Abstract
Regulated exocytosis in many cells is controlled by the SNARE complex, whose core includes three proteins that promote membrane fusion. Complexins I and II are highly related cytosolic proteins that bind tightly to the assembled SNARE complex and regulate neuronal exocytosis. Like somatic cells, sperm undergo regulated exocytosis; however, sperm release a single large vesicle, the acrosome, whose release has different characteristics than neuronal exocytosis. Acrosomal release is triggered upon sperm adhesion to the mammalian egg extracellular matrix (zona pellucida) to allow penetration of the egg coat. Membrane fusion occurs at multiple points within the acrosome but how fusion is activated and the formation and progression of fusion points is synchronized is unclear. We show that complexins I and II are found in acrosome-intact mature sperm, bind to SNARE complex proteins, and are not detected in sperm after acrosomal exocytosis (acrosome reaction). Although complexin I-deficient sperm acrosome-react in response to calcium ionophore, they do not acrosome-react in response to egg zona pellucida proteins and have reduced fertilizing ability. Complexin II is present in the complexin I-deficient sperm and its expression is increased in complexin I-deficient testes. Therefore, complexin I functions in exocytosis in two related but morphologically distinct secretory processes. Sperm are unusual because they express both complexins I and II but have a unique and specific requirement for complexin I.
Keywords: fertilization, zona pellucida, acrosome reaction, calcium, SNARE, secretion, oocyte, extracellular matrix, egg, membrane fusion
Introduction
Membrane fusion is a vital process for a variety of cell functions, including exocytosis. Although many cells undergo exocytosis, membrane fusion during this process has been studied most in nerve terminal synaptic vesicle release in which exocytosis is regulated by a family of proteins that form a stable tripartite complex known as the SNARE complex (Nickel et al., 1999; Sollner et al., 1993; Weber et al., 1998). The core components in the synaptic SNARE complex are the two plasma membrane proteins, syntaxin (Bennett et al., 1992) and SNAP-25 (synaptosomal-associated protein of 25 kDa) (Oyler et al., 1989), termed target SNARES (t-SNARES), and a SNARE protein on the vesicle membrane (v-SNARE), called vesicle-associated membrane protein 2 (VAMP-2) or synaptobrevin 2 (Sollner et al., 1993). Formation of the highly stable SNARE complex facilitates membrane fusion and exocytosis.
Following binding to the egg’s extracellular matrix, known as the zona pellucida, sperm undergo regulated exocytosis of the acrosome (Saling et al., 1979). Sperm are endowed with a single acrosome whose shape and size varies widely between species. The acrosome is located between the nucleus and plasma membrane and in mouse sperm envelopes 2/3 of the nucleus, extending 3-4 μm along each side of the head; it is much larger than a typical secretory vesicle (Yanagimachi, 1994). Zona pellucida binding activates signaling pathways that, in turn, increase the intracellular calcium concentration to trigger acrosomal exocytosis (Jungnickel et al., 2001). During this process, the outer acrosomal membrane fuses with the adjacent sperm plasma membrane at hundreds of points simultaneously, first forming fenestrae (Barros et al., 1967). As the fenestrae enlarge and fusion continues, hybrid vesicles form around the small amount of sperm cytosol and the membranes disintegrate, allowing the acrosomal contents, including hydrolytic enzymes, to disperse. Thus, in contrast to exocytosis at synapses, membranes are lost and there is no vesicle recycling after acrosomal exocytosis (Barros et al., 1967; Flaherty and Olson, 1991; Franklin et al., 1970; Nolan and Hammerstedt, 1997). Although the morphology of the acrosome reaction has been studied for many years and it is necessary for fertilization, a molecular understanding of this membrane fusion process remains elusive.
Despite the morphological differences, there are a number of similarities between sperm acrosomal exocytosis and synaptic vesicle exocytosis, including the requirements of increased intracellular calcium and SNARE complex formation (Jungnickel et al., 2001; Katafuchi et al., 2000; Ramalho-Santos et al., 2000; Sollner, 2003; Tomes et al., 2002). There is growing evidence that SNARE proteins are involved in the fusion of the outer acrosomal membrane and sperm plasma membrane during the acrosome reaction. Several SNARE proteins, including some syntaxins, VAMP-2, SNAP-23, and SNAP-25, are found in mammalian sperm and antibodies against some SNAREs inhibit acrosomal exocytosis (Ramalho-Santos et al., 2000; Tomes et al., 2002). Furthermore, syntaxins 1 and 2, SNAP-25 and VAMP-2 are believed to function during acrosomal exocytosis because botulinum neurotoxins BoNT/A, -E, -C, and -F, which cleave these SNARE proteins, inhibit the calcium induced acrosome reaction (Tomes et al., 2002). In addition, recombinant syntaxin 2 protein blocks acrosomal exocytosis in permeabilized mouse sperm (Hutt et al., 2005). Together, these results suggest that SNARE proteins are required for membrane fusion during acrosomal exocytosis.
The core ternary SNARE complex proteins are sufficient for membrane fusion in vitro (Weber et al., 1998). However, there are numerous proteins that bind to or associate with the SNARE complex to modulate its formation and function in vivo (Sollner, 2003). Complexins (also called synaphins) are small, highly charged proteins that form a family of four isoforms in mammals (McMahon et al., 1995; Reim et al., 2005). The more divergent complexins III and IV appear to be expressed predominantly in the retina (Reim et al., 2005), while complexins I and II are 83% identical 18-19 kDa proteins that are expressed abundantly in neurons (McMahon et al., 1995). Complexins I and II bind tightly to the VAMP-syntaxin groove of the SNARE complex in an anti-parallel fashion but do not bind to individual SNARE proteins (Chen et al., 2002; McMahon et al., 1995; Pabst et al., 2002). In mice, deletion of both complexins I and II impairs the fast synchronous calcium-triggered exocytosis but does not affect the slower calcium-triggered asynchronous exocytosis that also depends on SNAREs (Reim et al., 2001). Very recent studies suggest that complexins promote full assembly of SNARE complexes into a “super-primed” metastable state but then block completion of the fusion reaction in these complexes until a calcium signal is provided (Giraudo et al., 2006; Schaub et al., 2006; Tang et al., 2006). Formation of a “super-primed” metastable state is proposed to be necessary for synchronized exocytosis when calcium concentration is elevated (Tang et al., 2006).
Complexins I and II appear to have similar intracellular functions but, in the brain, are expressed by distinct subsets of neurons (Freeman and Morton, 2004). Therefore mice deficient in each complexin have different phenotypes (Glynn et al., 2003; Glynn et al., 2005; Glynn et al., 2007; Reim et al., 2001). Mice deficient in complexin II have normal reproductive ability (Reim et al., 2001; Takahashi et al., 1999). Mice lacking complexin I, on the other hand, are unable to reproduce, but it is unclear whether this is due only to their profound ataxia or also to an additional specific defect in the reproductive axis (Glynn et al., 2005; Reim et al., 2001).
