Abstract
The vector-borne, protistan parasite Trypanosoma brucei is the only known eukaryote with a multifunctional RNA polymerase I that, in addition to ribosomal genes, transcribes genes encoding the parasite's major cell-surface proteins—the variant surface glycoprotein (VSG) and procyclin. In the mammalian bloodstream, antigenic variation of the VSG coat is the parasite's means to evade the immune response, while procyclin is necessary for effective establishment of trypanosome infection in the fly. Moreover, the exceptionally high efficiency of mono-allelic VSG expression is essential to bloodstream trypanosomes since its silencing caused rapid cell-cycle arrest in vitro and clearance of parasites from infected mice. Here we describe a novel protein complex that recognizes class I promoters and is indispensable for class I transcription; it consists of a dynein light chain and six polypeptides that are conserved only among trypanosomatid parasites. In accordance with an essential transcriptional function of the complex, silencing the expression of a key subunit was lethal to bloodstream trypanosomes and specifically affected the abundance of rRNA and VSG mRNA. The complex was dubbed class I transcription factor A.
Keywords: class I transcription factor, DYNLL1, procyclin, Tryponosoma brucei, VSG
Introduction
The protistan parasite Trypanosoma brucei is transmitted by Glossina spp. and lives freely in the bloodstream and extracellular tissue spaces of its mammalian host, causing the lethal disease known as African Sleeping Sickness in humans. Key factors for successful T. brucei infection of mammalian and insect hosts are the parasite's variant surface glycoprotein (VSG) and procyclin, respectively. In the mammalian bloodstream, antigenic variation of the dense VSG coat is the parasite's means to evade the immune system (reviewed by Pays et al, 2007), whereas procyclins are important to establish an infection in the fly midgut (Ruepp et al, 1997).
VSG and procyclin genes are expressed with an extremely high efficiency in bloodstream and procyclic stages, respectively. The cell coat of a bloodstream trypanosome consists of 107 identical VSG molecules, which are derived from a single gene drawn from a large VSG repertoire. Accordingly, in vivo RNA labeling experiments indicated that VSG mRNA synthesis is approximately 100 times higher than that of a single β-tubulin gene (Ehlers et al, 1987). This level of VSG expression is essential for parasite growth and persistence of infection: RNAi-mediated VSG silencing caused rapid cell-cycle arrest in vitro and efficient clearance of trypanosomes from the bloodstream of infected mice (Sheader et al, 2005). The requirement for this extraordinarily high expression is probably the reason why T. brucei has evolved the unique capability of utilizing the high efficiency of RNA polymerase (pol) I-mediated transcription for VSG and procyclin genes (Kooter and Borst, 1984; Rudenko et al, 1989; Clayton et al, 1990; Günzl et al, 2003). In other eukaryotes, RNA pol I exclusively transcribes ribosomal RNA genes (RRNA), as is also the case in trypanosomes, whereas mRNA is invariably synthesized by RNA pol II. This clear division of function is most likely due to the specific association of the mRNA capping enzyme with RNA pol II and the co-transcriptional mode of mRNA capping (McCracken et al, 1997; Yue et al, 1997). In trypanosomes, however, protein-coding genes are arranged in long tandem arrays that are transcribed in a polycistronic manner. Individual mRNAs are resolved from their precursors by trans splicing a 39-nt 5′ leader sequence, derived from a capped RNA pol II-synthesized spliced leader donor RNA (SL RNA), and by polyadenylation. This post-transcriptional capping process uncouples capping from mRNA synthesis and enables T. brucei to efficiently express protein-coding genes by RNA pol I (Rudenko et al, 1991; Zomerdijk et al, 1991) or even by bacteriophage RNA polymerases (Wirtz et al, 1994).
The active VSG gene is always located in a telomeric expression site (ES), whereas there are two chromosome-internal loci of procyclin genes, termed GPEET and EP1. While the VSG promoter is very short with two distinct sequence boxes upstream of the transcription initiation site (TIS; Vanhamme et al, 1995; Pham et al, 1996), the RRNA, GPEET, and EP1 promoters are larger and consist of four distinct domains (Sherman et al, 1991; Brown et al, 1992; Janz and Clayton, 1994; Laufer and Günzl, 2001). Although there are no obvious sequence homologies between these promoters, an in vitro transcription competition study indicated that they bind a common trans-activating factor (Laufer and Günzl, 2001). In addition to the structural variation among T. brucei class I promoters, the nuclear distribution of RNA pol I makes class I transcription in this parasite even more complex. While in other eukaryotes, RNA pol I transcribes RRNA genes exclusively in the nucleolus, in bloodstream T. brucei RNA pol I is sequestered in the nucleolus and in a novel DNase I-resistant compartment dubbed the ES body (Navarro and Gull, 2001). It was hypothesized that this compartment accommodates only a single ES, ensuring mono-allelic VSG expression.
Despite the importance and multifaceted nature of class I transcription in T. brucei, a class I transcription factor has not been identified and our knowledge of the transcription machinery has been restricted to conserved subunits of RNA pol I (Walgraffe et al, 2005; Nguyen et al, 2006). Only very recently, a novel RNA pol I subunit with an essential transcriptional function was characterized (Nguyen et al, 2007). Our knowledge about class I transcription factors stems from research on mammals and the budding yeast Saccharomyces cerevisiae (recently reviewed in Grummt, 2003; Russell and Zomerdijk, 2005). In mammals, the basal RRNA promoter-binding factor is termed selectivity factor 1 (SL-1) in humans and TIF-IB in the mouse. It consists of the TATA-binding protein (TBP) and four TBP-associated proteins (TAFs). The interaction between RNA pol I and SL1 is mediated by a single polypeptide-dubbed RRN3 in humans or TIF-IA in the mouse, and activated RRNA transcription requires a dimer of the 94 kDa-large upstream binding factor UBF. In yeast, RRN3 is conserved and TBP stimulates RNA pol I transcription in the absence of TAFs, whereas the three subunits of the core factor, which is the functional equivalent of SL1, and the six subunits of the upstream activation factor, share no sequence similarity with the mammalian proteins.
Here, we have purified and characterized a protein that recognizes the VSG ES promoter with sequence specificity. We show by independent criteria that this protein is indispensable for class I transcription in vitro and for VSG and RRNA expression in vivo. Tandem affinity purification of the protein revealed a complex of seven subunits that contained five additional parasite-specific polypeptides and, surprisingly, a dynein light chain. We demonstrated that the protein complex is the functional entity that binds to the VSG ES promoter and conclude that we have characterized a novel multi-subunit transcription factor which we named class I transcription factor A (CITFA).
