Abstract
Drosophila telomeres do not have arrays of simple telomerase-generated G-rich repeats. Instead, Drosophila maintains its telomeres by occasional transposition of specific non-long terminal repeat (non-LTR) retrotransposons to chromosome ends. The genus Drosophila provides a superb model system for comparative telomere analysis. Here we present an evolutionary study of Drosophila telomeric elements to ascertain the significance of telomeric retrotransposons (TRs) in the maintenance of Drosophila telomeres. PCR and in silico surveys in the sibling species of Drosophila melanogaster and in more distantly related species show that multiple TRs maintain telomeres in Drosophila. In addition to TRs with two open reading frames (ORFs) capable of autonomous transposition, there are deleted telomeric retrotransposons that have lost their ORF2, which we refer to as half telomeric-retrotransposons (HTRs). The phylogenetic relationship among these telomeric elements is congruent with the phylogeny of the species, suggesting that they have been vertically inherited from a common ancestor. Our results suggest that an existing non-LTR retrotransposon was recruited to perform the cellular function of telomere maintenance.
The molecular study of Drosophila telomeres began initially by analyzing the plasmid cDm356 (Rubin 1978) and afterward the phage λT-A (Young et al. 1983). In both cases, the cloned DNA hybridized with all telomeres of Drosophila melanogaster. It took a long time to realize that these clones derived from heterochromatin: cDm356 from the centromeric region of chromosome Y (Agudo et al. 1999) and λT-A from the pericentromeric region of chromosome 3 (Losada et al. 1999). The unexpected findings of telomeric elements at the centromeric region of the Y could be understood if this region derived from an ancestral telomere, as has been proposed by Agudo et al. (1999).
It was not until 10 years after Gerald Rubin had observed hybridization at the telomeres with cDm356 that a telomere-specific retrotransposon, named HeT-A, was described as an element capable of healing broken or receding chromosome ends (Traverse and Pardue 1988; Biessmann et al. 1990, 1992a, b). The second element, TART, was discovered in the analysis of a native Drosophila telomere (Levis et al. 1993). The TART element has two open reading frames (ORF1 and ORF2), typical of non-LTR retrotransposons; ORF1 encodes a putative RNA-binding protein and ORF2 encodes a protein with domains related to both reverse transcriptase (RT) and endonuclease (Sheen and Levis 1994). However, HeT-A is an atypical element because it lacks ORF2, so the RT for its transposition is produced in trans from a yet-unknown source. This type of nonautonomous element has been previously described in several species. For example, half-elements derived from LINE elements, by deletion of its ORF2, have arisen in both the rat and mouse lineages (Smit 1999; Rat Genome Sequencing Project Consortium 2004). And, it has been shown that LINE-encoded proteins can act in trans to promote retrotransposition of half-LINE elements (Wei et al. 2001). In Drosophila telomeric arrays, HeT-A and TART are intermingled and frequently truncated at the 5′ end (Abad et al. 2004b). This could partially explain why it has been so difficult to clone and characterize complete copies of these elements. The HeT-A and TART elements never appear in euchromatin, but they are found in centromeric and pericentromeric heterochromatin (Traverse and Pardue 1988; Danilevskaya et al. 1993; Levis et al. 1993; Losada et al. 1997, 1999; Agudo et al. 1999; Abad et al. 2004a). Recently, the third and less abundant telomere-specific retrotransposon, TAHRE, was discovered during the sequence analysis of the telomeres of the y; cn bw sp strain of Drosophila melanogaster (Abad et al. 2004b, c). The 5′UTR, ORF1, and 3′UTR of TAHRE are very similar to the corresponding sequences of HeT-A, and its ORF2 encodes a RT that has some similarity to the RT of TART (Abad et al. 2004c). The structural and phylogenetic analyses of HeT-A, TAHRE, and TART imply that HeT-A derived from ancestral TAHRE elements (Abad et al. 2004c). However, Pardue and collaborators favor the hypothesis that HeT-A derived from telomerase-encoding sequences (Pardue et al. 1996, 2005; Pardue and DeBaryshe 1999, 2002, 2003).
The identification of TART and HeT-A elements in Drosophila yakuba and Drosophila virilis has shown that these elements have been performing the cellular function of telomere maintenance during more than 45 million yr (Danilevskaya et al. 1998b; Casacuberta and Pardue 2002, 2003a, b).
The molecular characterization of Drosophila telomeres has been so difficult, in part, because of the intrinsic difficulty in isolating large DNA clones containing chromosome ends (Adams et al. 2000; Celniker et al. 2002; Hoskins et al. 2002). But, recently, the use of sheared DNA libraries has allowed the cloning of these chromosomal regions from D. melanogaster (Abad et al. 2004b) as well as from other species (Osoegawa et al. 2006).
In this report we have used a phylogenetic analysis of Drosophila telomeric DNA to demonstrate that multiple autonomous and nonautonomous telomere-specific retrotransposons have diverged from the common ancestor that was recruited to perform the cellular function of telomere maintenance. The novel vision of the telomeres in Drosophila, presented in this work, could have never been achieved without the sequencing and the comparative analysis of the genomes of the 12 Drosophila species.
Results
TAHRE elements in the D. melanogaster species subgroup
To investigate the rate and nature of the evolution of Drosophila telomeres, it is essential to analyze telomere sequences from species that are at various levels of divergence. Thus, we initiated PCR (see Methods) and in silico surveys in the sibling species of D. melanogaster and in more distant species. To identify TAHRE homologs in silico, we performed TBLASTN searches using the ORF2 of Dmel\TAHRE as query for the genomic databases. Subsequent to the in silico survey, we have principally used scaffolds in which the telomeric retrotransposons (TRs) appear in the typical telomeric head-to-tail arrays with no interspersed nontelomeric elements. Using this approach we have identified scaffolds in which a subtelomeric minisatellite (named telomere-associated sequences or TAS) often appears between the most proximal telomeric retrotransposon in the array and the most distal nonrepetitive sequences. In these cases we identified the putative telomere from which they derived, validating our approach. As the success of our analyses depends on the coverage and assembly quality, the number and nature of telomeric contigs we have obtained vary among the sequenced species (Figs. 1B, 2).