Because acrosomal exocytosis appears to have many similarities to neuronal exocytosis, we hypothesized that complexin I would bind to the SNARE complex and regulate membrane fusion during sperm acrosomal exocytosis. To test this hypothesis, we determined if complexin I is present in mature sperm and examined acrosomal exocytosis in sperm from mice lacking complexin I. Finally, we investigated whether complexin I associates with the SNARE complex in sperm.
Materials and Methods
RT-PCR
Total RNA was prepared from frozen brain or testis using a single step guanidinium method. Total RNA was reverse-transcribed into cDNA using the RETROscript kit (Ambion, Austin, TX). Primers were designed to amplify the entire coding region of complexin I (UP: 5’-CCTGAGGAACCAAGCCATCA-3’ and DOWN 5’-CTGCTCCCAACATCCCTTGT-3’) or complexin II (UP: 5’-CCAGCCAGGAGTGCTGAAT-3’ and DOWN: 5’-CGGAGGAGGATGGGTTACTT-3’) based on published sequences (Genbank accession numbers NM_007756 and NM_009946 respectively) (Takahashi et al., 1995). One μl of reverse-transcribed cDNA or RNA was amplified in a 30 μl reaction containing 1x SUPERTaq complete buffer, 200 μM dNTPs, 30 pmoles of each primer, and 1 U of SUPERTaq Plus (Ambion, Austin, TX). PCR amplifications were performed using an initial denaturation step of 95°C for 5 min followed by 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min. A final extension step was carried out at 72°C for 10 min. The testis PCR products obtained with complexin I and II primers were sequenced by the University of Illinois Biotechnology Center.
Western Blots
Mature males were killed by CO2 asphyxiation. Cauda epididymides were placed in 2 ml of c-TYH medium (120 mM NaCl, 4.78 mM KCl, 1.71 mM CaCl2, 25.07 mM NaHCO3, 1.2 mM MgSO4, 1.2 mM KH2PO4, 5.56 mM glucose, 0.5 mM sodium pyruvate, 10 units/ml penicillin, 10 μg/ml streptomycin, 1 mg/ml polyvinylalcohol, 0.75 mM methyl-β-cyclodextrin, pH 7.3) (Choi and Toyoda, 1998; Visconti et al., 1999). Cauda epididymides were macerated with a 30-gauge needle and placed at 37°C for 5 min to release sperm. Sperm motility was assessed and was normally 50-70% for cauda epididymal sperm. Sperm were incubated at 37°C for 1 hr for capacitation and a second hr with 10 μM A23187 to induce the acrosome reaction. The sperm were washed twice in 15 ml of PBS by centrifugation at 600×g for 10 min and solubilized in RIPA buffer (150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 1% SDS, 0.01 M NaH2PO4, 2 mM EDTA, pH 7.2) with protease inhibitors (0.1 mM PMSF, 0.5 μg/ml leupeptin, and 1 μM pepstatin) at 0°C for 1 hr. The extracted samples were centrifuged at 10,000×g for 10 min and the supernatant collected.
For protein isolation from brain and testis, tissues were snap frozen in liquid nitrogen, homogenized in 1 ml of solubilization buffer (150 mM NaCl, 20 mM HEPES, 1 mM EDTA, 1% NP-40, 0.1% SDS, 10% glycerol) with protease inhibitors at 0°C for 20 min and heated at 95°C for 10 min. The samples were centrifuged at 14,000×g for 15 min and the supernatant collected.
Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in TBST (100 mM Tris pH 7.4, 0.9% NaCl, 0.1% Tween-20) containing 3% nonfat dry milk and 1% BSA. Purified complexin rabbit IgG made against a peptide corresponding to amino acid residues 41-91 of complexin II (Antibody A, Synaptic Systems, Göttingen, Germany) was used. This antibody reacts with both complexin I and II (Reim et al., 2001). The antibody was diluted 1:2000 in TBST and added to blocked membranes followed by horseradish peroxidase-labeled goat anti-rabbit secondary antibody diluted 1: 20,000 in TBST. Blots were washed, incubated for 5 min with Supersignal Pico chemiluminescent substrate (Pierce, Rockford, IL) and detected with film.
Complexin I Deficient Mice
Complexin I-deficient mice that had been generated previously by homologous recombination in embryonic stem cells were used in these experiments (Reim et al., 2001). These mice had been derived from stem cells in which the first coding exon of CPX I had been replaced by a neomycin cassette (Reim et al., 2001). Tail biopsies were collected for genotyping. DNA was extracted and used for PCR using the REDExtract-N-Amp Tissue PCR Kit (Sigma, St. Louis, MO). Separate primer sets were used for the wild type CPX I gene (UP: 5’ AGT ACT TTT GAA TCC CCT GGT GA 3’ and DOWN: 5’ TAG CTA TCC CTT CTT GTC CTT GTG 3’) and mutant CPX-I gene (UP: 5’ CGC GGC GGA GTT GTT GAC CTC G 3’ and DOWN: 5’ CTG GCT TGT CCC TGA ATC CTG TCC 3’).
Zona Pellucida Protein Purification
Zona pellucida proteins from ovarian homogenates were prepared as described (Lu and Shur, 1997). The zona pellucida proteins were collected into 10 mM phosphate buffer with 1 M NaCl, pH 7.0 and centrifuged. The zona pellucida pellet was washed in PBS, resuspended in 50 μl of PBS and dissolved at 60°C for 10 min.
Induction of the Acrosome Reaction
Sperm were capacitated at 37°C for 1 hr in dmKRBT (Larson and Miller, 1999) and the concentration was adjusted to 2 × 106 sperm/ml. Fifty μl of each sample were aliquoted into triplicate tubes and incubated in the presence of 10 μM A23187 (Sigma, St. Louis, MO), 20 ng/μl zona pellucida glycoproteins or vehicle control. Sperm were allowed to acrosome-react for 1 hr at 37°C and stained with Coomassie Blue G-250 or fluorescein isothiocyanate-labeled Pisum sativum agglutinin (FITC-PSA) to detect the acrosomes (Larson and Miller, 1999). At least 200 sperm were assessed in each experiment.
In vitro Fertilization
Mature oocytes were collected from superovulated mice. One or two oocyte-cumulus complexes were placed in a 50 μl drop of HTF medium in a culture dish (101.6 mM NaCl, 4.69 mM KCl, 0.37 mM KH2PO4, 0.2 mM MgSO4·7H2O, 21.4 mM Na lactate 60% syrup (ml/liter), 0.33 mM Na pyruvate, 2.78 mM glucose, 25.0 mM NaHCO3, 2.04 mM CaCl2, 0.4% BSA, 10 units/ml penicillin, 10 μg/ml streptomycin, 0.02% Phenol red, pH 7.3) covered by mineral oil. Sperm were collected from the cauda epididymis of wild type or complexin I deficient mice and capacitated in HTF medium at 37°C for 1 hr. Sperm at a final concentration of 1 × 104 or 105 cells/ml were co-incubated with oocytes for 42 hr at 37 °C, 5% CO2. From 100 to 250 oocytes were used for each group. After co-incubation, oocytes and zygotes were passed through a pipette to remove cumulus cells, fixed with 2% paraformaldehyde for 10 min and stained with 10 μg/ml propidium iodide in PBS for 15 min at room temperature. Oocytes and zygotes were carefully transferred into 2 μl of fluorescent mounting medium on slides (DakoCytomation, Carpinteria, CA). The slides were covered and observed under fluorescence microscopy, as described above. The presence of two pronuclei, two polar bodies or development to the two-cell stage was considered evidence of fertilization.
