Abstract
Background
One of the important factors of the demineralization and remineralization equilibrium of enamel is the pH of the surrounding solutions. Effort has been laid in the formulation of different fluoride compounds and the fluoride content in toothpastes but much less is known about the influence of the pH of the toothpastes on their effectiveness. It was therefore the aim of this study to investigate the influence of different pH levels on enamel remineralization in an in vitro experiment using polarization light microscopy and EDX quantitative element analysis.
Methods
A 5 × 5 mm window on the enamel surface of 40 caries free extracted human premolars was demineralized in a hydroxyethylcellulose solution at pH 4.8. The teeth were divided into 8 groups and the lower half of the window was covered with varnish serving as control. Each group was then immersed in toothpaste slurry containing amine fluoride (1400 ppm) at pH 4.1, 4.5, 5.1 and 6.9 or control toothpaste slurry without fluoride at pH 4.3, 4.7, 5.3 and 7.0. Serial sections were cut through the lesions and investigated with polarization light microscopy and quantitative EDX element analysis.
Results
The PLM results showed a decreased porous volume of the body of the lesion after incubation with fluoridated toothpaste at pH 4.53 and 5.16. No differences between the experimental window and the control window were found in the other groups. The quantitative element analysis showed no differences in the element content of any of the groups.
Conclusion
From the results it can be concluded that slightly acidified fluoridated dentifrices may have a certain positive effect on enamel remineralization.
Background
Dental caries progression or reversal depends upon the balance between demineralization and remineralization [1]. This balance is depended from several factors e.g. salivary Ca and P concentration, bioavailability of fluoride and pH. Remineralization occurs when the pH raises and Ca and P from saliva together with fluoride are forming new hydroxyapatite crystals on the enamel surface and the body of the lesion [2,3]. Mineral loss of incipient caries lesions is inversely proportional to the degree of saturation of Ca and P ions and the pH of the solution The critical pH range for demineralization and remineralization is between 4.3 and 5.0 where lesions with well defined surface layers occur whereas at pH levels around 6.0 no surface layers are forming [4].
Fluoride plays an important role in the remineralization process. Although a dose-response effect of fluoride enhancing enamel remineralization has been found [5,6] also small amounts of fluoride (<1 ppm) act on remineralization [7,8]. Demineralization and remineralization experiments have shown that fluoride enhances mineral uptake during continuous remineralization [9]. It is now acknowledged that fluoride acts as catalyst and influences reaction rates with dissolution and transformation of various calcium phosphate minerals. At low fluoride levels (<0.1 ppm) calcium uptake during remineralization is enhanced, while at this concentration no effect of fluoride on enamel demineralization is observed [10]. Below the critical pH hydroxyapatite is dissolved, but the released mineral ions could be reprecipitated as fluorapatite which is less soluble and may provide additional protection onto the apatite crystals. Even at a physiological pH fluorapatite precipitation is greater than that of hydroxyapatite also at low fluoride levels [11]. However, TenCate and Duijsters [12,13] showed that fluorapatite per se did not affect the overall mineral loss in enamel but calcium fluoride which is more effective in inhibiting enamel demineralization than fluorapatite.
Most of the studies concerning the effects of fluoridated toothpastes on enamel remineralization have been carried out with respect to the amount of fluoride [6,14-16] or different fluoride compounds [17-20]. Only little attention has been paid to the influence of different pH values of fluoridated toothpastes on enamel remineralization [21,22]. From a physico-chemical point of view it seems to be reasonable to investigate the influence of fluoride toothpastes on enamel remineralization under various pH conditions.
Methods
Forty for orthodontical reasons extracted caries free premolars were covered with varnish leaving a 5 × 5 mm window and randomly divided into 8 groups of 5 teeth in each group. They were kept in a demineralizing gel (hydroxyethylcellulose) at pH 4.95 for 50 days. After demineralization the lower half of the window was also covered with varnish serving as positive control. Each group was then incubated in amine fluoride (1400 ppm) containing toothpaste slurries and control slurries without amine fluoride at different pH levels for 48 hours which is equivalent to 2 years tooth brushing 2 times for 2 minutes per day [23]. For the slurries 40 cm3 of experimental toothpaste was mixed with 160 ml dist. water. The pH of the slurries was checked prior to the incubation of the teeth, after 24 hours and after 48 hours prior to the termination of the incubation. Incubation media are summarized in Table 1.
Table 1.