Results
Identification of two VSG ES promoter-binding proteins
In a previous study, an electrophoretic mobility shift assay (EMSA) was used to convincingly demonstrate specific protein binding to the VSG ES promoter in crude trypanosome extracts (Pham et al, 1997). These extracts were prepared from procyclic trypanosomes because, in contrast to bloodstream forms, procyclics can easily be grown in large numbers in vitro and because the VSG ES promoter can direct accurate transcription in procyclic cells (Lee and Van der Ploeg, 1997, and references therein) and in procyclic extracts (Laufer et al, 1999). To identify factors that specifically interact with the VSG ES promoter, we grew 30 l of procyclic forms, prepared a crude extract and, by testing individual fractions with EMSA, purified the VSG ES promoter-binding activity by a combination of ion exchange, heparin affinity, and DNA affinity chromatography (data not shown). Mass spectrometric analysis of the promoter-binding activity revealed 17 proteins with calculated masses ranging from 41.3 to 181.5 kDa (Figure 1A). While some of these proteins probably co-purified due to nonspecific DNA–protein interactions, a protein with an apparent size of ∼50 kDa could be specifically UV-crosslinked to VSG ES promoter DNA (Figure 1B). For further analysis, we therefore chose four proteins (Figure 1A arrowheads) with a predicted size in the 50 kDa range that had been annotated as ‘conserved hypothetical proteins' in the T. brucei genome database (http://www.genedb.org/genedb/tryp/index.jsp). The four proteins were C-terminally tagged in individual cell lines either with the HA epitope or the 19-kDa PTP tag (Schimanski et al, 2005b), which is suitable for tandem affinity purification. The DNA-binding activity of these proteins was tested by promoter pull-down and immunoblot experiments, using a VSG ES promoter containing the wild-type sequence or a few point mutations in either or both of the two essential sequence elements (Figure 1C), and a nonspecific control DNA. In addition, DNAs comprising the complete SLRNA, RRNA and GPEET promoters were co-analyzed. The proteins encoded by Tb927.2.3800 and Tb927.4.1310 bound to DNA in a nonspecific manner (data not shown), whereas the wild-type VSG ES promoter bound the Tb09.211.3440 protein very efficiently, and the nonspecific control and the class II SLRNA promoter DNA did not (Figure 1D: compare lane 3 with lanes 2 and 7). Binding of this protein to the VSG ES promoter required the integrity of both promoter elements, because mutation of either element strongly diminished the binding activity, and mutation of both elements abolished it completely (lanes 4–6). Albeit to a lesser extent, Tb09.211.3440 reproducibly bound to the other two class I promoters, which raised the possibility that this protein is a general class I transcription factor in T. brucei (compare lane 3 with lanes 8 and 9). The pull-down results with protein Tb11.47.0008 were similar but the binding specificity was not as clear (data not shown). We therefore continued our analysis with Tb09.211.3440, which from now on we refer to as CITFA-2 (as will become apparent below, CITFA-2 is the second largest subunit of the complex).
Figure 1.
Identification of VSG ES promoter-binding proteins. (A) List of identified proteins with their GeneDB gene accession numbers, calculated molecular weights, and current GeneDB annotations. Arrowheads indicate proteins that were further analyzed. (B) Autoradiograph of proteins from S-Sepharose or Resource Q (Res Q) fractions that were UV crosslinked to radio-labeled VSG ES promoter, DNAse-digested, and separated by SDS–PAGE. –pa, no polyamines added. (C) Sequence of the VSG ES 118 promoter from position −67 to +1 relative to the TIS. The important residues of boxes 1 and 2 are underlined and the point mutations are indicated below the wild-type sequence. (D) Immunoblot of PTP-CITFA-2 subsequent to promoter pull-downs carried out with a nonspecific DNA, with VSG ES promoter DNAs as indicated above, and with wild-type SLRNA, RRNA, and GPEET promoter DNAs. The numbers indicate the end positions of the DNA fragments relative to the TIS.
Silencing of CITFA-2 expression is lethal
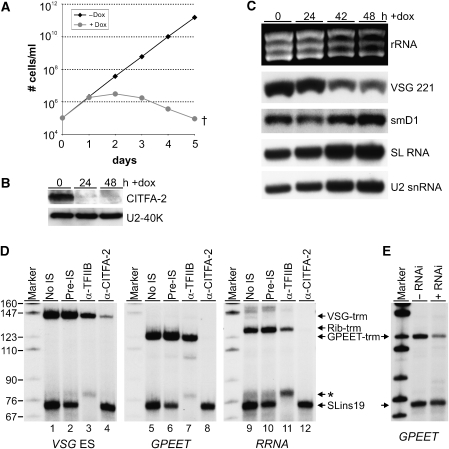
We silenced the expression of CITFA-2 in procyclic and bloodstream T. brucei by using an inducible RNA interference system that is based on stable transfection of cells expressing both the tetracycline repressor and T7 RNA polymerase (Wirtz et al, 1999). We first cloned 530 bp of the CITFA-2-coding region into the inducible construct pZJM, which harbors T7 but no class I promoters (Wang et al, 2000). While this strategy led to inducible procyclic cell lines, it failed to generate corresponding bloodstream forms. We therefore modified the available and more tightly regulated stem-loop vector for dsRNA synthesis (Shi et al, 2000) by replacing the inducible class I promoter with an inducible T7 promoter and by inserting two T7 terminators downstream of the dsRNA cassette before cloning the CITFA-2 sequence in opposite orientations into this T7-stl vector (Supplementary Figure S1). While T. brucei growth is not affected by the doxycycline concentrations used (Luu et al (2006) and data not shown), procyclic and bloodstream cell lines that were stably transfected with these plasmids rapidly ceased growth when CITFA-2 dsRNA expression was induced (Figure 2A and data not shown). Typically, cell growth was inhibited after 24 h and cell cultures died out after day 5, indicating that CITFA-2 is essential for both life-cycle stages. Semiquantitative RT–PCR revealed that CITFA-2 mRNA abundance was specifically reduced in induced cells within the first 24 h (data not shown). To evaluate the CITFA-2 protein levels in these cells, we raised a rat polyclonal antiserum against the N-terminal half of CITFA-2, which recognizes the endogenous protein with high specificity (Supplementary Figure S2). On immunoblots, CITFA-2 levels were seen to be strongly reduced, 24 and 48 h after RNAi induction, which confirmed effective silencing of CITFA-2 expression in these cells (Figure 2B). Analysis of total RNA prepared from noninduced and induced bloodstream cells showed that the abundance of RNA pol I-synthesized 18S, 28Sα and 28Sβ rRNAs and of VSG 221 mRNA was clearly reduced 42 and 48 h after induction (Figure 2C). Concomitant with the decrease of these major RNAs, the RNA pol II transcripts smD1 mRNA and SL RNA and the RNA pol III transcript U2 snRNA increased in these samples of equal amounts. When standardized against smD1 signals, rRNA/VSG mRNA decreased to 56/44% and 30/29% 42 and 48 h after induction, respectively. The latter numbers are in close accordance with the 73% reduction of rRNA found upon silencing an essential RNA pol I subunit in procyclic trypanosomes (Nguyen et al, 2007). We therefore concluded that silencing of CITFA-2 specifically affected RNA pol I transcripts. Together with our finding that CITFA-2 specifically bound to class I promoters, these results suggested that this protein is involved in class I transcription.
Figure 2.
CITFA-2 is an essential class I transcription factor. (A) Growth curve of a bloodstream-form RNAi cell line in the presence (circles, gray) or absence (diamonds, black) of doxycycline, which induces the expression of CITFA-2 dsRNA. (B) Immunoblot of whole-cell lysates prepared from bloodstream RNAi cells before and 24 and 48 h after induction of CITFA-2 dsRNA synthesis. Detection of the nuclear protein U2-40K served as a loading control. (C) Analysis of total RNA prepared from noninduced cells and from cells 24, 42, and 48 h after induction. The rRNAs were visualized by ethidium bromide staining, VSG 221 and smD1 mRNAs by hybridization with appropriate probes, and the small SL and U2 RNAs by a primer extension assay. (D) In vitro transcription of the class I templates VSG-trm, GPEET-trm, and Rib-trm and the class II template SLins19 in the absence of rat serum (no IS) or in the presence of anti-CITFA-2 pre-immune serum (pre-IS), TFIIB antiserum (α-TFIIB), or CITFA-2 antiserum (α-CITFA-2). The indicated transcription signals were obtained by primer extension assays and extension products were separated by denaturing PAGE and visualized by autoradiography. The asterisk designates an aberrantly initiated SLins19 transcript caused by the TFIIB antiserum. Marker, pBR322-MspI. (E) Co-transcription of GPEET-trm and SLins19 in extracts of procyclic 29-13 cells in which CITFA-2 silencing was not induced (−RNAi) or induced for 36 h (+RNAi).