GST Pulldown Assay
Sperm from 50 adult mice were washed twice in 50 ml of PBS by centrifugation and solubilized in 5 ml of solubilization buffer (150 mM NaCl and 20 mM HEPES, pH 7.4) containing 2.5% (w/v) Triton X-100 with protease inhibitors at 0°C for 1 hr. The samples were centrifuged at 14,000×g for 10 min and the supernatant transferred to a clean tube.
Recombinant complexin-GST vectors were kindly provided by Dr. Thomas Sudhof at the University of Texas Southwestern Medical Center, Dallas, Texas (McMahon et al., 1995). Sperm proteins were pre-cleared by incubation with 50% slurry of glutathione agarose beads and GST protein. Pre-cleared sperm proteins were divided into three tubes and mixed with 50 μg of GST protein or 50 μg of complexin I or complexin II-GST fusion proteins. The samples were incubated at 4°C for 2 hr and washed with PBS three times. Without prior boiling, the samples were analyzed by SDS-polyacrylamide gel electrophoresis.
The 120-kDa bands bound to complexins were excised. After destaining and drying, the proteins were reduced, alkylated with iodoacetamide, and digested with trypsin (Sequencing Grade, Promega, Madison, WI). Peptides were recovered from the gel by extraction with trifluoroacetic acid and acetonitrile and were dried in a vacuum centrifuge. The tryptic peptide mixtures were redissolved in 0.1% formic acid and analyzed by capillary liquid chromatography (LC) coupled to tandem mass spectroscopy (MS/MS). The LC MS/MS analysis was performed using a Waters capillary LC system interfaced to a quadrupole time-of-flight (Q-TOF) Micro mass spectrometer (Waters Corporation, Milford, MA). The instrumentation setting and parameters were described previously (Fu et al., 2005). The raw data file was processed by ProteinLynz software (version 2.1, Micromass) and was submitted to the MASCOT search engine (www.matrixscience.com) to identify proteins.
For all the protein identifications, sequence-specific fragmentation analysis was performed for tryptic peptides using ESI QTOF MS/MS. Proteins in the gel were digested with trypsin, loaded to nanoLC coupled to QTOF MS/MS to generate molecular mass and sequence-specific fragment ion information for database searches. After searching the experimental mass values against a calculated peptide mass database, the score for each entry was calculated from the Mowse factor elements for each match between the experimental data and peptide masses calculated from the entry. Mascot incorporates a probability based Mowse algorithm to accurately model the behavior of a proteolytic enzyme. The “positive identifications” were determined based on the number of tryptic peptides and the sequence coverage for each tryptic peptide. Individual ions scores > 40 indicate identity or extensive homology (p<0.05) and P value <0.05 was used as cut off for a positive hit. The “false positive” was rejected if the P value for search hit was greater than 0.05. The details of Mowse scoring algorithm is described (Pappin et al., 1993).
As another control, we also cut out the gel band at the same position on the control lane, performed trypsin digestion, and QTOF MS/MS analysis the same way as the experimental group. Neither VAMP-2 nor SNAP-25 was identified in the control groups, ensuring that these proteins were not from contamination on the gel.
Results
We first confirmed that complexin I and II genes were expressed in mouse testis (Fig. 1A). Primers specific to complexin I or complexin II were used to amplify reverse transcribed cDNA from murine testis or brain. A 509 bp complexin I product and a 489 bp complexin II product were amplified from both brain and testis cDNA. Both products obtained from mouse testis were sequenced and were 100% identical to the published murine complexin I and II sequences (McMahon et al., 1995; Takahashi et al., 1995).
Figure 1.
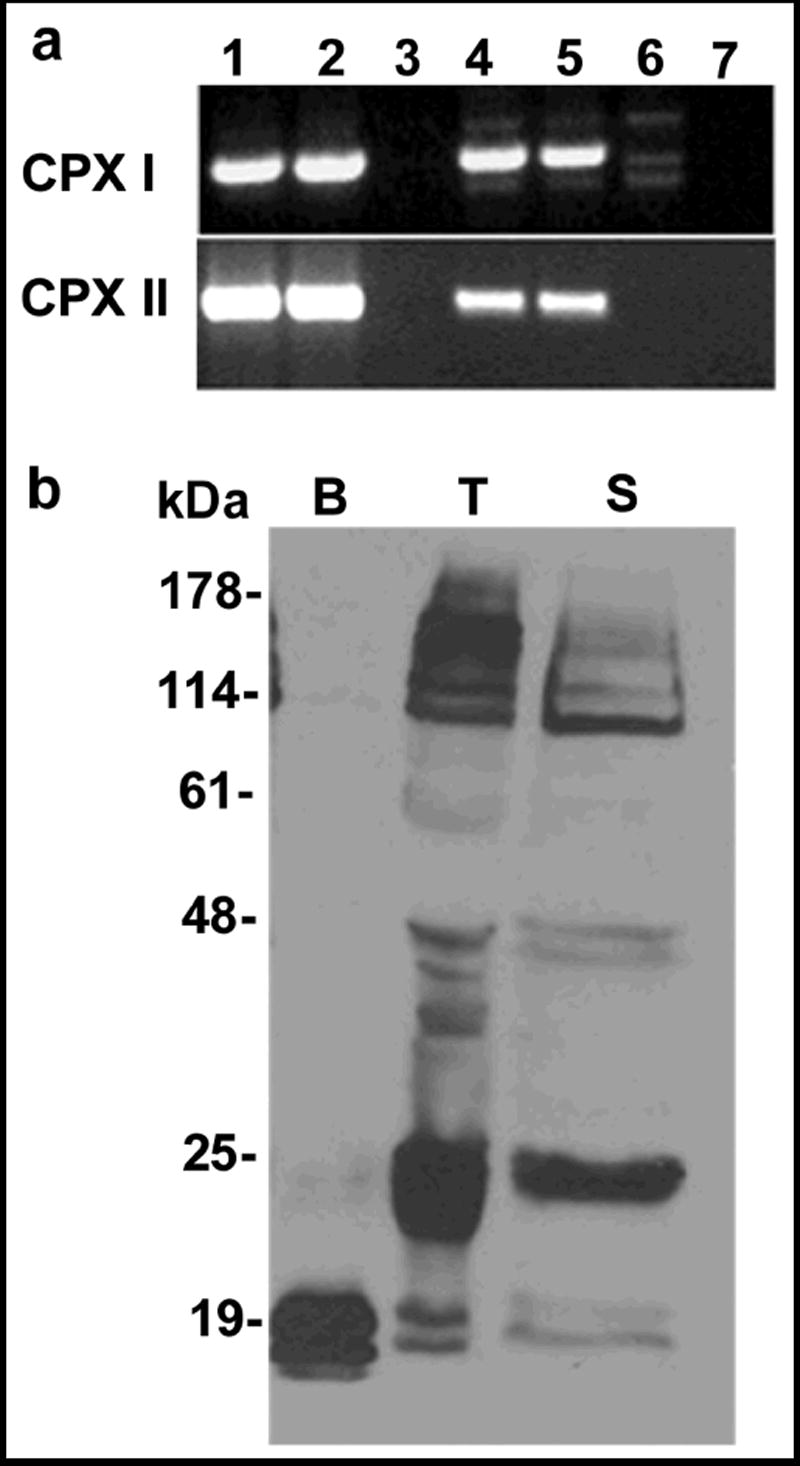
Complexins I and II are expressed in mouse testis and the proteins are found in mature acrosome-intact sperm. (A) Complexin RT-PCR. Primers were designed to amplify the entire complexin I and complexin II cDNAs (cpx 1 and 2). Lanes were loaded as follows: oligo dT-primed brain cDNA (1), random-primed brain cDNA (2), brain RNA (3), oligo dT-primed testis cDNA (4), random-primed testis cDNA (5), testis RNA (6), and water negative control (7). Complexin I primers amplified a 509 bp product from testis and brain cDNA. Complexin II primers amplified a 483 bp product from both brain and testis cDNA. Negative controls were RNA without reverse transcriptase or water (no cDNA). (B) Complexin western blot. Antibodies to complexins I and II detected the 18 kDa complexin I and 19 kDa complexin II proteins in mouse brain (B, 1 μg protein loaded), mouse testis (T, 60 μg protein loaded), and mouse cauda epididymal sperm (S, 80 μg protein loaded). Both complexins were much less abundant in extracts from testis and sperm compared to brain. The antibodies non-specifically bound to proteins larger than 25 kDa. These results are representative of more than 5 experiments.