Figure 1.
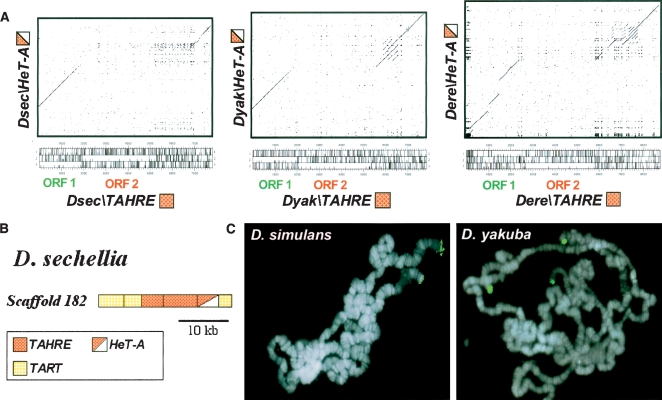
TAHRE elements in the D. melanogaster species subgroup. (A) Dot-matrix comparisons of the nucleotide sequence of each TAHRE (Dsec\TAHRE scaffold 182, 15703-7717; Dyak\TAHRE and Dere\TAHRE are listed in Supplemental Fig. S12) with its corresponding HeT-A (Dsec\HeT-A scaffold 182, 7716-2747; Dyak\HeT-A AF043258; Dere\HeT-A scaffold 4836, 38316–32346). The three-frame ORF maps of TAHRE elements are indicated (the tall tick marks correspond to stop codons; the short tick marks correspond to methionines; the ORFs are the long stretches without tall tick marks). (B) Diagram of scaffold 182 from D. sechellia. The color code for the TRs is indicated in the box below. (C) FISH of TAHRE-ORF2 probes to polytene chromosomes of D. simulans and D. yakuba. Hybridization signals are shown in green on the chromosomes counterstained with DAPI (blue).
Figure 2.
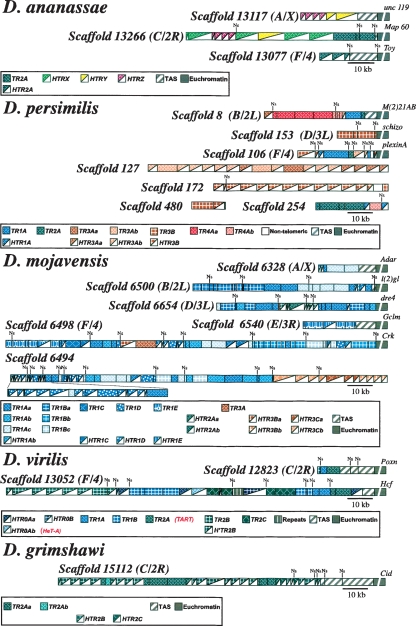
Diagrams of scaffolds containing head-to-tail arrays of telomeric retrotransposons from D. ananassae, D. persimilis, D. mojavensis, D. virilis, and D. grimshawi. The putative origin of each scaffold appears between brackets. The color code for TRs, HTRs, TAS, and euchromatin is indicated in boxes. In the euchromatin regions a distal gene is indicated. Ns indicates the presence of unsequenced regions.
In the Drosophila sechellia genome we found a scaffold that contains a typical tandem array of two Dsec\TART, two Dsec\TAHRE, one Dsec\HeT-A, and one Dsec\TART (Fig. 1B). However, in D. yakuba and Drosophila erecta there were no single scaffolds containing long TAHRE sequences, so we have constructed representative TAHREs by connecting scaffolds that had overlapping regions that were >97% identical (see Methods). Dot-matrix comparisons of each TAHRE with its corresponding HeT-A clearly show that the HeT-A elements are TAHREs without their ORF2 (Fig. 1A). To determine the chromosomal location and ascertain whether these elements are found at telomeres, we performed fluorescence in situ hybridization using TAHRE-ORF2 probes to polytene chromosomes of Drosophila simulans and D. yakuba. The results showed the presence of TAHRE sequences at two telomeres of D. simulans and at three of D. yakuba (Fig. 1C). These elements are not found in all telomeres, similar to the situation in D. melanogaster (Abad et al. 2004c), but they seem to be present across the melanogaster subgroup. As the PCR analysis has obvious limitations (see Methods), and the sequences of the 12 species are at their first assembly stage (Comparative Analysis Freeze 1), it is not surprising that a full-length TAHRE has only been found in D. melanogaster, in which identified telomeric clones have been sequenced (Abad et al. 2004c).
Recurrent evolution of HTRs from TRs across the Drosophila genus
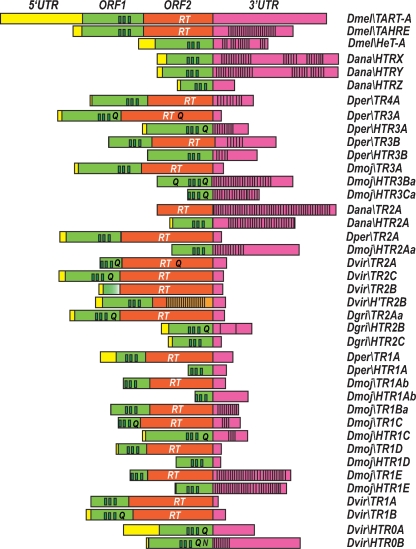
During our analyses in more distant species, we have found up to 37 phylogenetically distinct telomeric elements, of which 19 were half TRs (HTRs) because they had lost their ORF2. The names of the new elements were given after performing phylogenetic analysis. In order to help the reading and comprehension, a color code based on the phylogenetic relationships was added to the graphical representation of the elements. Thus, elements from different species that share the same color in Figure 2 are closely related to each other since they appear in the same cluster in the trees (see Figs. 5, 6, below). In addition to the 37 previously mentioned elements, we have also detected several subfamilies when the differences between the elements were low. The elements belonging to subfamilies were designated by adding a lower case letter to the name of the family and appear in the figures with the same color but different intensity. Given the high number of new telomeric retrotransposons, only a brief overview of the elements is given for each species.
Figure 5.