CITFA-2 is essential for class I transcription in vitro
To directly evaluate the role of CITFA-2 in class I transcription, we employed a homologous in vitro transcription system. This system, which is based on a crude mix of cytoplasmic and extracted nuclear components of procyclic trypanosomes, is active for both class I and class II SLRNA transcription (Laufer et al, 1999). The template plasmids VSG-trm, GPEET-trm and Rib-trm, which contain a VSG ES, the GPEET procyclin and an RRNA promoter, respectively, were co-transcribed with the SLRNA template SLins19. These templates contain insertions of unrelated oligonucleotide sequences downstream of the transcription initiation site, which allow specific detection of transcripts by primer extension assays. In a first analysis, we tested whether the addition of CITFA-2 antiserum affected RNA pol I-mediated transcription in vitro. In these reactions, pre-immune serum did not significantly affect transcription of either the class I templates or of SLins19 (Figure 2D, compare lanes 2, 6, and 10 with lanes 1, 5, and 9, respectively). When we added, as another control, a similarly derived antiserum directed against the general class II transcription factor TFIIB of T. brucei (Schimanski et al, 2006), correctly initiated SLins19 transcription was abolished whereas class I transcription was only mildly affected, most likely due to a nonspecific effect from this particular serum (lanes 3, 7, and 11; Nguyen et al, 2007). In contrast, anti-CITFA-2 serum specifically interfered with class I transcription. While VSG-trm transcription was strongly reduced (lane 4), GPEET-trm and Rib-trm transcription was abolished (lanes 8 and 12). This differential and reproducible effect may be a consequence of higher affinity binding of CITFA-2 to the VSG ES promoter (see Figure 1D).
To confirm this observation, we prepared transcription extracts from procyclic cells in which CITFA-2 expression was silenced for 36 h, and from non-RNAi-induced cells. CITFA-2 silencing was less effective in procyclic than in bloodstream RNAi cells and reduced the CITFA-2 protein level by only 64% (Supplementary Figure S3). Nevertheless, when GPEET-trm, which drives the most efficient class I transcription in this in vitro system (Laufer et al, 1999), was co-transcribed with SLins19, GPEET-trm transcription was decreased by ∼73% when CITFA-2 was silenced, whereas the same extract supported SLins19 transcription nearly as well (∼13% reduction) as the extract prepared from noninduced cells (Figure 2E). Moreover, as a third criterion, depletion of CITFA-2 from the extract by IgG affinity chromatography specifically abolished class I transcription (see below, Figure 6). We therefore concluded that CITFA-2 is a general class I transcription factor in T. brucei.
Six proteins specifically co-purify with CITFA-2
While a single CITFA-2 ortholog is encoded in the genomes of the trypanosomatids Trypanosoma vivax, Trypanosoma cruzi, Leishmania major and Leishmania infantum (for a sequence alignment, see Supplementary Figure S4), we were unable to identify homologous sequences outside of this taxonomic family or to detect a characteristic sequence motif. Hence, by primary structure, CITFA-2 and its orthologs appear to be trypanosomatid-specific proteins. In the next step, we therefore asked whether this protein is associated with more conserved proteins resembling known transcription factors. We generated the procyclic cell line TbT2 which exclusively expressed CITFA-2 with an N-terminal PTP tag at a level corresponding to wild-type CITFA-2 expression (PTP-CITFA-2; Figure 3A and B). Since we had shown that CITFA-2 is encoded by an essential gene and TbT2 cells grew normally, we concluded that the tag did not interfere with protein function. The PTP tag is a modified tandem affinity purification tag consisting of a protein A tandem domain and a protein C (ProtC) epitope separated by a tobacco etch virus (TEV) protease cleavage site. PTP fusion proteins can be sequentially purified by IgG chromatography, TEV protease elution, anti-ProtC immunoaffinity chromatography, and elution with an EGTA-containing buffer (Schimanski et al, 2005b). According to the anti-ProtC immunoblot analysis, both PTP-CITFA-2 purification steps were highly efficient and resulted in the near depletion of the protein from input material (Figure 3C). In the final eluate, 13.7% (360 ng) of the CITFA-2 protein was recovered from the input material. Since approximately 81% of CITFA-2 was extracted from cells (data not shown), we estimate that a single trypanosome harbors ∼750 CITFA-2 molecules. Coomassie staining of the final eluate detected several distinct protein bands (Figure 3D), whose excision and analysis by liquid chromatography–tandem mass spectrometry (LC–MS/MS) led to the identification of P-CITFA-2 and six co-purified proteins (identified peptides are listed in Supplementary Table S1). The mix of bands around 55 kDa contained tagged CITFA-2 and proteins encoded by genes Tb11.47.0008 and Tb11.47.0010. While Tb11.47.0008 had been purified by our initial chromatography approach, Tb11.47.0010 was a new identification. Similarly, the bands with apparent sizes of 43 and 23 kDa were the products of genes Tb11.01.0240 and Tb927.5.970, respectively, and were not identified before. The band with an apparent size of 28 kDa reproducibly appeared to be fuzzy, suggesting that it contained more than one protein or differently modified versions of the same protein. Interestingly, the identified protein is encoded by three genes, which are part of a tandem repeat of five genes on chromosome 8. Tb927.8.4030 and Tb927.8.4080 encode the same protein, whereas the product of gene Tb927.8.4130 has a different C-terminal region. According to four diagnostic peptide identifications (Supplementary Table S1), both protein versions co-purified with P-CITFA-2.
Figure 3.
Six proteins co-purify with CITFA-2. (A) Schematic depiction (not to scale) of the CITFA-2 gene locus in cell line TbT2. In one allele, the CITFA-2-coding region was replaced by a hygromycin-resistance gene (HYG-R) and in the second allele the PTP sequence was fused to the 5′ end of the coding region by targeted insertion of pPURO-PTP-CITFA-2. Coding regions are represented by open boxes, the PTP tag by a black box, and introduced gene flanks by small gray boxes. (B) Immunoblot analysis of PTP-CITFA-2 in extracts of wild-type (WT) and TbT2 cells. The tagged protein was detected with the protein A-specific PAP reagent (top panel) or with the polyclonal anti-CITFA-2 serum (middle panel). Protein loading was controlled by reprobing the same blot with an antibody against T. brucei TFIIB. (C) Immunoblot monitoring of PTP-CITFA-2 purification. Aliquots of the input material (INP), the flow-through of the IgG affinity chromatography (FT-IgG), the TEV protease elution (Elu TEV), the flow-through of the anti-ProtC affinity chromatography (FT-ProtC), and the final EGTA eluate (Elu) were separated on a 10% SDS/polyacrylamide gel, blotted, and probed with anti-ProtC antibody. The relative amount of each sample to the input material is specified. It should be noted that the size of the tagged protein (PTP-CITFA-2) was reduced by ∼15 kDa after protease cleavage (P-CITFA-2). (D) Coomassie staining of purified proteins. The total eluate of a standard PTP-CITFA-2 purification was separated on a 15% SDS/polyacrylamide gel and stained with Coomassie. For comparison, 0.003% of the input material (Inp) and 5% of the TEV protease eluate (TEV) were loaded. On the right, proteins identified by mass spectrometry are specified by their GeneDB accession numbers or protein name. The asterisk marks a minor IgG kappa light chain contamination of the anti-ProtC matrix. (E) Immunoblot of whole-cell lysates derived from cell lines that express the proteins of the indicated genes as C-terminal PTP fusions. In a control cell line, the spliceosomal smD1 protein was PTP-tagged. (F) Co-precipitation of CITFA-2 with PTP-tagged proteins. Proteins were eluted from IgG beads either with glycine (Tb11.47.0008 and Tb11.47.0010) or by TEV protease digest (smD1, Tb11.01.0240, Tb927.8.4130, Tb927.5.970) that reduced the protein sizes. In the precipitates (P), the tagged proteins were detected with the anti-ProtC antibody, and CITFA-2 and TFIIB with polyclonal antisera. For comparison, 20% of input material (INP) was co-analyzed.