Western blots were performed to determine if complexin I and II proteins are present in whole testis and sperm. An affinity-purified antibody (Antibody A) detected both proteins in testis and sperm (Fig. 1B). This antibody was raised against a peptide corresponding to amino acid residues 41-91 of complexin II and detects both complexins I and II in brain extracts. Although the antibody bound to several proteins larger than 25 kDa, the results indicated that complexins I and II are expressed in testis and sperm. Both complexins were considerably less abundant in testis and sperm than in brain tissue. This is in agreement with previous reports that found low levels of both complexins in testis (McMahon et al., 1995; Redecker et al., 2003).
During the acrosome reaction, the plasma membrane and outer acrosomal membrane fuse to form fenestrae that enlarge to create hybrid vesicles. These vesicles and the acrosomal contents disperse. If cytosolic complexin proteins were present in the relatively small cytosolic space (<10 nm) between the outer acrosomal membrane and the plasma membrane, they would be trapped inside the hybrid vesicles as these vesicles form and would be released during the acrosome reaction (Watson and Plummer, 1986; Yanagimachi, 1994). To provide evidence for the localization of complexins, sperm were treated with calcium ionophore to induce the acrosome reaction. Prior to and immediately following a 60-min capacitation period, 93% and 85% of sperm retained their acrosomes, respectively (Fig. 2A). However, after ionophore treatment, only 10% of sperm retained their acrosomes (Fig. 2B). Protein extracted from the same number of acrosome-intact and acrosome-reacted sperm was subjected to immunoblotting. Complexin antibodies did not detect the 18-19 kDa complexin I and II in acrosome-reacted sperm (Fig. 2C). Thus, both complexins were found in acrosome-intact sperm and absent from acrosome-reacted sperm, suggesting that complexins were localized properly for a role in acrosomal exocytosis.
Figure 2.
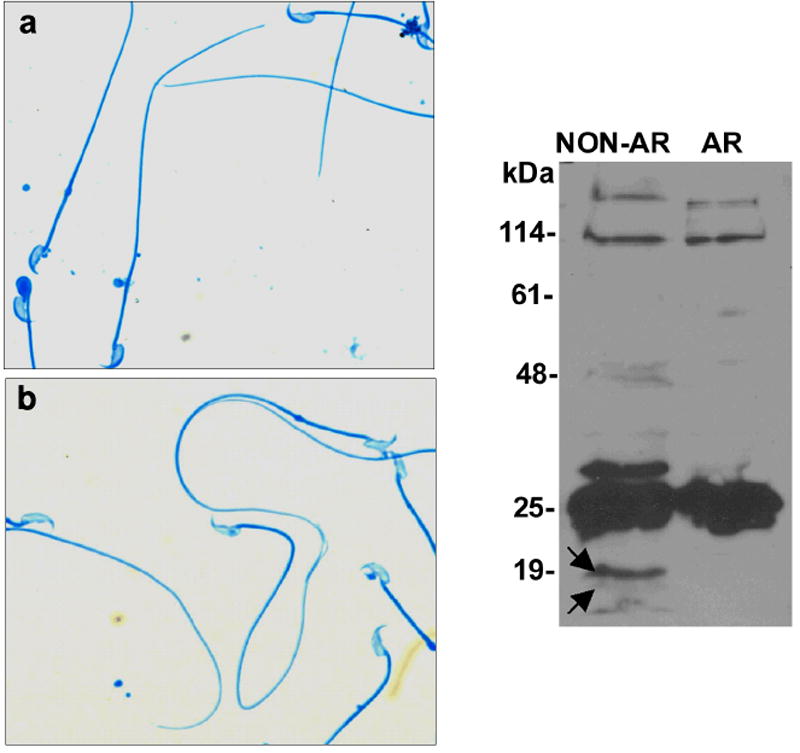
Complexins I and II are found in acrosome-intact but not acrosome-reacted sperm. Mouse sperm were incubated at 37°C for 60 min for capacitation and the acrosome reaction was induced by calcium ionophore A23187 for 60 min. Coomassie Blue staining to show acrosomal status in control (A, uncapacitated) and A23187-treated (acrosome-reacted) sperm (B). (C) Western Blots show complexins were not observed in sperm following the acrosome reaction. Proteins from the same number of control (NON-AR) and A23187-treated sperm (AR) were loaded for Western blots. Complexins were not detected in A23187-treated sperm. These results are representative of three experiments.
Previous studies generated mice deficient in complexin I by inserting a neomycin resistance cassette into the first exon of the CPX I gene (Reim et al., 2001). Mice that lack complexin I are unable to reproduce (Reim et al., 2001), whereas complexin II deficient mice are fertile (Takahashi et al., 1999; Reim et al., 2001). We have never observed vaginal plugs in female mice that have been housed with male complexin I deficient mice so it appears that the severe ataxia observed in complexin I deficient mice prevents the males from mating (Glynn et al., 2005; Reim et al., 2001). In contrast, mice that are heterozygous for the null mutation reproduce normally. Due to ataxia in the complexin I deficient mice, the fertility of their sperm could not be assessed in vivo. As an alternative, we used an in vitro approach. To determine if deletion of complexin I affects the acrosome reaction, complexin I deficient sperm were incubated with the isolated soluble zona pellucida or calcium ionophore A23187. When the acrosome reaction was physiologically induced by soluble zona pellucida proteins, 42.1±2.9% of capacitated wild type sperm acrosome reacted, compared to 25.7±2.9% for sperm lacking complexin I (Fig. 3A). There was no increase in acrosome reaction frequency of complexin I deficient sperm in response to zona pellucida proteins, demonstrating that complexin I is necessary for the zona pellucida-induced acrosome reaction.