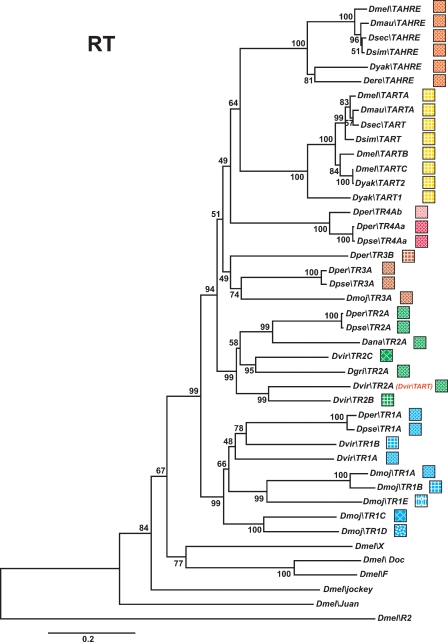
Phylogenetic relationships of TRs based on their RT domains. The tree was inferred using the neighbor-joining method. Bootstrap values are given as percentage numbers. The color code for TRs is indicated. TART and TAHRE have not been renamed to keep their historical names.
Figure 6.
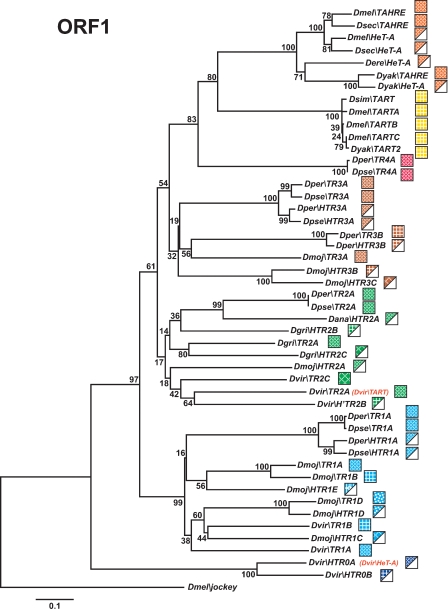
Phylogenetic relationships of TRs and HTRs based on their ORF1 domains. The tree was inferred using the neighbor-joining method. Bootstrap values are given as percentage numbers. The color code for TRs and HTRs is indicated. TART, TAHRE, and HeT-A have not been renamed to keep their historical names.
Drosophila ananassae, a species in the melanogaster group, has the second largest genome of the 12 sequenced species, around 231 Mb. After the analysis of its genome, we have found that three scaffolds end in arrays of TRs and HTRs (Fig. 2). In these telomeric arrays, the element Dana\TR2A and its corresponding deleted element Dana\HTR2A have a characteristic long 3′UTR made of complex repeats (see dot-matrix comparison between them in Fig. 3).
Figure 3.
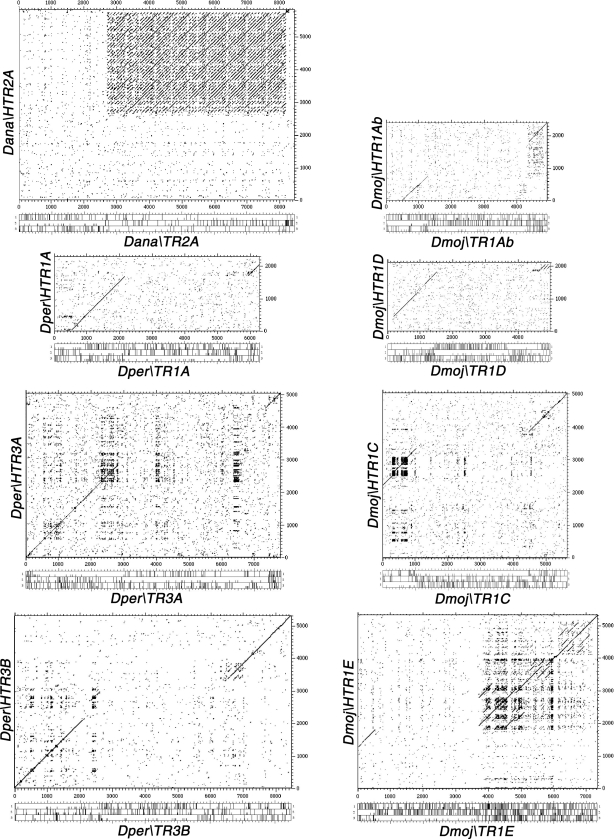
Dot-matrix analysis of TRs with HTRs. Dot-matrix comparisons of the nucleotide sequence of Dana\TR2A: Dana\HTR2A, Dper\TR1A: Dper\HTR1A, Dper\TR3A: Dper\HTR3A, Dper\TR3B: Dper\HTR3B, Dmoj\TR1Ab: Dmoj\HTR1Ab, Dmoj\TR1D: Dmoj\HTR1D, Dmoj\TR1C: Dmoj\HTR1C, Dmoj\TR1E: Dmoj\HTR1E. The three-frame ORF maps are indicated.
The N-terminal part of the ORF1 of retroelements evolved quickly due to base substitutions and small deletions. However, in D. ananassae there are three HTRs (Dana\HTRX, Dana\HTRY, and Dana\HTRZ; Supplemental Fig. S1a) with an additional large deletion that removed the conserved motif called major homology region (MHR) (Supplemental Fig. S1b). Moreover, Dana\HTRX and Dana\HTRY have long 3′UTRs with several repeats (Supplemental Fig. S1a). Apart from those telomeric scaffolds, we found other scaffolds containing decayed TRs interspersed with other transposable elements. Interestingly, Jennifer Kirkland, John Belote, and Robert Levis had previously isolated a D. ananassae clone having those TRs (DQ114943) (Kirkland 2005). They hypothesized that it was probably derived from centromeric heterochromatin after observing that the elements were decayed and that the clone did not give any telomeric signal when hybridized to polytene chromosomes (R. Levis, pers. comm.).