These six identified proteins are conserved among trypanosomatids (Supplementary Figures S4–S9), but they exhibit no similarity to proteins of other organisms. Nor could we identify a conserved sequence motif using the InterPro (http://www.ebi.ac.uk/interpro), Motif scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan), SMART (http://smart.embl-heidelberg.de/), and Minimotif miner (http://sms.engr.uconn.edu/servlet/SMS SearchServlet) search engines. Hence, based on primary structure, these six proteins appear to be specific to trypanosomatid parasites. In contrast, the smallest co-purified protein with an apparent size of 9 kDa was identified by a single peptide as the motor protein subunit dynein light chain DYNLL, which is also known as LC8 (Pfister et al, 2006). In T. brucei, this protein is encoded by the genes Tb11.50.0007 and Tb11.0845. Among several T. brucei DYNLL paralogs, this dynein light chain is most closely related to human DYNLL1 and 2 (data not shown). Accordingly, we propose to name the identified protein TbDYNLL1.
Previous PTP purifications of several nuclear protein complexes, which included RNA pol I (Nguyen et al, 2006, 2007) and the transcription factors TRF4/SNAPc/TFIIA (Schimanski et al, 2005a) and TFIIH (Lee et al, 2007) did not reveal any of the proteins that co-purified in this study. This strongly suggested that these proteins are specifically associated with CITFA-2. Nevertheless, we independently confirmed the interaction of each protein with CITFA-2 by co-immunoprecipitation. Except for DYNLL1, we C-terminally PTP-tagged each co-purified protein and, as a control, smD1 in individual cell lines (Figure 3E). As expected, precipitation of each PTP-tagged protein by IgG beads co-precipitated CITFA-2, whereas the control precipitation of smD1-PTP exhibited no detectable amounts of CITFA-2 (Figure 3F). Hence, we had correctly identified five proteins that co-purified with CITFA-2 and, at this stage, had obtained evidence that the sixth co-purified protein is DYNLL1.
CITFA-2 and the co-purified proteins form a complex
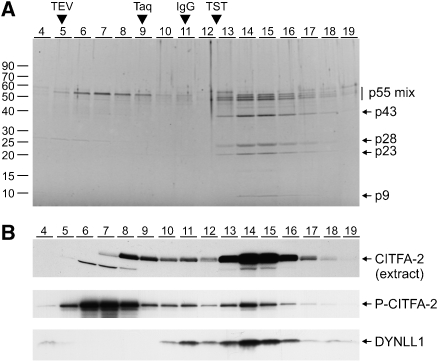
Next, we asked whether these proteins purified independently with CITFA-2 or as a complex. The final eluate of the PTP-CITFA-2 purification was therefore sedimented in a linear 10–40% sucrose gradient by ultracentrifugation and the proteins from individual fractions taken from top to bottom were separated by SDS–PAGE and visualized by Sypro Ruby staining (Figure 4A). A protein with the expected size of P-CITFA-2 sedimented by itself in fractions 6–8 and together with the other six co-purified proteins in fractions 14 and 15, indicating that CITFA-2 is part of a protein complex of seven subunits. It is unlikely that two independent complexes co-sedimented in the higher fractions because, under identical conditions, the ∼230 kDa transcription factor complex TRF4/SNAPc/TFIIA (Schimanski et al, 2005a) sedimented slower than the CITFA complex, which, assuming a stoichiometry of one for all of its component proteins, has an expected size of ∼250 kDa. When extract from wild-type cells was sedimented in an equivalent gradient and fractions were analyzed by immunoblotting, CITFA-2 sedimentation peaked in fractions 14 and 15 virtually identical to the sedimentation of the purified complex (Figure 4B). Furthermore, CITFA-2 sedimentation remained the same when the potassium chloride concentration of the extract was increased from 150 to 400 mM, suggesting that the protein complex is stable (data not shown). Although we cannot exclude the possibility that an important subunit of the complex was lost during purification, these results strongly indicated that we had purified an intact protein complex.
Figure 4.
Sedimentation analysis of the CITFA complex. (A) Sucrose gradient sedimentation of purified P-CITFA-2. Gradients were fractionated from top (fraction 4) to bottom (fraction 19) and the proteins of each fraction were separated on a 15% SDS/polyacrylamide gradient gel and stained with SYPRO Ruby. For comparison, sedimentations of TEV protease (29 kDa), Taq DNA polymerase (95 kDa), IgG (150 kDa), and TRF4/SNAPc/TFIIA (TST, ∼230 kDa) were co-analyzed. (B) Immunoblot analysis of sucrose gradient fractions. CITFA-2 was detected by anti-CITFA-2 serum in gradient fractions in which extract from wild-type cells (top panel) or purified CITFA complex (middle panel) was sedimented. The latter blot was reprobed with polyclonal anti-C. reinhardtii LC8 antibody to detect DYNLL1 in the purified material (bottom panel).
We used the fractionated material and a polyclonal antibody directed against the Chlamydomonas reinhardtii LC8 to verify that DYNLL1 is part of this complex. DYNLL1 is highly conserved among all eukaryotes and the heterologous antibody detected a protein of the correct size in unfractionated extract and only p9 in the purified CITFA complex (data not shown). In immunoblots of sucrose gradient fractions of purified P-CITFA-2, nearly all DYNLL1 co-sedimented with P-CITFA-2 in higher fractions and only a minor amount of free DYNLL1 was detected at the top of the gradient (Figure 4B and data not shown). Furthermore, we confirmed by immunoblotting that DYNLL1 did not co-purify in PTP purifications of the transcription factor TFIIH and of a cytoplasmic kinase (data not shown). These findings make it very unlikely that DYNLL1 was a contaminating protein in the CITFA-2 purification and therefore confirmed our identification of DYNLL1 as a subunit of the CITFA complex.