Figure 3.
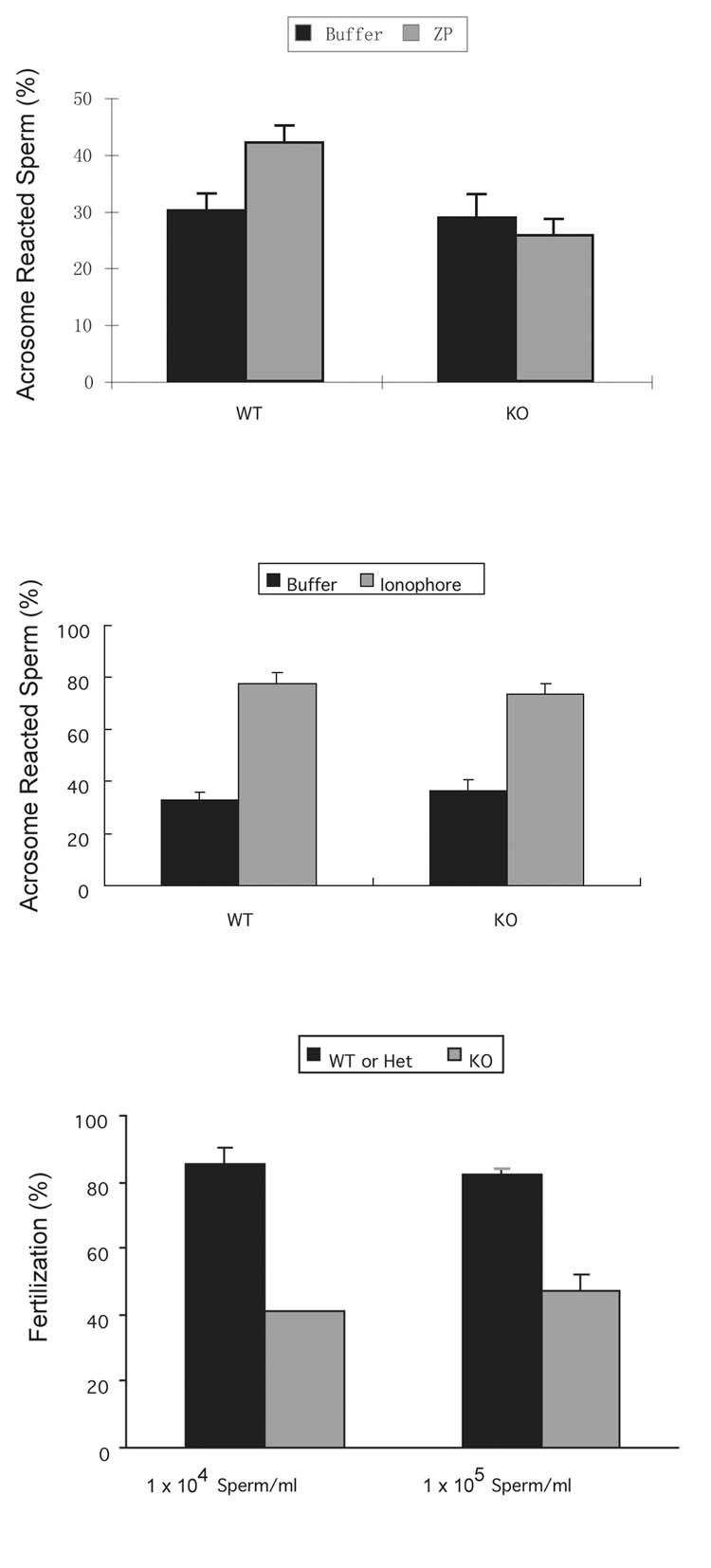
Sperm from complexin I deficient mice are unable to acrosome react in response to zona pellucida proteins and display reduced fertility in vitro. (A) Sperm from complexin I deficient mice (KO) and wild type (WT) animals of the same age were incubated with soluble mouse zona pellucida proteins to induce the acrosome reaction. Fluorescein isothiocyanate-labeled Pisum sativum agglutinin (FITC-PSA) was used to detect acrosomes. Means and s.e.m. of five experiments performed in duplicate are shown. The asterisk denotes that wild type sperm responded to zona pellucida proteins with acrosome reactions, as assessed by the Student t test (p < 0.05). (B) Sperm from complexin I deficient mice (KO) and wild type (WT) animals of the same age were incubated with 10 μM calcium ionophore A23187. After 60 min, aliquots of sperm were fixed and stained with FITC-PSA to detect acrosomes. Means and s.e.m. of three experiments performed in triplicate are shown. Asterisks indicate that sperm from both WT and KO underwent acrosome reactions induced by calcium ionophore (Student t test, P < 0.05) but the frequency was not different between sperm from WT and complexin I KO mice. (C) Mouse oocytes were co-incubated with 1 × 104/ml or 1 × 105/ml of sperm from complexin I deficient mice or wild type mice for 42 hours. After co-incubation, embryos and oocytes were fixed and stained with propidium iodide to detect nuclei. The presence of two-cell embryos or two pronuclei or polar bodies was considered as evidence of fertilization. Results are means and s.e.m. of five experiments.
To ascertain if sperm from complexin I-deficient sperm could respond to an influx of cytosolic calcium, sperm were incubated with calcium ionophore A23187. Complexin I-deficient sperm had an acrosome reaction frequency of 73.4±4.2% after incubation with A23187 for 60 min and the acrosome reaction frequency of wild type sperm was 77.8±4.1% (Fig. 3B). Thus the acrosome reaction frequency induced by calcium ionophore was not different between sperm from wild type and complexin I-deficient mice. These results suggest that complexin I functions upstream of or at the calcium influx step during acrosomal exocytosis. Complexin I-deficient sperm were capable of triggering exocytosis in response to the large calcium influx induced by A23187.
To determine the effect of a complexin I null mutation on sperm fertility, in vitro fertilization experiments were performed. Ovulated mature oocytes were incubated with two concentrations of capacitated sperm from wild type, complexin I heterozygous or complexin I deficient mice. Two sperm concentrations were used to determine if including additional sperm could compensate for any defect. The fertilization rate was not different between sperm from wild type and complexin I heterozygous mice so these data were pooled. However, the fertilization rate of sperm from complexin I deficient mice was reduced dramatically to 40.8±0.2% (sperm concentration of 1 × 104sperm/ml) and 47.3±5.0% (1 × 105sperm/ml), compared to 85.5±4.4% (1×104sperm/ml) and 82.1±2.1% (1 × 105sperm/ml) for wild type sperm (Fig. 3C). These results showed that fertilization is impaired in sperm lacking complexin I; thus, complexin I is required for normal sperm fertility.