Test and control slurries with different pH levels.
| Group | pH |
| Group 1 with amine fluorid 1400 ppm | 4,1 |
| Group 2 with amine fluorid 1400 ppm | 4,5 |
| Group 3 with amine fluorid 1400 ppm | 5,1 |
| Group 4 with amine fluorid 1400 ppm | 6,9 |
| Group 5 without amine fluorid (control) | 4,3 |
| Group 6 without amine fluorid (control) | 4,7 |
| Group 7 without amine fluorid (control) | 5,3 |
| Group 8 without amine fluorid (control) | 7,0 |
After treatment with slurries the teeth were embedded in Technovit 9100 (Kulzer, Germany) and serial sections through the lesions with a thickness of 80 μm were cut using a saw microtome (Leica 1600, Germany). All sections were investigated with polarization light microscopy (PLM) and three central sections of each lesion were categorized according to their morphological appearance and to each category was a numerical index number assigned into: not present (1), single porosities (2), interrupted band (3), inhomogeneous (4), completely homogeneous (5), more than 60 μm depth (6). The numerical values were statistically compared using the nonparametric Mann-Whitney test.
Three sections of each lesion were then coated with carbon and examined with a scanning electron microscope (Philips XL 30 FEG) at 20 kV using the backscattered electron detector In each experimental and control window of the different teeth 3 spot measurements (spot size 2 nm) were carried out on the enamel surface, within the body of the lesion and sound enamel, resulting in a total number of 9 measuring points per window. Element content in weight % of Ca, P, C, and F was measured with energy dispersive X-ray analysis (EDX) with a S-UTW detector (EDAX INC, Mahwah, NJ, USA). The count rate of the EDX detector was between 1800 and 2000 counts per second with a dead time of 30%. Measuring time was 30 s (live seconds) with a resolution of 135.8 eV and an amplification time of 100 μs. Line scans through the lesions were made at 256 points with a dwell time of 1000 ms and amplification time of 100 ms. The values of the spot measurements were statistically evaluated using the nonparametric ANOVA test for repeated measurements.
Results
Lesion morphology
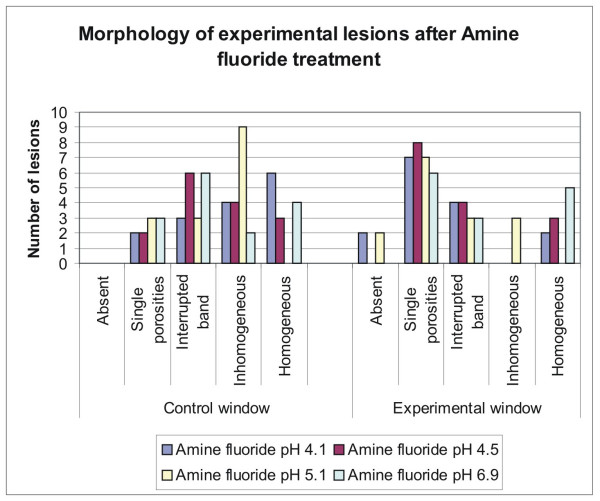
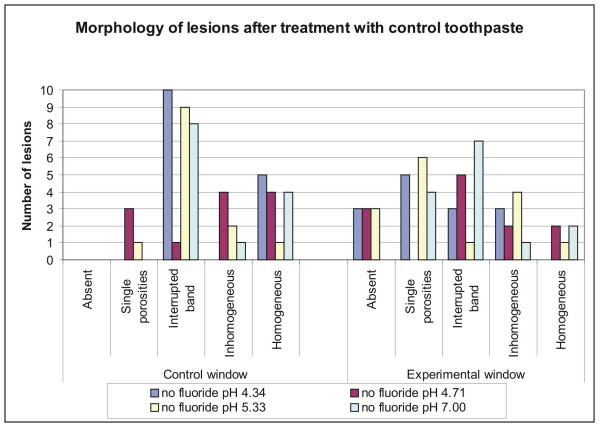
Morphological analysis of the sections with PLM revealed variable expression of the lesions after incubation at different pH values (Fig. 1). They were mainly expressed as inhomogeneous or homogeneous lesions, interrupted bands and as single porosities. In the experimental windows most of the lesions were expressed as single porosities and interrupted bands after treatment with fluoride Fig. 2), whereas with non fluoridated toothpastes the experimental lesions were mostly inhomogeneous or homogeneous Fig. 3). Significant differences in the lesion morphology between the control window and the experimental window were found in group 2 (pH 4.53; p = 0.032) and group 3 (pH 5.16; p = 0.014) after fluoride treatment. In the experimental windows a larger number of lesions with single porosities or interrupted bands was found than in the control windows. In all other groups no significant difference was found (p > 0.05).
