity over frontal midline sites (400–600 ms), suggesting that it may reflect the inverse of the dipole responsible for the late ITN.
Together, the overall pattern at these sites mirrored the pattern observed in posterior regions for arrow targets, in that the invalid condition was associated with a relative increase in amplitude of an early ERP component, followed by a relative decrease in amplitude at a later ERP component that occurred immediately before the behavioral response. Given that these differential modulations occurred at two successive negative-going potentials, it is less likely that this pattern is the result of a single effect, as may have been the case in the arrow task N1/P290 effects. This lends credence to the possibility that these potentials, and their corresponding processes, reflect dissociable effects of validity.
A dissociation between two consecutive ERPs was also observed by Nobre et al. (1994) in a semantic priming task, although in that study, primed words elicited a greater inferior frontal negativity at about 320 ms followed by an attenuated anterior midline N400. While those findings also provide general support for the view that there are multiple stages associated with categorization of verbal stimuli, the reason that they are in the opposite direction to the results in the present study is not entirely clear, although it may be due to the specificity of the “cue.” As we described previously, the N400 is usually attenuated to the extent that the number of possible semantic associates has been restricted by a semantically related prime. However, in the present study, the cue serves to activate only the broad category of nouns. The present findings also differ from those of Miniussi et al. (2005), who found that valid trials were associated with similar enhancement of the N3 and N400, both of which were maximal over the anterior midline. Our use of an average reference rather than a mastoid reference may have improved our ability to resolve ERP effects at inferior frontal and temporal sites nearby where intracranial EEG and fMRI studies have localized some of the important sources of the N400 (Nobre & McCarthy, 1995; Nobre et al., 1994; Matsumoto et al., 2005; Marinkovic, 2004; also see Van Petten & Luka, 2006).
Further evidence that the ITN components are associated with semantic processing and can be modulated by top-down control comes from a recent study of cueing effects on verbal processing in a spatial Stroop task in which directional words (e.g., ‘up’, ‘down’) conflicted with their position around a central crosshair (Stern & Mangels, 2006). In that study, two successive left-hemisphere inferior temporal negativities (early ITN: 200–400 ms; late ITN: 400–600 ms) were enhanced when responses were made based on the meaning of the word, rather than on its position relative to the crosshair. This effect was observed only when the manual responses to the words were fast and efficient. Importantly, in support of the view that these enhanced stimulus-locked negativities were related to successful pre-stimulus biasing of verbal processing, positive correlations were found between the amplitude of the “top-down” frontal pre-stimulus activity and the relative enhancement of the ITNs associated with fast vs. slow decisions.
4.3 Domain-general task-set switching
Invalidly cued targets elicited a greater P3a between 270–370 ms relative to both valid and neutrally cued targets. This effect was observed at the anterior frontal site (Fz) for both word and arrow targets, but also extended to more central sites in the word task (Figure 5). These differences in extent may reflect quantitative differences in activity within a single set of neural generators, which plausibly could have arisen from the greater effort required to switch to the more complex set of rules associated with the semantic task. Alternatively, re-configuring the neural resources for the semantic task may have engaged additional, qualitatively different brain regions. Nonetheless, the similarity of the waveforms and validity-effects at anterior electrode Fz suggests that the anterior P3a reflects an aspect of task-set switching that was common to both tasks.
Enhancement of the P3a has been associated with the initiation of task-set switching in other tasks (Barcelo et al., 2002; Rushworth et al., 2002; Kepp et al., 2006) and may be indicative of an attentional re-orienting response that is fundamental to this process. Indeed, a recent study showed an enhancement of the P3a specifically for re-orienting processes, by comparing the amplitude of this component for switch-cues that appeared either before, or after the presentation of cards (but before response) in a modified Wisconsin Card-Sort task (Kopp et al., 2006). Our results are consistent with this view in that P3a enhancement was observed only for invalidly-cued targets (10% probability), and not for neutrally-cued targets even though neutral-cues were also relatively rare (30% probability). Indeed, if the P3a was measuring only general novelty detection or orienting per se, it should have been modulated to some extent by the neutrally-cued targets as well.