Inactivation of a single gene can affect the abundance of other gene products in sperm. For example, deletion of certain members of the ADAM family, some of which form heterocomplexes and some do not, affects the abundance of other gene products in sperm (Nishimura et al., 2001; Nishimura et al., 2007). To determine if complexin I deficiency affected complexin II abundance, we collected protein from brain and testis and performed immunoblots. As expected, complexin Antibody A that detects both complexin I and II proteins in wild type tissues only detected complexin II in testes of complexin I deficient mice (Fig. 4). However, rather than a decrease, there was an increase in complexin II abundance in testes deficient in complexin I. In addition, the abundance of complexin I protein was reduced markedly in testes of heterozygote mice. Therefore, complexin II expression was upregulated upon loss of complexin I. The large compensatory increase in complexin II was not sufficient to maintain normal fertility; complexin II cannot replace the function of complexin I in sperm.
Figure 4.
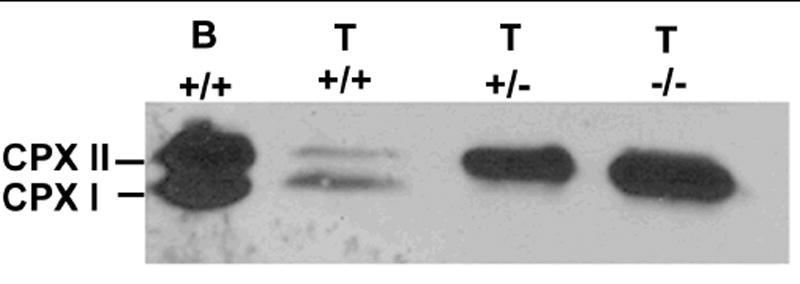
Complexin II expression is upregulated in complexin I deficient mice. Complexin antibodies detected the 18 kD complexin I and 19 kD complexin II proteins in wild type mouse brain (B, 1 μg protein), wild type mouse testes (T, +/+; 60 μg protein); but only detect 19 kD complexin II in testis tissue from complexin I heterozygote mice (T, +/-; 20 μg protein) and homozygote null mice (T, -/-; 20 μg protein). The abundance of complexin II was increased when complexin I was deficient in the testis. This result represents three experiments.
The main function of complexins I and II in neurons is to bind the SNARE complex to regulate membrane fusion during fast synchronous synaptic vesicle exocytosis (Chen et al., 2002; Pabst et al., 2000; Pabst et al., 2002; Tang et al., 2006). If complexin I plays a similar role in acrosomal exocytosis, it should bind to the SNARE complex in sperm. To test this, we performed complexin GST pulldown assays with mouse sperm proteins. The core SNARE complex is stable in SDS unless it is heated above 80°C (Fasshauer et al., 1998; Hayashi et al., 1994). Therefore, mouse sperm proteins were isolated with mild detergent and the precipitated material from the GST pulldown assays was not boiled before loading onto SDS-PAGE gels. Figure 5 shows a 120-kDa protein band from mouse sperm that bound to complexin I or II GST fusion protein but not any control pulldown reactions. This indicates that proteins in the 120-kDa band would form a complex specifically with complexin I or II. Proteins in these bands were identified by peptide mapping and on-line liquid chromatography mass spectrometry. Five peptides found in VAMP-2 were detected (LSELDDR, VLERDQK, YWWKNLK, KYWWKNLK, VNVDKVLER). Seven peptides found in SNAP-25 were detected (LKSSDAYK, QIDRIMEK, MLQLVEESK, EQMAISGGFIR, IMEKADSNKTR, ADSNKTRIDEANQR, TLVMLDEQGEQLER).
Figure 5.
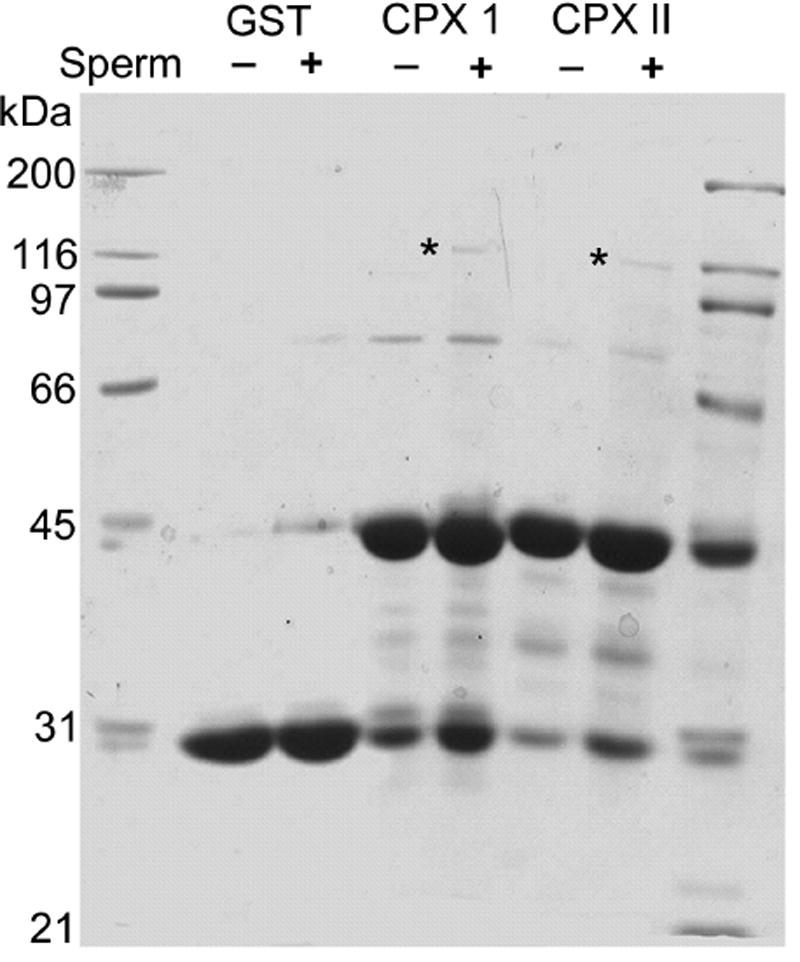
Complexins I and II bind a 120-kDa protein complex. Mouse sperm protein (3.3 mg) was incubated with purified GST protein (50 μg), or recombinant GST-complexin I (50 μg) or GST-complexin II (50 μg). Bound material was separated and bands identified by SDS-PAGE (samples were not boiled) and Coomassie Blue staining. The asterisks show the 120-kDa protein complex that interacts specifically with complexin I or II. Each band was excised and analyzed by mass spectrometry. The numbers on the left correspond to the migration of standards loaded in the outside lanes. Similar SDS-PAGE results were obtained in two experiments.
The results showed that the most abundant peptides originating from proteins bound to GST-complexin I were mouse VAMP-2 (synaptobrevin-2) and SNAP-25. These two proteins were also found in protein bound to GST-complexin II (Fig. 5). Dynamin 2 was detected in complexes precipitated with both complexin I and II (Zhao et al., 2007). Other proteins were present but not sufficiently abundant for definitive identification. These data provide the first evidence that complexins I and II bind to sperm SNARE complexes.