The genome sequence of Drosophila persimilis, a sister species of Drosophila pseudoobscura, provides an opportunity to study whether a recent speciation, in the obscura group, has affected the structure of telomeric elements. Although, the Drosophila persimilis genome has only been sequenced at the 3× level, we have been able to recognize telomeric scaffolds. Thus, we found three scaffolds containing arrays of TRs and HTRs that correspond to chromosome ends (Fig. 2). We have also found four additional scaffolds that are not linked to telomeres. In these scaffolds we identified five TRs (Dper\TR1A, Dper\TR2A, Dper\TR3A, Dper\TR3B, and Dper\TR4A) and three HTRs (Dper\HTR1A, Dper\HTR3A, and Dper\HTR3B). The dot-matrix comparisons between these TRs and their corresponding HTRs are shown in Figure 3. Dper\TR1A, Dper\TR2A, and Dper\TR3A have short 3′UTRs, Dper\TR3B has a long 3′UTR, and Dper\TR4A has a large repeated region in its 3′UTR (Fig. 4; Supplemental Figs. S2–S4). The ORF1 of the Dper\TR3A element contains dispersed short runs of polyglutamines (poly Qs) after the three zinc knuckle domains, whereas ORF2 has a nonhomogenous long run of poly Qs after the RT domain (Fig. 4).
Figure 4.
Diagrams of the structure of TRs and HTRs. Diagrams are approximately to scale. The 5′UTR regions appear in yellow, the ORF1 in green, the ORF2 in orange, and the 3′UTR in magenta. The three zinc knuckles domain in ORF1 and the RT domain in ORF2 are indicated. The polyglutamines appear as a single “Q” and the polyasparagines as a single “N.” The presence of repeats in the 3′UTR regions is also indicated.
As expected for two species whose separation has occurred recently, the telomeric elements found in D. persimilis are very similar (∼98% of nucleotide identity) to those in D. pseudoobscura (data not shown).
Drosophila willistoni belongs to the willistoni group and has the largest genome of the 12 sequenced species (∼237 Mb). Unfortunately, we could not identify any scaffolds containing TR sequences that, by our criteria, derive from a telomere. Nevertheless, we found small scaffolds that encoded partial TRs phylogenetically related to TR2 elements.
Drosophila mojavensis, a cactophilic species in the repleta group, is a good model of incipient speciation. Among the new sequenced species, the D. mojavensis genome assembly appears to be the most complete (Gilbert 2006). As a consequence, we found five scaffolds linked to telomeres and one additional very long scaffold not linked (Fig. 2). We have identified up to six TRs (Dmoj\TR1A, Dmoj\TR1B, Dmoj\TR1C, Dmoj\TR1D, Dmoj\TR1E, and Dmoj\TR3A) and, among the HTRs found, four (Dmoj\HTR1Ab, Dmoj\HTR1B, Dmoj\HTR1C, and Dmoj\HTR1D) have their corresponding TR (see dot-matrix comparison between them in Fig. 3). We have also found three additional HTRs whose TRs counterparts were not detected (Dmoj\HTR2A, Dmoj\HTR3B, and Dmoj\HTR3C).
The TRs and HTRs in D. mojavensis display a number of different features. Five of the six TRs found have a short 3′UTR (Dmoj\TR1A, Dmoj\TR1B, Dmoj\TR1C, Dmoj\TR1D, and Dmoj\TR3A) and only Dmoj\TR1E has a long 3′UTR, with three repeated regions along it (Fig. 4; Supplemental Figs. S4–S7). The ORF1 of Dmoj\TR1C has two long runs of poly Qs following the zinc knuckles domain (Fig. 4). Dmoj\HTR2Aa has a long 3′UTR with two duplications along it (Fig. 4; Supplemental Fig. S4). Dmoj\HTR3Ba has a long 3′UTR with a large repeated region covering the majority of the 3′UTR, and its ORF has several tracts of poly Qs before and after the zinc knuckles domain (Fig. 4; Supplemental Fig. S7). Dmoj\HTR3Ca has two large repeated regions covering almost the entire length of its 3′UTR, and its ORF has two tracts of poly Qs after the zinc knuckles domain (Fig. 4; Supplemental Fig. S7). With this data on hand, it would be interesting to analyze whether diverging populations of D. mojavensis show clear differences in the structure of the telomeres.
The genome size of D. virilis is about twice as large as that of D. melanogaster, but this seems to be due to an increase in the amount of simple and complex satellite DNAs. In D. virilis, we have found two scaffolds linked to telomeres (Fig. 2). In both cases, between the proximal telomeric element and the euchromatic sequences there are subtelomeric repeats of 370 bp, corresponding to the TAS of D. virilis. Interestingly, these are the repeats that Biessmann et al. (2000) found at the telomeres and at internal euchromatic regions. These telomeres have five autonomous elements: Dvir\TR1A, Dvir\TR1B, Dvir\TR2A (previously called Dvir\TART by Casacuberta and Pardue 2003a), Dvir\TR2B, and Dvir\TR2C. They also have three half telomeric-retrotransposons: Dvir\HTR0A (previously called Dvir\HeT-A by Casacuberta and Pardue 2003b), Dvir\HTR0B, and Dvir\H’TR2B. The TRs and HTRs of D. virilis have short and long 3′UTRs, respectively (Fig. 4; Supplemental Fig. S8). The ORF1s of Dvir\TR1B, Dvir\TR2C, and Dvir\HTR0A have short tracts of poly Qs following the three zinc knuckle domains (Fig. 4; Casacuberta and Pardue 2003b). However, the ORF1 of Dvir\TR2A has long runs of poly Q after the zinc knuckles domain (Casacuberta and Pardue 2003a) and the ORF of Dvir\HTR0B has long runs of poly Qs and a long tract of polyasparagines (poly Ns) following the zinc knuckles domain (Fig. 4). In D. virilis, only the ORF2 of Dvir\TR2A has long runs of poly Qs after the RT domain (Fig. 4; Casacuberta and Pardue 2003a).
The Hawaiian Drosophila lineage contains >1000 species. Drosophila grimshawi, the sequenced picture wing species, is representative of a lineage that began to rapidly diversify 26 million yr ago. In D. grimshawi, we identified a single telomeric scaffold (Fig. 2). This scaffold contains arrays made of one TR (Dgri\TR2A) and two HTRs (Dgri\HTR2B and Dgri\HTR2C). The Dgri\TR2A elements have tracts of poly Qs after the three zinc knuckles of the ORF1 and a short 3′UTR (Fig. 4; Supplemental Fig. S9). The Dgri\HTR2B has long tracts of poly Qs after the zinc knuckles domain and a large duplication in its 3′UTR (Fig. 4; Supplemental Fig. S9). The Dgri\HTR2C does not contain extended glutamine repeats and has a short 3′UTR (Fig. 4; Supplemental Fig. 9).