The temporal sequence of target-specific stimulus processing and domain-general task-set switching components may shed light on the nature of the interaction between these two mechanisms. Specifically, the onset of the P3a relative to the P1/N1 complex suggests that reorienting was initiated following initial detection of incongruity between expected and actual perceptual information. Task-specific categorization then appeared to commence at or just after the peak of the P3a. This is consistent with the view that prefrontal mechanisms responsible for updating task-set representations work to bias stimulus-response coordination in posterior regions (Miller & Cohen, 2001; Brass et al., 2005; Crone et al., 2006). For the arrow task, which required decisions to be made based only on perceptual form information, categorization could commence almost immediately after the switch took place, as suggested by the latency of the parieto-occipital P290. For the word task, however, the P3a overlapped with the early ITN (N320), which may index initial lexical/semantic processing of the verbal stimulus. It is unlikely that the N320 simply represents the inverse of the P3a, given that while the P3a was observed for both word and arrow tasks, the N320 was observed only for word targets.4 Thus the N320 appears to represent an additional level of stimulus identification specific to the word task that takes place before semantic categorization, which we argue may be indexed by the subsequent late ITN (400–600 ms).
4.5 Conclusions
By employing a hybrid cued selective attention and task-switching task design, this study provides some of the first evidence from scalp recorded ERPs that expectancy can have differential effects on identification and categorization stages of task-specific stimulus processing. Across both perceptual and semantic categorization tasks, we observed greater activity for invalid targets at identification stages, followed by greater activity for valid targets at putative categorization stages. The particular target-specific ERPs that were modulated by validity depended on the nature of the stimulus categorization, however. For arrows, these effects were observed at a posterior N1/P290 complex, where as for words they were observed at a sequence of inferior temporal negativities (N320/late ITN). We argue that the earlier components in each task (N1 and N320) represent the initial identification of the target as an arrow or word. Because in validly-cued trials this information is already known, processing at these regions occurs rapidly, facilitating processing at later stages (represented by the P290 and late ITN) that are involved in the actual categorical decision-making process necessary to perform the task.
Object processing in the ventral visual stream follows a hierarchy of distinct stages that process successively more conceptual aspects of visual stimuli, from differentiation of basic perceptual features to categorization of semantically distinct attributes (Grill-Spector, et al. 1998; Grill-Spector, 2003). Similarly, semantic processing of visually presented word stimuli is carried out in spatio-temporally distinct stages, from initial word-form processing in the visual cortex to a series of language-processing regions in the temporal and inferior prefrontal cortices (Hauk et al., 2006; for review, see Fiez & Peterson, 1998; Marinkovic, 2004). The present results suggest that top-down expectation and stimulus-driven input interact along these levels of processing to bias both perception and categorization processes toward task-relevant information (see Pessoa, et al., 2003; Yantis, 2005).
Acknowledgments
This research was supported by an NIH grant R21MH066129 to J. A. Mangels. We are grateful to Matt Greene who provided assistance with programming, and to Anna Nobre, Bernadette Sibuma, and two anonymous reviewers for comments on previous drafts of this manuscript. Aspects of this data were presented at the 2005 meeting of the Cognitive Neuroscience Society (CNS), New York, NY.
Footnotes
The Standard Frequency Index (SFI) is a word frequency measure taken from the Educator’s Word Frequency Guide (Zeno et al., 1995). It is an index of a word’s frequency across common American secondary school textbooks that also takes into account how widely that word is used across different subject areas (i.e., dispersion). This measure provides a good estimate of word frequency for our undergraduate population. SFI values reported in Zeno et al. (1995) range between 3.5 and 88.3.
Small (7–10 ms), but significant latency differences between the invalid condition and the other two conditions were found for the P1 to the arrow, F(2, 36) = 7.5, p < 0.005, as well as the word, F(2, 36) = 8.6, p < 0.005. Although it might be expected that invalidly-cued stimuli would have a slower P1, this was the direction of effect only for the word stimuli. For arrow stimuli, invalidly-cued stimuli elicited a faster P1. These differences were not predicted, and the functional significance of these effects is currently unclear. Thus, we will focus on amplitude measurements made at these peaks.