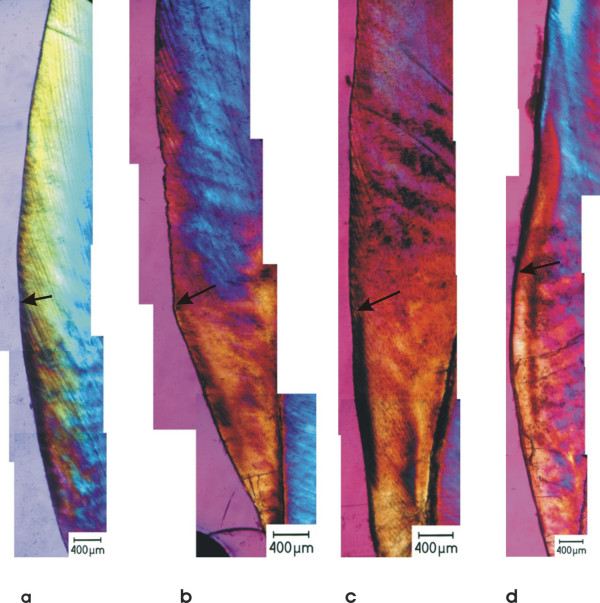
Figure 1.
Polarization light microscopy of experimental lesions. Experimental caries-like lesions after treatment with fluoridated toothpaste at different pH levels. a) pH 4.1; the upper experimental window shows an interrupted band. b) pH 4.5; the upper experimental window shows single porosities. c) pH 5.1; the upper experimental window shows an inhomogeneous lesion. d) pH 6.9; no difference can be seen between the upper experimental window and the lower control window.
Figure 2.
Quantitative distribution of the different experimental lesions. Quantitative distribution of lesions according to their morphological appearance in control and experimental windows after fluoride application at different pH levels. There is a clear shift of the lesion morphology towards the less expressed lesions in the experimental windows.
Figure 3.
Quantitative distribution of the different control lesions. Quantitative distribution of lesions according to their morphological appearance in control and experimental windows after application of control toothpaste at different pH levels. The different morphologies are more or less equally distributed.
EDX analysis
EDX element analysis showed no statistically significant differences in the element content for Ca, P, C and F in the body of the lesion and the superficial enamel layer. The mean element content in the body of the lesion of Ca was between 33 wt% and 41.9 wt%, for P the content was between 16.6 wt% and 19.9 wt%, for C it was between 6.5 wt% and 12.6 wt% and for F it was between 0.27 wt% and 0.68 wt%. All results are summarized in Table 2 for the body of the lesion and Table 3 for the superficial enamel layer.
Table 2.
Mean element content in the body of the lesion of control and experimental window.
| Experimental window | Control window | |||||||
| Group/element | Ca | P | C | F | Ca | P | C | F |
| Amine fluoride pH 4.1 | 34.1 ± 3.6 | 18.3 ± 1.3 | 9.3 ± 2.5 | 0.33 ± 0.22 | 34.2 ± 3.6 | 18.5 ± 1.1 | 8.5 ± 2.0 | 0.34 ± 0.25 |
| Amine fluoride pH 4.5 | 34.5 ± 5.5 | 19.0 ± 2.7 | 8.5 ± 5.4 | 0.47 ± 0.4 | 33.5 ± 6.0 | 18.3 ± 1.6 | 9.1 ± 2.7 | 0.42 ± 0.3 |
| Amine fluoride pH 5.1 | 35.5 ± 4.4 | 19.1 ± 1.4 | 7.4 ± 3.8 | 0.48 ± 0.39 | 35.0 ± 5.1 | 18.9 ± 1.6 | 8.0 ± 3.6 | 0.57 ± 0.45 |
| Amine fluoride pH 6.9 | 34.6 ± 5.5 | 18.6 ± 2.2 | 9.4 ± 7.4 | 0.4 ± 0.26 | 33.7 ± 6.4 | 18.5 ± 2.9 | 8.8 ± 9.6 | 0.57 ± 3.4 |
| no fluoride pH 4.3 | 34.8 ± 5.5 | 18.9 ± 2.1 | 8.0 ± 6.6 | 0.35 ± 0.28 | 35.1 ± 4.3 | 18.9 ± 1.2 | 8.2 ± 4.5 | 0.41 ± 0.33 |
| no fluoride pH 4.7 | 35.45 ± 5.8 | 18.7 ± 2.1 | 9.5 ± 4.5 | 0.3 ± 0.28 | 35.1 ± 3.2 | 18.8 ± 1.1 | 7.8 ± 3.1 | 0.35 ± 0.27 |
| no fluoride pH 5.3 | 35.0 ± 4.7 | 18.7 ± 1.6 | 9.0 ± 4.1 | 0.34 ± 0.25 | 34.9 ± 3.9 | 18.6 ± 1.2 | 9.6 ± 2.2 | 0.46 ± 0.32 |
| no fluoride pH 7.0 | 41.9 ± 19.8 | 17.4 ± 4.6 | 5.5 ± 4.8 | 0.28 ± 0.3 | 40.3 ± 20.0 | 16.6 ± 4.3 | 7.7 ± 4.0 | 0.38 ± 0.31 |
Table 3.