Since we have analyzed whole-genome shotgun draft sequence that contains many gaps within telomeric regions, we cannot exclude the possibility that corresponding TRs may exist in the genomes where only orphan HTRs have been detected. Nor can we exclude the possibility that additional elements and even additional families may exist in all of the sequenced species. Future analyses, when further sequencing has been done, are likely to find more telomere-specific elements than the ones described in this article.
Phylogenetic relationships among Drosophila telomeric retrotransposons
Normally, phylogenetic analyses of non-LTR retrotransposons are restricted to the RT domain, the only domain common to all elements. Therefore, we have initially constructed the phylogenetic relationships between the TRs based on their entire RT domains (for alignments see Supplemental Fig. S10). The limits of the RT domains were defined according to Malik et al. (1999). The phylogenetic tree identifies multiple families of TRs that belong, as expected, to the jockey clade (Fig. 5).
In Drosophila, the C-terminal half of ORF1 of TRs and the ORF of HTRs contains three conserved domains: the MHR, the zinc knuckles (also called CCHC), and the leucine zipper-like region (Rashkova et al. 2003). To verify the TRs phylogeny based on the RT domain, we have also analyzed the phylogenetic relationships between TRs and HTRs using these ORF1 domains (for alignments see Supplemental Fig. S11). In this analysis, the ORF1s from the D. ananassae HTRs were omitted, as they lack MHR domains. Importantly, although the ORF1 tree has less resolution than the RT tree, because the ORF1 region is smaller and less conserved than the RT domain, the phylogeny of the TR ORF1s is in agreement with the RT phylogeny (Fig. 6). Moreover, it is in this type of analysis that the evolutionary relationships between TRs and their corresponding HTRs appear more clearly. Our finding of multiple pairs (Fig. 6) of TRs and HTRs is strong evidence that HTRs, like HeT-A, have derived from ancestral TRs, rather than from telomerase.
The phylogenetic relationships among the telomere-specific elements are congruent with the phylogeny of the species, which suggests vertical transmission of these elements from a common ancestor recruited to perform the cellular function of telomere maintenance.
Discussion
Drosophila telomeric sequences do not behave as regular heterochromatin sequences. While in centromeric heterochromatin there are multiple amplifications, deletions and insertions of transposable elements that produce the decay of the elements present in it, in terminal retrotransposon arrays amplifications and internal deletions still occur, but never interrupt the reading frames of the telomeric elements. In noncoding regions, the amplification events principally occur in the 3′UTRs, and it is possible that multiple amplifications events occur in the same 3′UTR (Fig. 4; Supplemental figures). In coding regions, amplifications in ORF1 and/or ORF2 can take place, where repeats of the triplets CAG and CAA encode for tracts of polyglutamines (or AAC for polyasparagines). These polyglutamines or polyasparagines are not part of known functional domains and they have been suggested to provide a molecular basis for fast adaptation to environmental changes (Trifonov 2004). As the glutamine-rich and asparagine-rich regions form polar zippers, Perutz et al. (2002) proposed that they mediate interaction between different proteins. It is tempting to speculate that the initial expansion of triplets coding for poly Qs/Ns could have been selected because of the improvement in the ability to interact with other telomeric proteins. However, since extremely long expansions cause the formation of protein aggregates, the elimination of these tracts by unequal crossing-over is expected to happen over time. This could explain why some elements have poly Qs and most of them do not.
In telomere-specific elements it is normal to find deletions of varied sizes in different upstream regions with respect to the zinc knuckles domains of the ORF1. However, the most outstanding event that occurs in telomeric heterochromatin is a recurrent loss of most of the TRs’ ORF2, giving rise to HTR elements. This finding is consistent with the suggested origin of HeT-A by deletion of the ORF2 of an ancestral TAHRE (Abad et al. 2004c).
The recent discoveries of the telomerase reverse transcriptase (TERT) of Bombyx mori (Lepidoptera), Tribolium castaneum (Coleoptera), and Apis mellifera (Hymenoptera) has confirmed the absence of telomerase orthologs in the available Drosophila and Anopheles genomes (Osanai et al. 2006; Robertson and Gordon 2006). This is consistent with the absence of TTAGG telomeric repeats in flies and mosquitoes. The phylogenetic relationships using the core RT domain of the three insect TERTs showed, as expected, that the insect telomerases cluster together, and that A. mellifera appears basal to T. castaneum (Robertson and Gordon 2006) in the same way that the Hymenoptera is basal to the Coleoptera in the Holometabola (Honey Bee Genome Sequencing Consortium 2006). These insect TERTs lack the N-terminal domain implicated in processivity in others telomerases, and this feature could explain why telomerase activity is low in the bee and virtually undetectable in the silkworm.
The unexpected complexity of the Drosophila telomeric sequences seems to challenge the requirement for the conventional telomerase-synthesized repeats with G-tracts. But, the 3′ noncoding DNA of Dmel\HeT-A elements have the same strand bias: the strand running 5′–3′ toward the end of the chromosome is G+T rich (Danilevskaya et al. 1998a). Moreover, the 3′ UTR of Dmel\HeT-A elements contains sequences with the propensity to form G-quadruplex DNA structures (Abad and Villasante 1999). The RNA templates of TRs and HTRs found in the genus Drosophila have a C+A bias not restricted to the noncoding regions. Interestingly, the RNA template of other members of the jockey clade such as, Dmel\Doc, Dmel\F, Dmel\Juan, and Dmel\X also has a C+A bias. Therefore, in Drosophila, the universal telomere strand bias seems to be maintained by repeated reverse transcription of telomere-specific C+A biased RNA templates primed by a 3′OH on the end of the chromosomal DNA.