The purified CITFA complex is active in VSG ES promoter binding and class I transcription
To test the activity of the purified proteins, we first conducted EMSA with a radio-labeled VSG ES promoter probe (Figure 5A). The purified proteins efficiently shifted the probe to a single position, and the shift was effectively competed by adding unlabeled VSG ES promoter DNA (compare lanes 3 with lanes 4–6). In contrast, mutation of either upstream sequence element drastically diminished the competitive effect, which confirmed the promoter pull-down result that both elements are important for the interaction with CITFA-2 (compare lane 6 with lanes 7 and 8). Unlabeled DNA fragments comprising the RRNA and GPEET promoters were also able to compete, but with different efficiencies. Whereas the RRNA promoter competed for complex formation almost as efficiently as the VSG ES promoter (lane 9), the competitive effect of the GPEET promoter was much weaker (lane 10), suggesting that purified CITFA-2 in the absence of other factors binds to the T. brucei class I promoters, which have distinct sequences, with different affinities. Furthermore, in a time-course experiment, the DNA–protein complex was rapidly formed without an intermediate state, which indicated that the gel shift was caused either by CITFA-2 or by the protein complex (lanes 11–16). To discriminate between these two possibilities, EMSA was conducted with sucrose gradient fractions of the purified protein. While the lower fractions harboring CITFA-2 alone did not detectably shift the VSG probe, incubation of this probe with the complex containing fractions 14–17 reproduced the shift (Figure 5B). Hence, CITFA-2 does not bind to the DNA by itself but requires other subunits of the protein complex to achieve this task.
Figure 5.
The purified CITFA complex binds to the VSG ES promoter. (A) EMSA with radio-labeled VSG −85/−3 DNA which was incubated in the absence (free probe) or presence of P-CITFA-2 eluate. The gel shift was competed with the indicated molar excess of specified unlabeled promoter DNAs. For the time course, the binding reaction was upscaled five-fold and aliquots were taken from the reaction at specified time points. (B) Equivalent gel shift assays with proteins from specified sucrose gradient fractions.
In a final step, we analyzed whether the purified proteins are functional in class I transcription. For this, we prepared extract from TbT2 cells (described in Figure 3A) and depleted PTP-CITFA-2 from the extract by IgG chromatography (Figure 6A). As expected, transcription of GPEET-trm, VSG-trm, and Rib-trm was nearly abolished in the depleted extract while SLRNA transcription was not affected (Figure 6B, compare lanes 1, 9, and 13 with lanes and 2, 10, and 14, respectively). In previous studies, we have used the same technique to deplete the essential class II transcription factors SNAP2 (Schimanski et al, 2005a) and TFIIB (Schimanski et al, 2006), and found that depletion inhibited SLRNA transcription but not GPEET-trm transcription. In contrast, depletion of an essential RNA pol I subunit from the extract resulted in the specific inhibition of class I promoter transcription in the system (Nguyen et al, 2007). Hence, it is very unlikely that IgG or the PTP tag caused a nonspecific depletion effect on class I template transcription. In agreement with these results, the addition of PTP-purified TFIIB, which had previously been shown to be functional in reconstituting SLRNA transcription (Schimanski et al, 2006), had no effect on the transcription of the three class I templates (lanes 3, 11, and 15). In contrast, class I transcription in the depleted extract was partially reconstituted by adding back the tandem affinity-purified P-CITFA-2 (Figure 3D, lanes labeled depl+P-CITFA-2). As we showed for the efficient GPEET-trm transcription, this reconstitution was dose-dependent (lanes 4–6). The reconstitution signals for GPEET, VSG, and RRNA promoter transcription were 21, 17, and 13%, respectively (Figure 6B and data not shown). Since we added purified CITFA in an amount comparable to that present in the cell extract, the purified complex was not fully active, perhaps because elution of the complex from anti-ProtC beads by chelating divalent cations caused an inactivating conformational change in the complex that was partially rescued in the subsequent dialysis against transcription buffer. This is a likely explanation because we have observed EGTA-mediated deactivation of a T. brucei class II transcription factor before (Schimanski et al, 2005a). Alternatively, it is possible that an essential component of the complex was partially lost during the purification and not identified in our biochemical characterization. Interestingly, the P-CITFA-2 eluate did not reconstitute GPEET-trm transcription in extract prepared from cells in which CITFA-2 expression was silenced by RNAi (data not shown), suggesting that CITFA-2 silencing caused a downstream effect that could not be rescued by adding back a functional CITFA complex. In summary, these data showed that tandem affinity-purified CITFA bound to the VSG ES promoter in a specific manner and was partially active in reconstituting class I transcription in a depleted extract.
Figure 6.
The purified CITFA complex is transcriptionally active. (A) Immunoblot of mock-treated and PTP-CITFA-2-depleted (depl) transcription extract (Textract). PTP-CITFA-2 and TFIIB were co-detected with the PAP reagent and a polyclonal antibody, respectively. (B) Co-transcription of class I templates and SLins19 in mock-treated or CITFA-2-depleted extract. The depleted extract was complemented with final eluate of TFIIB (depl+TFIIB-P) or CITFA-2 (depl+P-CITFA-2) PTP purifications. Marker, pBR322-MspI.
Discussion
In this study, we have characterized a protein complex that is indispensable for multifunctional class I transcription in T. brucei. The complex specifically bound to VSG ES, RRNA, and GPEET promoters and as shown for the VSG ES promoter, this binding depended on the integrity of the two upstream promoter elements. Furthermore, transcription from all three class I promoters was strongly affected or even abolished when anti-CITFA-2 serum was added to the extract, or when CITFA-2 was depleted from extract either through CITFA-2 expression silencing or through antibody affinity chromatography. In accordance with these strong effects observed in vitro, CITFA-2 silencing led to a rapid cessation of procyclic and bloodstream cell growth and to reduced abundance of class I transcripts in bloodstream forms. The complex consists of at least seven proteins, six of which are conserved within trypanosomatids but have no resemblance to proteins of other organisms. The seventh subunit is the dynein light chain TbDYNLL1, which has not previously been identified as part of the class I transcription machinery in other organisms. Hence, we have characterized a novel protein complex, which we propose to name T. brucei class I transcription factor A (TbCITFA). We also propose to number individual subunits of this complex according to their predicted molecular weight, because a terminology based on apparent sizes is ambiguous for the three largest subunits (Table 1).
Table 1.
T. brucei CITFA subunits
| Subunit | GeneDB acc no. | MW (kDA) | No. aa | Th. pI | Apparent size (kDa) |
|---|---|---|---|---|---|
| CITFA-1 | Tb11.47.0010 | 52.9 | 465 | 8.9 | ∼55 |
| CITFA-2 | Tb09.211.3440 | 48.2 | 421 | 4.7 | ∼55 |
| CITFA-3 | Tb11.47.0008 | 47.0 | 416 | 6.1 | ∼55 |
| CITFA-4 | Tb11.01.0240 | 42.8 | 388 | 8.6 | 43 |
| CITFA-5a | Tb927.8.4030 | 25.8 | 232 | 5.5 | 28 |
| Tb927.8.4080 | |||||
| CITFA-5b | Tb927.8.4130 | 25.7 | 231 | 5.3 | 28 |
| CITFA-6 | Tb927.5.970 | 21.7 | 190 | 6.7 | 23 |
| DYNLL1 | Tb11.50.0007 | 10.4 | 90 | 6.3 | 9 |
| Tb11.0845 | |||||
| Th. pI, theoretical pI. | |||||
T. brucei CITFA is the first characterized class I transcription factor of a protistan organism. To the best of our knowledge, partially characterized factors are only known from Acanthamoeba castellani, where, with the exception of TBP, the proteins have not been identified (Al Khouri and Paule, 2002). The general lack of sequence conservation among class I transcription factors of distantly related species may explain the parasite-specific sequences of trypanosomatid CITFA subunits and it is possible that functional and structural analyses of CITFA subunits will reveal orthologs or functional equivalents of known class I transcription factors. On the other hand, the number of subunits, the absence of a TBP homolog, and the presence of DYNLL1 clearly distinguish T. brucei CITFA from known class I transcription factors.