There were no significant differences in RT to the arrows as a function of direction (right, left), nor interaction between condition and direction (all ps > 0.2). Although there were significant mean RT differences between living/nonliving items, F(1, 18) = 53.0, p < 0.001, there was no condition x item interaction (p = 0.8). Therefore, for both arrow and word stimuli, all subsequent analyses were collapsed over item type.
Although it could be argued that the left-temporal negativity (300–500 ms) in the arrow task may be related to the N320, this component showed opposite effects of validity to the P3a, further arguing against the possibility that the temporal negativities during this period simply reflect the inverse of the midline P3a.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barcelo F, Perianez JA, Knight RT. Think differently: a brain orienting response to task novelty. NeuroReport. 2002;13:1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DEJ. Attentional systems in target and distractor processing: a combined ERP and fMRI study. NeuroImage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cromon DY, Philips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. Journal of Cognitive Neuroscience. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- Butterfield B, Mangels JA. Neural correlates of error detection and correction in a semantic retrieval task. Cognitive Brain Research. 2003;17:793–817. doi: 10.1016/s0926-6410(03)00203-9. [DOI] [PubMed] [Google Scholar]
- Carrasco M, McElress B. Covert attention accelerates the rate of visual information processing. PNAS. 2001;98(9):5363–5367. doi: 10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dormeyer S, Shulman GL, Peterson SE. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990;248:1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Crone FA, Wendelken C, Donohue SF, Bunge SA. Neural evidence for dissociable components of task-switching. Cerebral Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Curran T, Tanaka JW, Weiskopf DM. An electrophysiological comparison of visual categorization and recognition memory. Cognitive, Affective & Behavioral Neuroscience. 2002;2:1–18. doi: 10.3758/cabn.2.1.1. [DOI] [PubMed] [Google Scholar]
- Curran T, Tucker DM, Kutas M, Posner MI. Topography of the N400: brain electrical activity reflecting semantic expectancy. Electroencephalography and clinical Neurophysiology. 1993;88:188–209. doi: 10.1016/0168-5597(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Downing CJ. Expectancy and visual-spatial attention: Effects on perceptual quality. Journal of Experimental Psychology: Human perception and performance. 1988;14:188–202. doi: 10.1037//0096-1523.14.2.188. [DOI] [PubMed] [Google Scholar]
- Johannes S, Munte TF, Heinze HJ, Mangun GR. Luminance and spatial attention effects on early visual processing. Cognitive Brain Research. 1995;2:189–205. doi: 10.1016/0926-6410(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Johnson BW, Hamm JP. High-density mapping in an N400 paradigm: evidence for bilateral temporal lobe generators. Clinical Neurophysiology. 2000;111:532–545. doi: 10.1016/s1388-2457(99)00270-9. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowics YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Peterson SF. Neuroimaging studies of word reading. PNAS. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Human Brain Mapping. 1998;6:316–328. doi: 10.1002/(SICI)1097-0193(1998)6:4<316::AID-HBM9>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins HL, Shafto MG, Richardson K. Effects of target luminance and cue validity on the latency of visual detection. Perception and Psychophysics. 1988;44:484–492. doi: 10.3758/bf03210434. [DOI] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Ford M, Pulvermuller F, Marslen-Wilson WD. The time course of visual word recognition as revealed by linear regression analysis of ERP data. NeuroImage. 2006;30:1383–1400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Henderson Stimulus discrimination following covert attentional orienting to an exogenous cue. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:91–106. doi: 10.1037//0096-1523.17.1.91. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anll-Vento L. Event-related brain potentials in the study of visual selective attention. PNAS. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J, Bott L, Posada A. Modulations in the degree of synchronization during ongoing oscillatory activity in the human brain. European Journal of Neuroscience. 2005;21:2547–2554. doi: 10.1111/j.1460-9568.2005.04069.x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Tabeling S, Moschner C, Wessel K. Fractionating the neural mechanisms of cognitive control. Journal of Cognitive Neuroscience. 