Mean element content in the superficial enamel layer of control and experimental window.
| Experimental window | Control window | |||||||
| Group/element | Ca | P | C | F | Ca | P | C | F |
| Amine fluoride pH 4.1 | 36.6 ± 4.5 | 18.4 ± 1.5 | 9.6 ± 2.8 | 0.42 ± 0.43 | 35.0 ± 5.5 | 19.0 ± 1.6 | 8.4 ± 2.9 | 0.3 ± 0.31 |
| Amine fluoride pH 4.5 | 37.4 ± 3.3 | 19.9 ± 1.2 | 6.5 ± 3.3 | 0.36 ± 0.28 | 34.9 ± 6.1 | 19.0 ± 1.5 | 7.6 ± 2.9 | 0.45 ± 0.34 |
| Amine fluoride pH 5.1 | 35.9 ± 4.8 | 19.1 ± 1.1 | 7.5 ± 3.5 | 0.49 ± 0.63 | 36.5 ± 5.6 | 19.4 ± 1.8 | 7.6 ± 4.2 | 0.58 ± 0.41 |
| Amine fluoride pH 6.9 | 35.3 ± 3.7 | 19.0 ± 1.7 | 9.8 ± 7.5 | 0.53 ± 0.54 | 35.7 ± 4.6 | 19.4 ± 1.2 | 6.7 ± 4.1 | 0.48 ± 0.29 |
| no fluoride pH 4.3 | 35.7 ± 4.6 | 18.9 ± 1.6 | 8.5 ± 4.9 | 0.46 ± 0.34 | 36.4 ± 4.9 | 19.5 ± 1.2 | 7.4 ± 4.1 | 0.68 ± 1.9 |
| no fluoride pH 4.7 | 35.9 ± 3.1 | 18.9 ± 1.2 | 9.0 ± 3.6 | 0.4 ± 0.24 | 35.4 ± 3.5 | 18.7 ± 1.2 | 9.0 ± 3.2 | 0.43 ± 0.35 |
| no fluoride pH 5.3 | 33.6 ± 7.0 | 17.9 ± 3.3 | 12.6 ± 10.6 | 0.45 ± 0.31 | 35.0 ± 4.4 | 18.6 ± 1.5 | 9.5 ± 4.2 | 0.34 ± 0.21 |
| no fluoride pH 7.0 | 41.7 ± 19.8 | 17.2 ± 4.5 | 8.0 ± 5.7 | 0.27 ± 0.24 | 41.5 ± 19.6 | 17.0 ± 4.5 | 7.7 ± 7.1 | 0.29 ± 0.26 |
Discussion
One of the main causes for enamel demineralization is undoubtedly the drop of pH below the critical point for hydroxyapatite dissolution [24]. The equilibrium between enamel demineralization and remineralization maintains an intact enamel surface [1]. At the critical pH for hydroxyapatite dissolution fluorapatite and calcium fluoride are supersaturated and may be deposited in lesion pores of enamel reducing further demineralization [11]. On the other hand, fluoride has been discussed being a catalyst for the transformation of different phosphate minerals rather than forming fluorapatite [7]. The results of this investigation support the latter as there were no differences in the element content between the experimental and control caries like lesions. Furthermore, no increased fluoride content could be determined which would be likely in fluorapatite formation.