In D. melanogaster, as in any eukaryote, recombination-based mechanisms maintain chromosome termini normally (Kahn et al. 2000). Therefore, it is plausible that a progressive telomerase inactivation (as seems to happen now in B. mori) would have led to its loss if another telomerase-independent mechanism would have been able to replace telomere function progressively. Interestingly, to compensate for the extremely low activity of the T. castaneum and B. mori telomerase’s, these species seem to have co-opted non-LTR retrotransposons, which belong to the R1 clade, for the maintenance of telomeres. (Fujiwara et al. 2005; Osanai et al. 2006). A scenario for the origin of Drosophila telomeres has been recently proposed (Abad et al. 2004c): once the telomerase was lost, and the recombination-based mechanism to maintain telomeres was acting normally, telomere erosion could activate the mobilization of C+A biased non-LTR retroelements via a DNA-damage signaling pathway (Rudin and Thompson 2001) and their addition to the chromosome ends would restore telomere function (Yamamoto et al. 2003). In agreement with this model, Morrish et al. (2007) have detected endonuclease-independent LINE-1 retrotransposition at dysfunctional telomeres. Finally, the extensive and rapid evolution of Drosophila telomere-specific elements could give a wide range of situations for evolutionary innovations.
Methods
Drosophila strains and DNA preparation
Four Drosophila species were used for experimental studies: D. simulans (14021–0251.176 from the Tucson Stock Center [TSC]), D. mauritiana (14021–0241.05, TSC), D. sechellia (14021–0248.08, TSC), and D. yakuba (14021–0261.00, TSC). Genomic DNA was obtained from adult flies as previously described (Pirrotta et al. 1983).
PCR amplification and DNA cloning
The sequences of Dmel\TAHRE (AJ542581), Dmel\TART (AJ566116), and Dmel\HeT-A (U06920) were used to design primers for the PCR amplification of specific regions of telomeric retrotransposons. The sequence of these primers appears in Supplemental Table S1. Because of the great similarity of HeT-A and TAHRE, with HeT-A being much more abundant, we have not managed to PCR-amplify whole copies of TAHRE from genomic DNA. In all of the attempts we made, HeT-A was the only amplified product. To overcome these difficulties, we designed two sets of primers (Supplemental Table S1) to amplify two overlapping regions of TAHRE: one containing the ORF1–ORF2 junction and the other encompassing the ORF2–3′UTR junction, thus covering the entire ORF2 of TAHRE. Using this approach, overlapping fragments from D. simulans, D. mauritiana, and D. sechellia were isolated. The high degree of identity of the nucleotide sequence showed that the reconstructed elements encoded TAHRE homologs. However, while Dsim\TAHRE (AM040252) and Dsec\TAHRE (AM040246) sequences show nondecayed ORFs, suggesting a telomere origin, all cloned Dmau\TAHRE (AM040237, AM040238, and AM040239) and some of the Dsec\TAHRE (AM040247, AM040248, and AM040249) and Dsim\TAHRE (AM040253) sequences have the typical deletions of the decayed telomeric elements found in centromeric and pericentromeric heterochromatin (Losada et al. 1997, 1999; Agudo et al. 1999; Abad et al. 2004a). When we tried the same approach in more distant species, we were unable to get PCR amplification using any set of primers. The amplified DNA was cloned in vector pGEM-T (Promega). DNA fragments containing the TAHRE ORF1–ORF2 junction were PCR amplified using genomic DNA form D. mauritiana, D. sechellia, and D. simulans and the pair of primers TH1 from the ORF1 and Tsc1.4 from ORF2. The amplified DNAs were cloned, sequenced, and named pThmau3.1 (AM040237), pThsch3.6 (AM040246), and pThsim3.6 (AM040252), respectively. In D. simulans a clone containing HeT-A sequences was also obtained (pHeTsim1.9; AM040254). The PCR amplifications of fragments containing the TAHRE ORF2–3′UTR junction were performed using the pair of primers TH5 from the ORF2 and TH9 from the 3′UTR. The cloned DNAs were sequenced and named pThmau2.1 (AM040238) and pThmau1.7 (AM040239) in D. mauritiana; pThsch2.1.0 (AM040247), pThsch2.1.1 (AM040248), and pThsch1.8 (AM040249) in D. sechellia, and pThsim2.1 (AM040253) in D. simulans. In D. yakuba, we have reconstructed the TAHRE homolog using two sequences encompassing the ORF1–ORF2 junction (AAEU02006918 and AAEU02009565), one with a portion of the ORF2 (AAEU02013304), and another one that contains a big region of the ORF2 and the whole 3′UTR (AAEU2000405). To confirm that the reconstructed ORF1–ORF2 junction truly exists in the genome, we PCR amplified a DNA fragment encompassing the junction using the pair of primers Thy1 and Thy2 (see Supplemental Table S1), and the PCR product was cloned (pThyak1.4) and sequenced (AM161543). Similarly, in D. erecta, we have found that scaffolds 3102 and 1433 contain an ORF1–ORF2 junction and an ORF2–3′UTR junction of a TAHRE homolog, respectively. The reconstructed sequences of Dyak\TAHRE and Dere\TAHRE are listed in Supplemental Figure S12. The unavailable TART ORF2 sequences from D. mauritiana, D. sechellia, and D. simulans were amplified with the primers T14 (AJ566116; 11235–11254) and T28 (AJ566116; 6997–7016), cloned, and sequenced: pTmau4.3 (AM040241), pTsch4.3 (AM040251), and pTsim4.3 (AM040256). The unavailable HeT-A sequences from different species were PCR amplified using the following pairs of primers: TH1 and Hs6 (AM040254, 1226–1246) for D. mauritiana, TH1 and Hs4 (AM040254, 1199–1216) for D. sechellia, and TH9 and Hs4 for D. simulans. The cloned DNA fragments were sequenced and named pHeTmau1.2 (AM040240), pHeTsch1.2 (AM040250), and pHeTsim2.7 (AM040255), respectively.