While we have shown that DYNLL1 is a CITFA subunit, we do not yet understand its function in this complex. In other organisms, DYNLL interacts with many cytoplasmic proteins, probably linking these interactors to the dynein or myosin V motors (Pfister et al (2006) and references therein). DYNLL has also been implicated in a number of regulatory roles such as inhibitor of nitric oxide synthase (Jaffrey and Snyder, 1996), regulator of apoptotic signaling (Puthalakath et al, 1999), or suppressor of the human transcription repressor TRPS1 (Kaiser et al, 2003). While in most cases, the mode of DYNLL-mediated regulation is unknown, some results suggest that it is by sequestering an interacting protein to cytoskeleton-associated motor complexes (Puthalakath et al, 1999). As the active VSG in bloodstream T. brucei is localized to the expression site body (Navarro and Gull, 2001) and procyclin gene units in procyclic cells are arranged at the nucleolar periphery (Landeira and Navarro, 2007), it is an intriguing possibility that TbDYNLL1 facilitates the positioning of these transcription units. Alternatively, the dynein light chain may directly promote efficient transcription as has been recently implicated for LC8 that binds to the phosphoprotein of rabies virus (Tan et al, 2007).
In conclusion, the identification and characterization of CITFA in T. brucei represents a major step towards deciphering the unique and essential properties of the multifunctional RNA pol I transcription machinery of both an early diverged eukaryote and a lethal human parasite.
Materials and methods
DNA sequences
The DNA sequence of the VSG ES 118 promoter spanning the region from position −3 to −85 relative to the TIS was amplified by PCR from plasmid MIG35 (Navarro M, Wirtz LE, Cross GAM, unpublished) with oligonucleotides 5′-GCCCTCGAGAACCGTCTAAAAGA-3′ and 5′-GCGAAGCTTGTCTGATAT CCGAGA-3′, which incorporate a XhoI and a HindIII site, respectively. The amplification product was cloned into the corresponding sites of plasmid pBluescript SKII+ (Stratagene), generating the plasmid pESP3. Similarly, plasmids pESP4 and pESP5, which contain the VSG ES promoter with mutations in box 2 (−62 CT −61 was mutated to AA) and box 1 (−38 CAGGG −34 was mutated to GGTAA), respectively, were amplified and cloned from plasmids pLEW115 and pLEW116, respectively (Navarro M, Wirtz LE, Cross GAM, unpublished). For N-terminal PTP tagging of CITFA-2, 387 bp of the 5′ terminal coding region, which excluded the initiation codon, were amplified from genomic DNA and inserted into the NotI and ApaI sites of plasmid pN-PURO-PTP (Schimanski et al, 2005b). For C-terminal PTP tagging of CITFA subunits, the following 3′ terminal coding regions were cloned into the NotI and ApaI sites of plasmid pC-PTP-NEO: 702 bp of Tb11.47.0010, 585 bp of Tb11.47.0008, 600 bp of Tb11.01.0240, 336 bp of Tb927.8.4130, and 313 bp of Tb927.5.970. pT7-stl is a derivative of pLew100 (Wirtz et al, 1999) and was derived by replacing the sequence between BsiWI and MluI restriction sites with two T7 transcription terminators and by inserting the sequence 5′-CTAATACGACTCACTATAGG GATCTCCCTATCAGTGATAGAGATCCCTATCAGTGATAGAGA-3′, which contains the T7 promoter and two tandem tetracycline operators, in the form of two hybridized oligonucleotides into the KpnI and HindIII sites. For CITFA-2 RNAi, the CITFA-2-coding sequence from position 121 to position 650 was inserted into pZJM (Wang et al, 2000) or the stem-loop cassette of pT7-stl following the established cloning strategy (Shi et al, 2000).
Cells
T. brucei cell culture, targeted integration of linear DNAs into cells by electroporation, and the generation of stable cell lines by selection and limiting dilution were described in detail previously (Wirtz et al, 1999; Günzl et al, 2000). Depending on the selectable markers present in procyclic lines, cells were kept in medium with 40 μg/ml of G418, 20 μg/ml of hygromycin, and/or 4 μg/ml of puromycin. Procyclic 29-13 cells (Wirtz et al, 1999) transfected with the CITFA-2 RNAi construct were cultured in the presence of 15 μg/ml of G418, 50 μg/ml of hygromycin, and 2.5 μg/ml of phleomycin. The corresponding ‘single marker' bloodstream cell line (Wirtz et al, 1999) was maintained in medium containing 2.5 μg/ml of G418 and 0.25 μg/ml of phleomycin. In RNAi experiments, dsRNA synthesis was induced with 2 μg/ml of doxycycline. Cells were counted and diluted to specific densities daily.
Initial purification and analysis of the promoter-binding complex
A nuclear extract was prepared from 30 l of procyclic cells and contained 20 mM HEPES, pH 7.6, 420 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, and protease inhibitors. The extract was subjected to chromatographic purification by a protocol that was devised by extensive empirical testing. All steps were carried out at 4°C and purification was monitored by EMSA. Purification was achieved consecutively by a 25–50% saturation ammonium sulfate precipitation, S-Sepharose cation exchange chromatography, heparin affinity chromatography, DNA affinity chromatography in which an 83-bp biotinylated VSG ES promoter, PCR-amplified from pESP3, was coupled to a Streptavidin Sepharose High Performance column (Amersham), and Resource Q ion exchange chromatography (Amersham). In a final step, the active fractions were loaded onto 4 ml 15–45% (v/v) linear glycerol gradients (10 mM HEPES, 150 mM NaCl, 0.1 mM EDTA, 1 mM DTT) and centrifuged at 50 000 rev/min in a Beckman SW 60 rotor for 16 h at 7°C. Purified proteins were resolved by 10% SDS–PAGE and silver-stained. Each visible band was excised, destained, dehydrated and treated with 100 ng of modified TPCK-trypsin for 8 h at 37°C. Peptides were subjected to LC–ESI–MS/MS analysis using an HPLC system (Smart System, Amersham). Protein searches were performed with SonarMSMS (Genomic Solutions) using locally collated versions of the available T. brucei genome data in 2001, with a re-analysis in May 2005 (a detailed protocol for this subheading is included in the Supplementary data).
Analysis of the protein complex by tandem affinity purification
Preparation of crude extract from 5 × 1010 procyclic cells and tandem affinity purification of PTP-tagged CITFA-2 was carried out according to the standard PTP purification protocol (Schimanski et al, 2005b). For immunoblot analyses, proteins were separated by SDS–PAGE, electroblotted onto a polyvinylidene difluoride membrane, and detected with specified antibodies and the BM Chemiluminescence Blotting substrate (Roche). PTP-tagged proteins were either detected with the PAP reagent (Sigma) or the monoclonal anti-ProtC antibody HPC4 (Roche). The antiserum against CITFA-2 was raised against recombinant protein. For this, the coding sequence of the 208 amino acid-long N-terminal half was fused to the ProtC epitope, a thrombin cleavage site, and a 6xHis tag in the expression vector pET100/D-TOPO (Invitrogen). The vector was transformed into Escherichia coli strain BL21 and protein expression was induced for 4 h at 37°C by adding 1 mM IPTG. Recombinant protein was tandem affinity-purified in subsequent steps by TALON metal affinity chromatography (BD Biosciences), imidazole elution, and anti-ProtC immunoaffinity chromatography. Recombinant protein was finally eluted in phosphate-buffered saline (PBS) containing 10 mM EGTA and 5 mM EDTA and dialyzed against PBS. The protein was used for immunizing rats as detailed elsewhere (Schimanski et al, 2006).