Discussion
Results herein show that, although the acrosome reaction is a morphologically distinct event, at the molecular level membrane fusion during acrosomal exocytosis shares some similarities with synaptic vesicle exocytosis. Some SNARE proteins have been found in the acrosomal region of sperm and SNARE complex formation appears to be necessary for the acrosome reaction (Katafuchi et al., 2000; Ramalho-Santos et al., 2000; Tomes et al., 2002). Moreover, some SNARE regulatory proteins, including α-SNAP, NSF, rab3a and synaptotagmins also are detected around the acrosomal area and may regulate acrosomal exocytosis (Michaut et al., 2000; Tomes et al., 2005; Yunes et al., 2000). Our data provide indirect support for a role of the SNARE complex in acrosomal exocytosis and demonstrate directly that complexin I is required for membrane fusion during the sperm acrosome reaction. Complexins have recently been proposed to bind SNARE complexes to allow them to move to a “super-primed” state and simultaneously hold SNARE complexes in a release-competent state until calcium concentrations are increased (Tang et al., 2006). This implies a sequential positive role (for stability) and then negative role, preventing premature fusion. In sperm, perhaps complexin I allows SNARE complexes at each acrosomal membrane fusion site to form the super-primed state properly, a positive role. Our results are consistent with this putative function. If complexins are required for formation of the super-primed complex, complexin I-deficient sperm would not develop the super-primed complexes and so we could not assess a blocking effect of complexins (a negative role in the second step) prior to a calcium signal. But it is apparent that complexin I-deficient sperm do not prematurely acrosome-react, as would be predicted if the only role of complexin I was a negative role, preventing premature fusion (Figs. 2 and 3). Because it is complexin-dependent, the acrosome reaction resembles the fast synchronous release in neurons rather than the slower asynchronous calcium-induced release (Tang et al., 2006) but this hypothesis requires further investigation. For example, the fast synchronous release in neurons requires synaptotagmin I as a calcium sensor but whether synaptotagmin I functions in sperm is unknown. Synaptotagmin I expression has not been detected in the testis (Hutt et al., 2002).
Mouse sperm lacking complexin I did not undergo acrosomal exocytosis by the physiological inducer (zona pellucida) but underwent the reaction in response to calcium ionophore, which triggers a single massive calcium influx in the presence of 2 mM extracellular calcium. Fertility was significantly reduced in complexin I deficient sperm as assessed by in vitro fertilization (Fig. 3C), despite the increase in complexin II abundance (Fig. 4). Because fertility was reduced when in vitro fertilization was used, a technique not requiring sperm transport, defective sperm transport in the female reproductive tract was not the cause. These results indicate that complexin I is required for the physiological acrosome reaction. The observation that calcium ionophore induces acrosomal exocytosis in complexin I deficient sperm suggests possible roles for complexin I. One is that complexin I functions upstream of the increase in cytosolic calcium, and the high intracellular calcium induced by ionophore can bypass the absence of complexin I and induce exocytosis. This is consistent with the proposed role of complexins to promote formation of a super-primed complex awaiting a calcium signal for secretion (Tang et al., 2006). An abnormally large increase in cytosolic calcium induced by A23187 could bypass the formation of this complex, as maybe be true for neurotransmitter release. A second possibility is that the extremely large increase in cytosolic calcium generated by the ionophore may activate a completely different, nonphysiological pathway to trigger the acrosome reaction. Curiously, although complexin I is apparently required for acrosomal exocytosis, in neurons either complexin I or II can function at this step.
We found that complexins I and II can associate with sperm VAMP-2 and SNAP-25 in a 120-kDa protein complex (Fig. 5). Although we did not detect syntaxin in the 120-kDa protein complex, it is likely that syntaxins were present in these complexes because complexins I and II bind with highest affinity to the pre-assembled SNARE complex in neurons (Hu et al., 2002; Pabst et al., 2000). Perhaps several syntaxin isoforms were present, making detection of each more difficult.
This is the first report of a specific and required function for complexin I in a cell that expresses complexin II. Acrosome-intact sperm contain complexins I and II in equal abundance and both proteins were absent from acrosome-reacted sperm, suggesting they were located in the same sperm compartment. However, sperm from complexin I deficient mice, despite the elevated level of complexin II (Fig. 4) are defective in acrosomal exocytosis. In contrast, neurons lacking complexin I undergo normal secretion unless complexin II is also absent (Reim et al., 2001). This unique requirement for complexin I is unprecedented and quite surprising because even the more sequence-divergent complexins III and IV can replace the function of complexins I and II in neurons (Reim et al., 2005). The absolute requirement for complexin I in sperm may be explained if complexin II is incapable of binding the appropriate sperm SNARE complex; however, our initial experiment did not detect a difference in the SNAREs bound by complexin I/II (Fig. 5). There may be other proteins in this complex that we did not detect by mass spectrometry that may be unique to complexin I; although the detected protein complexes precipitated by complexins I and II are the same size, the composition of the complexes may be different. Further study of this interesting finding is required to understand the unique requirement for complexin I in acrosomal exocytosis.
In conclusion, our results demonstrate that complexin I is in the acrosomal region of sperm and is necessary for acrosomal exocytosis and normal fertility. The requirement for complexins in both synaptic vesicle and sperm acrosomal exocytosis indicates a key role for these molecules in regulated exocytosis in very different and specialized cell types. Although it would appear that sperm and neurons have little in common, it is becoming increasingly clear that these very distinct cells share surprising similarities in gene expression patterns and functions (Meizel, 2004). Complexin I has a unique function in acrosomal exocytosis because complexin II cannot compensate for the absence of complexin I during this process, despite being much more abundant in sperm deficient in complexin I. This is the first example of a differential role of and II in cells that express both genes.