To perform standard PCR amplification, Taq DNA polymerase and reaction buffer from Promega were used in a Perkin-Elmer Thermal Cycler. The 50-μL reaction mix contained 2 mM MgCl2, 5 U of Taq polymerase, 100 μm of dNTPs, 500 ng of genomic DNA, and 30 nmol of each primer. The amplification program was 30 cycles of 1 min, 20 sec at 94°C, 1 min at 50°C, and 2 min, 30 sec at 72°C, increasing the extension step of the last cycle to 7 min. To perform long PCR, the Expand Long Template PCR System kit from Roche Diagnostics was used. The 50-μL reaction mix contained 1.75 mM MgCl2, 3.75 U of Taq DNA polymerase, 350 μm of dNTPs, 500 ng of genomic DNA, and 300 nmol of each primer. The amplification program was 30 cycles of 1 min, 20 sec at 94°C, 4 min at 52°C, and 4 min 30 sec at 68°C, increasing the extension step of the last cycle to 8 min.
DNA sequencing, sequence analyses, and annotation
The sequencing was done using the primers listed in Supplemental Table 1. All sequencing was performed using big dye-termination reagents and ABI 3700 automated sequencers. Sequence analyses were performed online against the DroSpeGe comparative genome database, the FlyBase database, and the database at the National Center for Biotechnology Information. The TBLASTN program (Altschul et al. 1990) was used for these searches. The major problem during the annotation of TRs and HTRs inside the positive scaffolds was to identify the boundaries of the elements. This task was performed manually using principally dot-matrix comparisons. The name of the TR or HTR, its length, and the coordinates within its corresponding scaffold appear in Supplemental Table S2. Normally, the translated sequences revealed intact ORFs, but in some cases, ORFs had to be manually reconstructed due to frameshifts in the original sequence. Also, in some cases the ORFs had to be extended upstream of the first encountered methionine. Multiple-sequence alignments were performed with ClustalX (Thompson et al. 1997), followed by manual adjustments of gaps. Phylogenetic trees were generated with the MEGA 2.1 program (Kumar et al. 2001) using a neighbor-joining method with bootstrap statistics (1000) after removal of the gaps. Dot-matrix analyses were performed with the COMPARE and DOTPLOT programs from the CGC package (University of Wisconsin, Madison).
Fluorescence in situ hybridization
The Dsim\TAHRE probe used for FISH was pThsim2.1 (AM040253). The Dyak\TAHRE probe used for FISH was an ORF2 fragment obtained by PCR using pThyak1.4 as the template and the pair of primers: Thy2 and Ths11: 5′-ACAATATAAATCCG CAGCC-3′. Probes were labeled by nick translation using Biotin-16-dUTP (Roche Diagnostics). FISH experiments on polytene chromosomes were carried out under high-stringency conditions, as described in Pimpinelli et al. (2000). Post-hybridization washing was in 0.1× SSC at 60°C. For probe detection, a 3.3-μg/mL FITC-conjugated avidin (DCS grade, Vector Laboratories) diluted in SBT (1% BSA, 0.1% Tween-20, 4× SSC) solution was used. Chromosomes were counterstained with 4',6-diamino-2-phenylindole (DAPI). Digital images were obtained using a Zeiss Axiovert 200 microscope equipped with a cooled CCD camera. The fluorescent signals from the FITC and the DAPI were recorded separately as grayscale digital images and then pseudo-colored and merged using the Adobe Photoshop software.
Acknowledgments
We thank R. Levis, R. de Frutos, M.J. Martínez-Sebastián, J. Dopazo, H. Dopazo, and E. Torroja for critical comments and suggestions. We also thank the DroSpeGe comparative genome database and the FlyBase database for access to the 12 Drosophila draft genomes assemblies before publication. The work was supported by a grant from Ministerio de Educación y Ciencia (BFU2005-07690-C02-01) to A.V., an institutional grant from Fundación Ramón Areces to the CBMSO, and by a subcontract to Lawrence Berkeley National Laboratory (PI, S.C.) from UC Berkeley (HG000750, PI, G. Rubin) carried out under Departent of Energy Contract DE-AC0376SF00098, University of California, Berkeley.
Footnotes
[Supplemental material is available online at www.genome.org. The sequence data from this study have been submitted to GenBank under accession nos. AM161543, AM040250-6, AM040240-1, AM040246-9, and AM040237-9.]
Article published online before print. Article and publication date are at http://www.genome.org/cgi/doi/10.1101/gr.6365107
References
- Abad J.P., Villasante A., Villasante A. The 3′ non-coding region of the Drosophila melanogaster HeT-A telomeric retrotransposon contains sequences with propensity to form G-quadruplex DNA. FEBS Lett. 1999;453:59–62. doi: 10.1016/s0014-5793(99)00695-x. [DOI] [PubMed] [Google Scholar]
- Abad J.P., de Pablos B., Agudo M., Molina I., Giovinnazo G., Martín-Gallardo A., Villasante A., de Pablos B., Agudo M., Molina I., Giovinnazo G., Martín-Gallardo A., Villasante A., Agudo M., Molina I., Giovinnazo G., Martín-Gallardo A., Villasante A., Molina I., Giovinnazo G., Martín-Gallardo A., Villasante A., Giovinnazo G., Martín-Gallardo A., Villasante A., Martín-Gallardo A., Villasante A., Villasante A. Genomic and cytological analysis of the Y chromosome of Drosophila melanogaster: Telomere-derived sequences at internal regions. Chromosoma. 2004a;113:295–304. doi: 10.1007/s00412-004-0318-0. [DOI] [PubMed] [Google Scholar]
- Abad J.P., De Pablos B., Osoegawa K., De Jong P.J., Martin-Gallardo A., Villasante A., De Pablos B., Osoegawa K., De Jong P.J., Martin-Gallardo A., Villasante A., Osoegawa K., De Jong P.J., Martin-Gallardo A., Villasante A., De Jong P.J., Martin-Gallardo A., Villasante A., Martin-Gallardo A., Villasante A., Villasante A. Genomic analysis of Drosophila melanogaster telomeres: Full-length copies of HeT-A and TART elements at telomeres. Mol. Biol. Evol. 2004b;21:1613–1619. doi: 10.1093/molbev/msh174. [DOI] [PubMed] [Google Scholar]