For identification, purified proteins were collected by StrataClean resin (Stratagene), released into SDS loading buffer by boiling the samples for 5 min, separated on a 15% SDS/polyacrylamide gel, and stained with Gelcode Coomassie stain (Pierce). Individual bands were excised from the gel, proteins were digested with trypsin or substilisin, and peptides were identified by LC–MS/MS.
Sedimentation analysis was carried out in 4 ml 10–40% linear sucrose gradients (20 mM HEPES–KOH, pH 7.7, 150 mM potassium chloride, 20 mM potassium L-glutamate, 3 mM MgCl2, 0.1% Tween 20), which were centrifuged in a Beckman SW55 rotor for 19 h at 42 000 rev/min and 4°C. Twenty fractions were collected from top to bottom and protein was precipitated using StrataClean resin as described above. Proteins were separated on a 15% SDS/polyacrylamide gels and stained with SYPRO Ruby (Invitrogen) according to the manufacturer's protocol.
For co-immunoprecipitations of various PTP-tagged proteins, 100 μl of crude cell extract, corresponding to 8 × 108 cells, was mixed with 20 μl settled volume of IgG beads and incubated on ice for 1 h. The beads were washed four times with TET150 buffer (150 mM NaCl, 20 mM Tris–HCl, pH 8.0, 3 mM MgCl2, 0.1% Tween 20). For glycine elution of proteins, the beads were incubated twice in 150 μl of 100 mM glycine–HCl (pH 2) for 2 min. The two fractions were pooled and mixed with 1 ml of TET150 buffer. Alternatively, proteins were eluted by adding 4 units of AcTEV protease (Invitrogen) to the beads and by incubating the reactions at 28°C for 30 min. Immunoprecipitates were collected using StrataClean resin and analyzed by immunoblotting as described above.
DNA–protein interaction
Promoter pull-downs were carried out as described previously (Schimanski et al, 2004) except that the KCl concentration was increased to 80 mM. For EMSA, the 93 bp-long DNA (ESP3) generated from the digestion of plasmids pESP3 with XhoI and HindIII was 3′-end-labeled using the Klenow fragment of DNA polymerase and [α-32P]dCTP. The probe was purified on a NucTrap column (Stratagene) followed by phenol–chloroform extraction and ethanol precipitation. EMSA conditions were based on those described by Pham et al (1997) and carried out in 15 μl containing 6 mM HEPES, pH 7.6, 40 mM DTT, 4% Ficoll 400, 1 μg poly-dI/dC, 0.17 mM spermine, and 1.7 mM spermidine. Unless in exceptional cases, the final concentration of sodium or potassium chloride was kept between 50 and 150 mM. Protein extract was added to the binding reaction and the mixture incubated for 10 min at 25°C. Then, 1 ng of radio-labeled DNA probe was added and the reaction incubated for another 10 min at 25°C, before separation in a 4% polyacrylamide/0.5 × TBE gel at 150 V and 4°C for 3 h. Gels were dried onto filter paper and exposed for autoradiography. EMSAs with PTP-purified CITFA-2 were slightly modified and conducted with 15 ng of total protein and 25 ng of poly-dI/dC in the absence of spermine and spermidine. Competition assays were carried out in the same way, with the competitor being added before the protein. The unlabeled competitors ESP4 and ESP5 were excised as ESP3 from corresponding plasmids, whereas the RRNA and GPEET competitor DNAs, which extended from positions −257 to +106 and from −246 to +70 relative to the TIS, respectively, were generated by PCR.
For UV crosslinking, the VSG ES 118 sequence from position −85 to −3 was PCR amplified from plasmid ESP3 in the presence of [α-32P]dCTP and 5′-bromodeoxy uridine triphosphate. The binding reaction was carried out under the same conditions as for EMSA, and then irradiated with UV at 3000 μW/cm2 in a Stratalinker (Stratagene) for 30 min on ice. CaCl2 was added to final concentration of 15 mM and the DNA digested for 30 min at 37°C using 5 units of DNase I and 5 units of S7 micrococcal nuclease (Roche). The proteins were resolved on a 10% SDS–PAGE and visualized by autoradiography.
In vitro transcription and RNA analysis
The in vitro transcription system has been previously described in detail (Laufer et al, 1999; Laufer and Günzl, 2001). In brief, a standard reaction was conducted in a volume of 40 μl and contained 8 μl of extract, 20 mM potassium L-glutamate, 20 mM KCl, 3 mM MgCl2, 20 mM HEPES–KOH, pH 7.7, 0.5 mM of each nucleoside triphosphate, 20 mM creatine phosphate, 0.48 mg/ml of creatine kinase, 2.5% polyethylene glycol, 0.2 mM EDTA, 0.5 mM EGTA, 4 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 12.5 μg/ml vector DNA, 20 μg/ml class I template, and 7.5 μg/ml SLins19 template. In reactions with extract from 29-13 cells, modified template versions lacking T7 promoters were used (Lee et al, 2007). In reactions with antisera, the volume of the extract was reduced to 4 μl, and extract was pre-incubated with antiserum for 30 min on ice before reactions were started by adding templates and nucleotides. Reactions were incubated for 1 h at 27°C and stopped by the addition of 300 μl of solution D (4 M guanidinium thiocyanate, 25 mM sodium citrate, pH 7.0, 0.5% N-lauroylsarcosine). In total RNA preparations, specific transcripts were detected by Superscript II (Invitrogen)-mediated extension of 32P-end-labeled primers Tag_PE and SLtag which hybridize to unrelated oligonucleotide tags of the class I and SLins19 RNAs, respectively (Laufer et al, 1999).
Northern analysis and U2 and SL RNA primer extension assays of total RNA prepared from RNAi-induced cells were conducted exactly as recently described (Nguyen et al, 2007).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Miguel Navarro, Elizabeth Wirtz, and Stephen King for kindly providing the plasmid pMIG35, the plasmids pLew 115 and pLew 116, and the anti-LC8 antibody, respectively. We are also grateful to Mary Ann Gawinowicz (Protein Core Facility, Columbia University) for excellent mass spectrometric analysis. This work was supported by NIH grants AI059377 (AG), AI021729 (GAMC), and RR00862 (BTC).