Acknowledgments
We thank Dr. Thomas C. Südhof at University of Texas Southwestern Medical Center for the recombinant complexin-GST vectors. Financial support was provided by the National Institutes of Health (HD38311 to DM; T32 HD07028 to HB), the David H. and Norraine A. Baker Graduate Fellowship (to HB and LZ) and start-up funds from the School of Pharmacy and Wisconsin Alumni Research Foundation at the University of Wisconsin-Madison (LL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barros C, Bedford JM, Franklin LE, Austin CR. Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol. 1967;34:C1–5. doi: 10.1083/jcb.34.3.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Choi YH, Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod. 1998;59:1328–33. doi: 10.1095/biolreprod59.6.1328. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Eliason WK, Brunger AT, Jahn R. Identification of a minimal core of the synaptic SNARE complex sufficient for reversible assembly and disassembly. Biochemistry. 1998;37:10354–62. doi: 10.1021/bi980542h. [DOI] [PubMed] [Google Scholar]
- Flaherty SP, Olson GE. Ultrastructural analysis of the acrosome reaction in a population of single guinea pig sperm. Anat Rec. 1991;229:186–94. doi: 10.1002/ar.1092290205. [DOI] [PubMed] [Google Scholar]
- Franklin LE, Barros C, Fussell EN. The acrosomal region and the acrosome reaction in sperm of the golden hamster. Biol Reprod. 1970;3:180–200. doi: 10.1093/biolreprod/3.2.180. [DOI] [PubMed] [Google Scholar]
- Freeman W, Morton AJ. Differential messenger RNA expression of complexins in mouse brain. Brain Res Bull. 2004;63:33–44. doi: 10.1016/j.brainresbull.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Fu Q, Goy MF, Li L. Identification of neuropeptides from the decapod crustacean sinus glands using nanoscale liquid chromatography tandem mass spectrometry. Biochem Biophys Res Commun. 2005;337:765–78. doi: 10.1016/j.bbrc.2005.09.111. [DOI] [PubMed] [Google Scholar]
- Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–80. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Glynn D, Bortnick RA, Morton AJ. Complexin II is essential for normal neurological function in mice. Hum Mol Genet. 2003;12:2431–48. doi: 10.1093/hmg/ddg249. [DOI] [PubMed] [Google Scholar]
- Glynn D, Drew CJ, Reim K, Brose N, Morton AJ. Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Hum Mol Genet. 2005;14:2369–85. doi: 10.1093/hmg/ddi239. [DOI] [PubMed] [Google Scholar]
- Glynn D, Sizemore RJ, Morton AJ. Early motor development is abnormal in complexin 1 knockout mice. Neurobiol Dis. 2007;25:483–95. doi: 10.1016/j.nbd.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Sudhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. Embo J. 1994;13:5051–61. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Carroll J, Rickman C, Davletov B. Action of complexin on SNARE complex. J Biol Chem. 2002;277:41652–6. doi: 10.1074/jbc.M205044200. [DOI] [PubMed] [Google Scholar]
- Hutt DM, Baltz JM, Ngsee JK. Synaptotagmin VI and VIII and syntaxin 2 are essential for the mouse sperm acrosome reaction. J Biol Chem. 2005;280:20197–203. doi: 10.1074/jbc.M412920200. [DOI] [PubMed] [Google Scholar]
- Hutt DM, Cardullo RA, Baltz JM, Ngsee JK. Synaptotagmin VIII is localized to the mouse sperm head and may function in acrosomal exocytosis. Biol Reprod. 2002;66:50–6. doi: 10.1095/biolreprod66.1.50. [DOI] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Katafuchi K, Mori T, Toshimori K, Iida H. Localization of a syntaxin isoform, syntaxin 2, to the acrosomal region of rodent spermatozoa. Mol Reprod Dev. 2000;57:375–83. doi: 10.1002/1098-2795(200012)57:4<375::AID-MRD10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Larson JL, Miller DJ. Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Dev. 1999;52:445–9. doi: 10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lu Q, Shur BD. Sperm from β1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reaction and penetrate the zona pellucida poorly. Development. 1997;124:4121–4131. doi: 10.1242/dev.124.20.4121. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–9. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Meizel S. The sperm, a neuron with a tail: ‘neuronal’ receptors in mammalian sperm. Biol Rev Camb Philos Soc. 2004;79:713–32. doi: 10.1017/s1464793103006407. [DOI] [PubMed] [Google Scholar]
- Michaut M, Tomes CN, De Blas G, Yunes R, Mayorga LS. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc Natl Acad Sci U S A. 2000;97:9996–10001. doi: 10.1073/pnas.180206197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Weber T, McNew JA, Parlati F, Sollner TH, Rothman JE. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci U S A. 1999;96:12571–6. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev Biol. 2001;233:204–13. doi: 10.1006/dbio.2001.0166. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Myles DG, Primakoff P. Identification of an ADAM2/ADAM3 complex on the surface of mouse testicular germ cells and cauda epididymal sperm. J Biol Chem. 2007;282:17900–7. doi: 10.1074/jbc.M702268200. [DOI] [PubMed] [Google Scholar]
- Nolan JP, Hammerstedt RH. Regulation of membrane stability and the acrosome reaction in mammalian sperm. Faseb J. 1997;11:670–82. doi: 10.1096/fasebj.11.8.9240968. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–52. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst S, Hazzard JW, Antonin W, Sudhof TC, Jahn R, Rizo J, Fasshauer D. Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J Biol Chem. 2000;275:19808–18. doi: 10.1074/jbc.M002571200. [DOI] [PubMed] [Google Scholar]
- Pabst S, Margittai M, Vainius D, Langen R, Jahn R, Fasshauer D. Rapid and selective binding to the synaptic SNARE complex suggests a modulatory role of complexins in neuroexocytosis. J Biol Chem. 2002;277:7838–48. doi: 10.1074/jbc.M109507200. [DOI] [PubMed] [Google Scholar]
- Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–32. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J, Moreno RD, Sutovsky P, Chan AW, Hewitson L, Wessel GM, Simerly CR, Schatten G. SNAREs in mammalian sperm: possible implications for fertilization. Dev Biol. 2000;223:54–69. doi: 10.1006/dbio.2000.9745. [DOI] [PubMed] [Google Scholar]
- Redecker P, Kreutz MR, Bockmann J, Gundelfinger ED, Boeckers TM. Brain synaptic junctional proteins at the acrosome of rat testicular germ cells. J Histochem Cytochem. 2003;51:809–19. doi: 10.1177/002215540305100612. [DOI] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstatter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 2005;169:669–80. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling PM, Sowinski J, Storey BT. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: sequential relationship to the acrosome reaction. J Exp Zool. 1979;209:229–238. doi: 10.1002/jez.1402090205. [DOI] [PubMed] [Google Scholar]
- Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol. 2006;13:748–50. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–24. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sollner TH. Regulated exocytosis and SNARE function (Review) Mol Membr Biol. 2003;20:209–20. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ujihara H, Huang GZ, Yagyu KI, Sanbo M, Kaba H, Yagi T. Reduced hippocampal LTP in mice lacking a presynaptic protein: complexin II. Eur J Neurosci. 1999;11:2359–66. doi: 10.1046/j.1460-9568.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T, Miyata T, Kaba H, Higuchi T, Okutani F, Fujimoto S. Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Letters. 1995;368:455–460. doi: 10.1016/0014-5793(95)00713-j. [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–87. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Tomes CN, De Blas GA, Michaut MA, Farre EV, Cherhitin O, Visconti PE, Mayorga LS. α-SNAP and NSF are required in a priming step during the human sperm acrosome reaction. Mol Hum Reprod. 2005;11:43–51. doi: 10.1093/molehr/gah126. [DOI] [PubMed] [Google Scholar]
- Tomes CN, Michaut M, De Blas G, Visconti P, Matti U, Mayorga LS. SNARE complex assembly is required for human sperm acrosome reaction. Dev Biol. 2002;243:326–38. doi: 10.1006/dbio.2002.0567. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. β-Cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Watson PF, Plummer JM. Relationship between calcium binding sites and membrane fusion during the acrosome reaction induced by ionophore in ram spermatozoa. J Exp Zool. 1986;238:113–8. doi: 10.1002/jez.1402380114. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–72. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. Physiology of Reproduction. 2. Raven Press; New York: 1994. pp. A189–A317. [Google Scholar]
- Yunes R, Michaut M, Tomes C, Mayorga LS. Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol Reprod. 2000;62:1084–9. doi: 10.1095/biolreprod62.4.1084. [DOI] [PubMed] [Google Scholar]
- Zhao L, Shi X, Li L, Miller DJ. Dynamin 2 associates with complexins and is found in the acrosomal region of mammalian sperm. Mol Reprod Dev. 2007;74:750–7. doi: 10.1002/mrd.20660. [DOI] [PubMed] [Google Scholar]


