Abstract
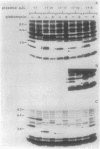
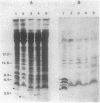
By oligonucleotide-directed mutagenesis, stop codon mutations were introduced at various sites in the pCloDF13-derived bacteriocin release protein (BRP) structural gene. The expression, lipid modification (incorporation of [3H]palmitate), and processing (in the presence and absence of globomycin) of the various carboxyl-terminal shortened BRPs were analyzed by a special electrophoresis system and immunoblotting with an antiserum raised against a synthetic BRP peptide, and their functioning with respect to release of cloacin DF13, lethality, and apparent host cell lysis were studied in Sup-, supF, and supP strains of Escherichia coli. All mutant BRPs were stably expressed, lipid modified, and processed by signal peptidase II, albeit with different efficiencies. The BRP signal peptide appeared to be extremely stable and accumulated in induced cells. Full induction of the mutant BRPs, including the shortest containing only 4 amino acid residues of the mature polypeptide, resulted in phospholipase A-dependent and Mg2+-suppressible apparent cell lysis. The extent of this lysis varied with the mutant BRP used. Induction of all mutant BRPs also prevented colony formation, which appeared to be phospholipase A independent. One shortened BRP, containing 20 amino acid residues of the mature polypeptide, was still able to bring about the release of cloacin DF13. The results indicated that the 8-amino-acid carboxyl-terminal segment of the BRP contains a strong antigenic determinant and that a small segment between amino acid residues 17 and 21, located in the carboxyl-terminal half of the BRP, is important for release of cloacin DF13. Either the stable signal peptide or the acylated amino-terminal BRP fragments (or both) are involved in host cell lysis and lethality.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty D., Lloubès R., Geli V., Lazdunski C., Howard S. P. Extracellular release of colicin A is non-specific. EMBO J. 1987 Aug;6(8):2463–2468. doi: 10.1002/j.1460-2075.1987.tb02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Baty D., Howard S. P., Verheij H. M., Lazdunski C. Lipoprotein nature of the colicin A lysis protein: effect of amino acid substitutions at the site of modification and processing. J Bacteriol. 1987 May;169(5):2187–2194. doi: 10.1128/jb.169.5.2187-2194.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J., Ray P. H. Inhibition of prolipoprotein signal peptidase by globomycin. J Biol Chem. 1985 May 25;260(10):5891–5894. [PubMed] [Google Scholar]
- Ghrayeb J., Inouye M. Nine amino acid residues at the NH2-terminal of lipoprotein are sufficient for its modification, processing, and localization in the outer membrane of Escherichia coli. J Biol Chem. 1984 Jan 10;259(1):463–467. [PubMed] [Google Scholar]
- Giam C. Z., Chai T., Hayashi S., Wu H. C. Prolipoprotein modification and processing in Escherichia coli. A unique secondary structure in prolipoprotein signal sequence for the recognition by glyceryl transferase. Eur J Biochem. 1984 Jun 1;141(2):331–337. doi: 10.1111/j.1432-1033.1984.tb08196.x. [DOI] [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J Biol Chem. 1980 Apr 25;255(8):3707–3712. [PubMed] [Google Scholar]
- James R., Jarvis M., Barker D. F. Nucleotide sequence of the immunity and lysis region of the ColE9-J plasmid. J Gen Microbiol. 1987 Jun;133(6):1553–1562. doi: 10.1099/00221287-133-6-1553. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Lau P. C., Hefford M. A., Klein P. Structural relatedness of lysis proteins from colicinogenic plasmids and icosahedral coliphages. Mol Biol Evol. 1987 Sep;4(5):544–556. doi: 10.1093/oxfordjournals.molbev.a040460. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Luirink J., Hayashi S., Wu H. C., Kater M. M., de Graaf F. K., Oudega B. Effect of a mutation preventing lipid modification on localization of the pCloDF13-encoded bacteriocin release protein and on release of cloacin DF13. J Bacteriol. 1988 Sep;170(9):4153–4160. doi: 10.1128/jb.170.9.4153-4160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., Watanabe T., Wu H. C., Stegehuis F., de Graaf F. K., Oudega B. Modification, processing, and subcellular localization in Escherichia coli of the pCloDF13-encoded bacteriocin release protein fused to the mature portion of beta-lactamase. J Bacteriol. 1987 May;169(5):2245–2250. doi: 10.1128/jb.169.5.2245-2250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., de Graaf F. K., Oudega B. Uncoupling of synthesis and release of cloacin DF13 and its immunity protein by Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):126–132. doi: 10.1007/BF00326547. [DOI] [PubMed] [Google Scholar]
- Luirink J., de Jong J., van Putten A. J., de Graaf F. K., Oudega B. Functioning of a hybrid BRP-beta-lactamase protein in the release of cloacin DF13 and lysis of Escherichia coli cells. FEMS Microbiol Lett. 1989 Mar;49(1):25–31. doi: 10.1016/0378-1097(89)90336-4. [DOI] [PubMed] [Google Scholar]
- Luirink J., van der Sande C., Tommassen J., Veltkamp E., De Graaf F. K., Oudega B. Effects of divalent cations and of phospholipase A activity on excretion of cloacin DF13 and lysis of host cells. J Gen Microbiol. 1986 Mar;132(3):825–834. doi: 10.1099/00221287-132-3-825. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Cole S. T. An unmodified form of the ColE2 lysis protein, an envelope lipoprotein, retains reduced ability to promote colicin E2 release and lysis of producing cells. J Gen Microbiol. 1987 Sep;133(9):2411–2420. doi: 10.1099/00221287-133-9-2411. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The immunity and lysis genes of ColN plasmid pCHAP4. Mol Gen Genet. 1988 Feb;211(2):335–341. doi: 10.1007/BF00330613. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part I: Production, and translocation across membranes. Microbiol Sci. 1984 Oct;1(7):168–175. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Suit J. L., Luria S. E. Expression of the kil gene of the ColE1 plasmid in Escherichia coli Kilr mutants causes release of periplasmic enzymes and of colicin without cell death. J Bacteriol. 1988 Oct;170(10):4963–4966. doi: 10.1128/jb.170.10.4963-4966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba M., Masaki H., Ohta T. Primary structures of the ColE2-P9 and ColE3-CA38 lysis genes. J Biochem. 1986 Feb;99(2):591–596. doi: 10.1093/oxfordjournals.jbchem.a135515. [DOI] [PubMed] [Google Scholar]
- Tokunaga H., Wu H. C. Studies on the modification and processing of prolipoprotein in Escherichia coli. Effects of structural alterations in prolipoprotein on its maturation in wild type and lpp mutants. J Biol Chem. 1984 May 25;259(10):6098–6104. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tiel-Menkvled G. J., Rezee A., De Graaf F. K. Production and excretion of cloacin DF13 by Escherichia coli harboring plasmid CloDF13. J Bacteriol. 1979 Nov;140(2):415–423. doi: 10.1128/jb.140.2.415-423.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- d'Enfert C., Pugsley A. P. A gene fusion approach to the study of pullulanase export and secretion in Escherichia coli. Mol Microbiol. 1987 Sep;1(2):159–168. doi: 10.1111/j.1365-2958.1987.tb00508.x. [DOI] [PubMed] [Google Scholar]
- de Geus P., van Die I., Bergmans H., Tommassen J., de Haas G. Molecular cloning of pldA, the structural gene for outer membrane phospholipase of E. coli K12. Mol Gen Genet. 1983;190(1):150–155. doi: 10.1007/BF00330338. [DOI] [PubMed] [Google Scholar]