References
- Al Khouri AM, Paule MR (2002) A novel RNA polymerase I transcription initiation factor, TIF-IE, commits rRNA genes by interaction with TIF-IB, not by DNA binding. Mol Cell Biol 22: 750–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Huang J, Van der Ploeg LH (1992) The promoter for the procyclic acidic repetitive protein (PARP) genes of Trypanosoma brucei shares features with RNA polymerase I promoters. Mol Cell Biol 12: 2644–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CE, Fueri JP, Itzhaki JE, Bellofatto V, Sherman DR, Wisdom GS, Vijayasarathy S, Mowatt MR (1990) Transcription of the procyclic acidic repetitive protein genes of Trypanosoma brucei. Mol Cell Biol 10: 3036–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers B, Czichos J, Overath P (1987) RNA turnover in Trypanosoma brucei. Mol Cell Biol 7: 1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I (2003) Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev 17: 1691–1702 [DOI] [PubMed] [Google Scholar]
- Günzl A, Bindereif A, Ullu E, Tschudi C (2000) Determinants for cap trimethylation of the U2 small nuclear RNA are not conserved between Trypanosoma brucei and higher eukaryotic organisms. Nucleic Acids Res 28: 3702–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzl A, Bruderer T, Laufer G, Schimanski B, Tu LC, Chung HM, Lee PT, Lee MG (2003) RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot Cell 2: 542–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH (1996) PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274: 774–777 [DOI] [PubMed] [Google Scholar]
- Janz L, Clayton C (1994) The PARP and rRNA promoters of Trypanosoma brucei are composed of dissimilar sequence elements that are functionally interchangeable. Mol Cell Biol 14: 5804–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser FJ, Tavassoli K, Van den Bemd GJ, Chang GT, Horsthemke B, Moroy T, Ludecke HJ (2003) Nuclear interaction of the dynein light chain LC8a with the TRPS1 transcription factor suppresses the transcriptional repression activity of TRPS1. Hum Mol Genet 12: 1349–1358 [DOI] [PubMed] [Google Scholar]
- Kooter JM, Borst P (1984) Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res 12: 9457–9472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeira D, Navarro M (2007) Nuclear repositioning of the VSG promoter during developmental silencing in Trypanosoma brucei. J Cell Biol 176: 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer G, Günzl A (2001) In-vitro competition analysis of procyclin gene and variant surface glycoprotein gene expression site transcription in Trypanosoma brucei. Mol Biochem Parasitol 113: 55–65 [DOI] [PubMed] [Google Scholar]
- Laufer G, Schaaf G, Bollgönn S, Günzl A (1999) In vitro analysis of alpha-amanitin-resistant transcription from the rRNA, procyclic acidic repetitive protein, and variant surface glycoprotein gene promoters in Trypanosoma brucei. Mol Cell Biol 19: 5466–5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Nguyen TN, Schimanski B, Günzl A (2007) SL RNA gene transcription in Trypanosoma brucei requires transcription factor TFIIH. Eukaryot Cell 6: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Van der Ploeg LH (1997) Transcription of protein-coding genes in trypanosomes by RNA polymerase I. Annu Rev Microbiol 51: 463–489 [DOI] [PubMed] [Google Scholar]
- Luu VD, Brems S, Hoheisel JD, Burchmore R, Guilbride DL, Clayton C (2006) Functional analysis of Trypanosoma brucei PUF1. Mol Biochem Parasitol 150: 340–349 [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley DL (1997) 5′-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev 11: 3306–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Gull K (2001) A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 414: 759–763 [DOI] [PubMed] [Google Scholar]
- Nguyen TN, Schimanski B, Günzl A (2007) Active RNA polymerase I of Trypanosoma brucei harbors a novel subunit essential for transcription. Mol Cell Biol 27: 6254–6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TN, Schimanski B, Zahn A, Klumpp B, Günzl A (2006) Purification of an eight subunit RNA polymerase I complex in Trypanosoma brucei. Mol Biochem Parasitol 149: 27–37 [DOI] [PubMed] [Google Scholar]
- Pays E, Salmon D, Morrison LJ, Marcello L, Barry JD (2007) Antigenic variation in Trypanosoma brucei. In: Trypanosomes—After the Genome, Barry JD, McCulloch R, Mottram J, Acosta-Serrano A (eds), pp 339–372. Norfolk, UK: Horizon Press [Google Scholar]
- Pfister KK, Shah PR, Hummerich H, Russ A, Cotton J, Annuar AA, King SM, Fisher EM (2006) Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet 2: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VP, Qi CC, Gottesdiener KM (1996) A detailed mutational analysis of the VSG gene expression site promoter. Mol Biochem Parasitol 75: 241–254 [DOI] [PubMed] [Google Scholar]
- Pham VP, Rothman PB, Gottesdiener KM (1997) Binding of trans-acting factors to the double-stranded variant surface glycoprotein (VSG) expression site promoter of Trypanosoma brucei. Mol Biochem Parasitol 89: 11–23 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 3: 287–296 [DOI] [PubMed] [Google Scholar]
- Rudenko G, Bishop D, Gottesdiener K, Van der Ploeg LH (1989) Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J 8: 4259–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G, Chung HM, Pham VP, Van der Ploeg LH (1991) RNA polymerase I can mediate expression of CAT and neo protein-coding genes in Trypanosoma brucei. EMBO J 10: 3387–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruepp S, Furger A, Kurath U, Renggli CK, Hemphill A, Brun R, Roditi I (1997) Survival of Trypanosoma brucei in the tsetse fly is enhanced by the expression of specific forms of procyclin. J Cell Biol 137: 1369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Zomerdijk JC (2005) RNA-polymerase-I-directed rDNA transcription, life and works. Trends Biochem Sci 30: 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski B, Brandenburg J, Nguyen TN, Caimano MJ, Günzl A (2006) A TFIIB-like protein is indispensable for spliced leader RNA gene transcription in Trypanosoma brucei. Nucleic Acids Res 34: 1676–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski B, Laufer G, Gontcharova L, Günzl A (2004) The Trypanosoma brucei spliced leader RNA and rRNA gene promoters have interchangeable TbSNAP50-binding elements. Nucleic Acids Res 32: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski B, Nguyen TN, Günzl A (2005a) Characterization of a multisubunit transcription factor complex essential for spliced-leader RNA gene transcription in Trypanosoma brucei. Mol Cell Biol 25: 7303–7313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski B, Nguyen TN, Günzl A (2005b) Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryot Cell 4: 1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheader K, Vaughan S, Minchin J, Hughes K, Gull K, Rudenko G (2005) Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc Natl Acad Sci USA 102: 8716–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DR, Janz L, Hug M, Clayton C (1991) Anatomy of the PARP gene promoter of Trypanosoma brucei. EMBO J 10: 3379–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Djikeng A, Mark T, Wirtz E, Tschudi C, Ullu E (2000) Genetic interference in Trypanosoma brucei by heritable and inducible double-stranded RNA. RNA 6: 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GS, Preuss MA, Williams JC, Schnell MJ (2007) The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc Natl Acad Sci USA 104: 7229–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, Pays A, Tebabi P, Alexandre S, Pays E (1995) Specific binding of proteins to the noncoding strand of a crucial element of the variant surface glycoprotein, procyclin, and ribosomal promoters of Trypanosoma brucei. Mol Cell Biol 15: 5598–5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walgraffe D, Devaux S, Lecordier L, Dierick JF, Dieu M, Van Den AJ, Pays E, Vanhamme L (2005) Characterization of subunits of the RNA polymerase I complex in Trypanosoma brucei. Mol Biochem Parasitol 139: 249–260 [DOI] [PubMed] [Google Scholar]
- Wang Z, Morris JC, Drew ME, Englund PT (2000) Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J Biol Chem 275: 40174–40179 [DOI] [PubMed] [Google Scholar]
- Wirtz E, Hartmann C, Clayton C (1994) Gene expression mediated by bacteriophage T3 and T7 RNA polymerases in transgenic trypanosomes. Nucleic Acids Res 23: 3887–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E, Leal S, Ochatt C, Cross GAM (1999) A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol 99: 89–101 [DOI] [PubMed] [Google Scholar]
- Yue Z, Maldonado E, Pillutla R, Cho H, Reinberg D, Shatkin AJ (1997) Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc Natl Acad Sci USA 94: 12898–12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerdijk JC, Kieft R, Borst P (1991) Efficient production of functional mRNA mediated by RNA polymerase I in Trypanosoma brucei. Nature 353: 772–775 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information