2006;18:949–965. doi: 10.1162/jocn.2006.18.6.949. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Marinkovic K. The spatiotemporal dynamics of word processing in the human cortex. The Neuroscientist. 2004;10:142–152. doi: 10.1177/1073858403261018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels JA, Picton TW, Craik FIM. Attention and successful episodic encoding: an event-related potential study. Cognitive Brain Research. 2001;11:77–95. doi: 10.1016/s0926-6410(00)00066-5. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Luck LA. Sustained visual-spatial attention produces costs and benefits in response time and evoked neural activity. Neuropsychologia. 1998;36:189–200. doi: 10.1016/s0028-3932(97)00123-1. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Iidaka T, Haneda K, Okada T, Sadto N. Linking semantic priming effect in functional MRI and event-related potentials. NeuroImage. 2005;24:624–634. doi: 10.1016/j.neuroimage.2004.09.008. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. Journal of Neuroscience. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Marzi CA, Nobre AC. Modulation of brain activity by selective task sets observed using event-related potentials. Neuropsychologia. 2005;43:1516–1528. doi: 10.1016/j.neuropsychologia.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. TRENDS in Cognitive Science. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nessler D, Mecklinger A, Penney TB. Perceptual fluency, semantic familiarity and recognition-related familiarity: an electrophysiological exploration. Cognitive Brain Research. 2005;22:265–88. doi: 10.1016/j.cogbrainres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, Mccarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy Language-related ERPs: scalp distributions and modulation by word type and semantic priming. Journal of Cognitive Neuroscience. 1994;6:233–255. doi: 10.1162/jocn.1994.6.3.233. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: 11. Effects of word type and semantic priming. Journal of Neuroscience. 1995;15:1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing top-down control. Journal of Neuroscience. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research. 2005;45:1867–1875. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Synder CRR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology: General. 1990;109:160–174. [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Passingham RF, Nobre AC. Components of switching intentional set. Journal of Cognitive Neuroscience. 2002;13:1139–1150. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- Soldan A, Mangels JA, Cooper LA. Evaluating models of object-decision priming: evidence from event-related potential repetition effects. Journal of Experimental Psychology: Learning Memory and Cognition. 2006;32:230–248. doi: 10.1037/0278-7393.32.2.230. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamics of human object recognition processing: an integrated high-density electrical mapping and functional imaging study of “closure” processes. NeuroImage. 2006;29:605–18. doi: 10.1016/j.neuroimage.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Shihui H, Wanzhan L, William YE, Woods D. Interactions between spatial attention and global/local feature selection: an ERP study. NeuroReport. 2000;11:2753–2758. doi: 10.1097/00001756-200008210-00029. [DOI] [PubMed] [Google Scholar]
- Stern ER, Mangels JA. An electrophysiological investigation of preparatory attentional control in a spatial stroop task. Journal of Cognitive Neuroscience. 2006;18:1–14. doi: 10.1162/jocn.2006.18.6.1004. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff M, Isnard I, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cerebral Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BL. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain and Language. 2006;97:279–293. doi: 10.1016/j.bandl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa M, Bobes MA, Rodriguez V, Pinilla T. Switching attention without shifting the spotlight: Object-based attentional modulation of brain potentials. Journal of Cognitive Neuroscience. 1998;10(1):137–151. doi: 10.1162/089892998563743. [DOI] [PubMed] [Google Scholar]
- Vogel ED, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;27:190–203. [PubMed] [Google Scholar]
- Yantis S. How visual salience wins the battle for awareness. Nature Neuroscience. 2005;8:975–977. doi: 10.1038/nn0805-975. [DOI] [PubMed] [Google Scholar]
- Zeno SM, Ivens SH, Millard RT, Duvvuri R. The educator’s Word Frequency Guide. Touchstone Applied Science Associates (TASA), Inc; USA: 1998. [Google Scholar]