The results of this investigation showed an increased remineralization at pH levels between 4.5 and 5.1 under the influence of amine fluoride because the porous volume of the body of the lesion was significantly reduced. Supersaturation of hydroxyapatite is limited to a pH range of 5.6–5.8 [4,11] with the consequence that hydroxyapatite formation at a lower pH would not be likely. However, in the presence of fluoride at a pH between 4.5 and 5.1 the released mineral ions could be reprecipitated as mixed fluor-hydroxyapatite enhancing remineralization of the body of the lesion and the enamel surface layer. The results of the quantitative EDX element analysis confirm the presence of hydroxyapatite in the body of the lesion and in the superficial enamel layer of both the control window and fluoride treated experimental window.
It could be argued that the application of slightly acidified toothpastes may result in erosion of the enamel surface. Erosion occurs at much lower pH levels where the solutions are undersaturated with respect to hydroxyapatite and also fluorapatite [25] and therefore, remineralization would not be possible with respect to thermodynamics. The presented results also showed no erosion of the enamel surface in any of the caries like lesions.
It has been shown that fluoride enhances mineral uptake during enamel remineralization, and inhibits mineral loss during demineralization [9,12,13]. Formation of Calcium fluoride plays an important role in the cariostatic effect of topical fluoride and is pH dependent. It is probably the major reaction product on dental hard tissues from short treatments with relatively concentrated fluoride agents and serves as fluoride reservoir [26]. Demineralization is significantly reduced below the saturation line of calcium fluoride and the formation of hydroyapatite is increased at lower pH values in the presence of calcium fluoride [11]. Calcium fluoride formation depends from pH and is less soluble at low pH values.
Conclusion
From the results of this study it can be concluded that the pH of the dentifrices also plays an important role in their effectiveness. Slightly acidified fluoride containing dentifrices may have a certain effect on enamel remineralization.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
WHA was the supervisor of the project and responsible for the manuscript draft.
AH carried out the PLM investigations
JH carried out the EDX measurements
ZG carried out the slurry experiments and F measurements
JB supported the experiments of ZG and contributed to the manuscript draft
PG contributed to the planning of the project, evaluation of the results and writing of the manuscript
All authors have read and approved the final version of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We would like to acknowledge the contribution of our coauthor Z. Gintner who died during the work on this investigation.
This study has been supported by GABA International, Münchenstein, Switzerland.
Contributor Information
Wolfgang H Arnold, Email: wolfa@uni-wh.de.
Anabel Haase, Email: a.haase@uni-wh.de.
Julia Hacklaender, Email: j.haclaender@uni-wh.de.
Zeno Gintner, Email: jbanoczy@axelero.hu.
Jolan Bánóczy, Email: jbanoczy@axelero.hu.
Peter Gaengler, Email: peter.gaengler@uni-wh.de.
References
- Gaengler P, Arnold WH, Steinberg D. Mikrobiologie und Biochemie der Zahnplaque. In: Gaengler, P., Hoffmann, Th., Willershausen, B., Schwenzer, N., Ehrenfeld M, editor. Koservierende Zahnheilkunde. Stuttgart, New York , Thieme; 2005. [Google Scholar]
- Aoba T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004;10:249–257. doi: 10.1111/j.1601-0825.2004.01030.x. [DOI] [PubMed] [Google Scholar]
- Featherstone JD. The caries balance: the basis for caries management by risk assessment. Oral Health Prev Dent. 2004;2 Suppl 1:259–264. [PubMed] [Google Scholar]
- Margolis HC, Zhang YP, Lee CY, Kent RL, Jr., Moreno EC. Kinetics of enamel demineralization in vitro. J Dent Res. 1999;78:1326–1335. doi: 10.1177/00220345990780070701. [DOI] [PubMed] [Google Scholar]
- Argenta RM, Tabchoury CP, Cury JA. A modified pH-cycling model to evaluate fluoride effect on enamel demineralization. Pesqui Odontol Bras. 2003;17:241–246. doi: 10.1590/S1517-74912003000300008. [DOI] [PubMed] [Google Scholar]
- Wiegand A, Krieger C, Attin R, Hellwig E, Attin T. Fluoride uptake and resistance to further demineralisation of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin Oral Investig. 2005;9:52–57. doi: 10.1007/s00784-005-0306-7. [DOI] [PubMed] [Google Scholar]
- Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3) J Clin Pediatr Dent. 2004;28:203–214. doi: 10.17796/jcpd.28.3.w0610427l746j34n. [DOI] [PubMed] [Google Scholar]
- Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries enamel structure and the caries process in the dynamic process of demineralization and remineralization (part 2) J Clin Pediatr Dent. 2004;28:119–124. doi: 10.17796/jcpd.28.2.617404w302446411. [DOI] [PubMed] [Google Scholar]
- Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;11:277–286. doi: 10.1159/000260279. [DOI] [PubMed] [Google Scholar]
- Gibbs CD, Atherton SE, Huntington E, Lynch RJ, Duckworth RM. Effect of low levels of fluoride on calcium uptake by demineralized human enamel. Arch Oral Biol. 1995;40:879–881. doi: 10.1016/0003-9969(95)00041-M. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur J Oral Sci. 1997;105:461–465. doi: 10.1111/j.1600-0722.1997.tb00231.x. [DOI] [PubMed] [Google Scholar]
- ten Cate JM, Duijsters PP. Influence of fluoride in solution on tooth demineralization. I. Chemical data. Caries Res. 1983;17:193–199. doi: 10.1159/000260667. [DOI] [PubMed] [Google Scholar]
- ten Cate JM, Duijsters PP. Influence of fluoride in solution on tooth demineralization. II. Microradiographic data. Caries Res. 1983;17:513–519. doi: 10.1159/000260711. [DOI] [PubMed] [Google Scholar]
- Seppa L, Salmenkivi S, Hausen H. Salivary fluoride concentration in adults after different fluoride procedures. Acta Odontol Scand. 1997;55:84–87. doi: 10.3109/00016359709115397. [DOI] [PubMed] [Google Scholar]
- Rosin-Grget K, Lincir I, Andrijanic A. In vitro fluoride uptake by enamel from different amine fluoride concentrations. Caries Res. 2002;36:266–269. doi: 10.1159/000063921. [DOI] [PubMed] [Google Scholar]
- Warrick JM, Miller LL, Doan EJ, Stookey GK. Caries-preventive effects of sodium and amine fluoride dentifrices. Am J Dent. 1999;12:9–13. [PubMed] [Google Scholar]
- Madléna M, Dombi C, Gintner Z, Bánóczy J. Effect of amine fluoride/stannous fluoride toothpaste and mouthrinse on dental plaque accumulation and gingival health. Oral Dis. 2004;10:294–297. doi: 10.1111/j.1601-0825.2004.01025.x. [DOI] [PubMed] [Google Scholar]
- Madléna M, Nagy G, Gabris K, Márton S, Keszthelyi G, Bánóczy J. Effect of amine fluoride toothpaste and gel in high risk groups of Hungarian adolescents: results of a longitudinal study. Caries Res. 2002;36:142–146. doi: 10.1159/000057873. [DOI] [PubMed] [Google Scholar]
- Rosin-Grget K, Lincir I, Tudja M. Effect of amine fluoride on enamel surface morphology. Coll Antropol. 2000;24:501–508. [PubMed] [Google Scholar]
- Holler BE, Friedl KH, Jung H, Hiller KA, Schmalz G. Fluoride uptake and distribution in enamel and dentin after application of different fluoride solutions. Clin Oral Investig. 2002;6:137–144. doi: 10.1007/s00784-002-0164-5. [DOI] [PubMed] [Google Scholar]
- Kardos S, Shi B, Sipos T. The in vitro demineralization potential of a sodium fluoride, calcium and phosphate ion-containing dentifrice under various experimental conditions. J Clin Dent. 1999;10:22–25. [PubMed] [Google Scholar]
- Eisenburger M, Addy M, Hughes JA, Shellis RP. Effect of time on the remineralisation of enamel by synthetic saliva after citric acid erosion. Caries Res. 2001;35:211–215. doi: 10.1159/000047458. [DOI] [PubMed] [Google Scholar]
- Tóth Z, Gintner Z, Bánóczy J, Phillips PC. The effect of fluoridated milk on human dental enamel in an in vitro demineralization model. Caries Res. 1997;31:212–215. doi: 10.1159/000262402. [DOI] [PubMed] [Google Scholar]
- Fejerskov O. Concepts of dental caries and their consequences for understanding the disease. Community Dent Oral Epidemiol. 1997;25:5–12. doi: 10.1111/j.1600-0528.1997.tb00894.x. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–329. doi: 10.1080/000163599428562. [DOI] [PubMed] [Google Scholar]
- Cruz R, Ogaard B, Rolla G. Uptake of KOH-soluble and KOH-insoluble fluoride in sound human enamel after topical application of a fluoride varnish (Duraphat) or a neutral 2% NaF solution in vitro. Scand J Dent Res. 1992;100:154–158. doi: 10.1111/j.1600-0722.1992.tb01732.x. [DOI] [PubMed] [Google Scholar]