- Abad J.P., De Pablos B., Osoegawa K., De Jong P.J., Martin-Gallardo A., Villasante A., De Pablos B., Osoegawa K., De Jong P.J., Martin-Gallardo A., Villasante A., Osoegawa K., De Jong P.J., Martin-Gallardo A., Villasante A., De Jong P.J., Martin-Gallardo A., Villasante A., Martin-Gallardo A., Villasante A., Villasante A. TAHRE, a novel telomeric retrotransposon from Drosophila melanogaster, reveals the origin of Drosophila telomeres. Mol. Biol. Evol. 2004c;21:1620–1624. doi: 10.1093/molbev/msh180. [DOI] [PubMed] [Google Scholar]
- Adams M.D., Celniker S.E., Holt R.A., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Celniker S.E., Holt R.A., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Holt R.A., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., Li P.W., Hoskins R.A., Galle R.F., Hoskins R.A., Galle R.F., Galle R.F., et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Agudo M., Losada A., Abad J.P., Pimpinelli S., Ripoll P., Villasante A., Losada A., Abad J.P., Pimpinelli S., Ripoll P., Villasante A., Abad J.P., Pimpinelli S., Ripoll P., Villasante A., Pimpinelli S., Ripoll P., Villasante A., Ripoll P., Villasante A., Villasante A. Centromeres from telomeres? The centromeric region of the Y chromosome of Drosophila melanogaster contains a tandem array of telomeric HeT-A- and TART-related sequences. Nucleic Acids Res. 1999;27:3318–3324. doi: 10.1093/nar/27.16.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J., Gish W., Miller W., Myers E.W., Lipman D.J., Miller W., Myers E.W., Lipman D.J., Myers E.W., Lipman D.J., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Mason J.M., Ferry K., d'Hulst M., Valgeirsdottir K., Traverse K.L., Pardue M.-L., Mason J.M., Ferry K., d'Hulst M., Valgeirsdottir K., Traverse K.L., Pardue M.-L., Ferry K., d'Hulst M., Valgeirsdottir K., Traverse K.L., Pardue M.-L., d'Hulst M., Valgeirsdottir K., Traverse K.L., Pardue M.-L., Valgeirsdottir K., Traverse K.L., Pardue M.-L., Traverse K.L., Pardue M.-L., Pardue M.-L. Addition of telomere-associated HeT DNA sequences “heals” broken chromosome ends in Drosophila. Cell. 1990;61:663–673. doi: 10.1016/0092-8674(90)90478-w. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Champion L.E., O'Hair M., Ikenaga K., Kasravi B., Mason J.M., Champion L.E., O'Hair M., Ikenaga K., Kasravi B., Mason J.M., O'Hair M., Ikenaga K., Kasravi B., Mason J.M., Ikenaga K., Kasravi B., Mason J.M., Kasravi B., Mason J.M., Mason J.M. Frequent transpositions of Drosophila melanogaster HeT-A transposable elements to receding chromosome ends. EMBO J. 1992a;11:4459–4469. doi: 10.1002/j.1460-2075.1992.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Valgeirsdottir K., Lofsky A., Chin C., Ginther B., Levis R.W., Pardue M.-L., Valgeirsdottir K., Lofsky A., Chin C., Ginther B., Levis R.W., Pardue M.-L., Lofsky A., Chin C., Ginther B., Levis R.W., Pardue M.-L., Chin C., Ginther B., Levis R.W., Pardue M.-L., Ginther B., Levis R.W., Pardue M.-L., Levis R.W., Pardue M.-L., Pardue M.-L. HeT-A, a transposable element specifically involved in “healing” broken chromosome ends in Drosophila melanogaster. Mol. Cell. Biol. 1992b;12:3910–3918. doi: 10.1128/mcb.12.9.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Zurovcova M., Yao J.G., Lozovskaya E., Walter M.F., Zurovcova M., Yao J.G., Lozovskaya E., Walter M.F., Yao J.G., Lozovskaya E., Walter M.F., Lozovskaya E., Walter M.F., Walter M.F. A telomeric satellite in Drosophila virilis and its sibling species. Chromosoma. 2000;109:372–380. doi: 10.1007/s004120000094. [DOI] [PubMed] [Google Scholar]
- Casacuberta E., Pardue M.-L., Pardue M.-L. Coevolution of the telomeric retrotransposons across Drosophila species. Genetics. 2002;161:1113–1124. doi: 10.1093/genetics/161.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta E., Pardue M.-L., Pardue M.-L. Transposon telomeres are widely distributed in the Drosophila genus: TART elements in the virilis group. Proc. Natl. Acad. Sci. 2003a;100:3363–3368. doi: 10.1073/pnas.0230353100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta E., Pardue M.-L., Pardue M.-L. HeT-A elements in D. virilis: Retrotransposon telomeres are conserved across the Drosophila genus. Proc. Natl. Acad. Sci. 2003b;100:14091–14096. doi: 10.1073/pnas.1936193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S.E., Wheeler D.A., Kronmiller B., Carlson J.W., Halpern A., Patel S., Adams M., Champe M., Dugan S.P., Frise E., Wheeler D.A., Kronmiller B., Carlson J.W., Halpern A., Patel S., Adams M., Champe M., Dugan S.P., Frise E., Kronmiller B., Carlson J.W., Halpern A., Patel S., Adams M., Champe M., Dugan S.P., Frise E., Carlson J.W., Halpern A., Patel S., Adams M., Champe M., Dugan S.P., Frise E., Halpern A., Patel S., Adams M., Champe M., Dugan S.P., Frise E., Patel S., Adams M., Champe M., Dugan S.P., Frise E., Adams M., Champe M., Dugan S.P., Frise E., Champe M., Dugan S.P., Frise E., Dugan S.P., Frise E., Frise E., et al. Finishing a whole-genome shotgun: Release 3 of the Drosophila melanogaster euchromatic genome sequence. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevskaya O., Lofsky A., Kurenova E.V., Pardue M.-L., Lofsky A., Kurenova E.V., Pardue M.-L., Kurenova E.V., Pardue M.-L., Pardue M.-L. The Y chromosome of Drosophila melanogaster contains a distinctive subclass of Het-A-related repeats. Genetics. 1993;134:531–543. doi: 10.1093/genetics/134.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevskaya O.N., Lowenhaupt K., Pardue M.-L., Lowenhaupt K., Pardue M.-L., Pardue M.-L. Conserved subfamilies of the Drosophila HeT-A telomere-specific retrotransposon. Genetics. 1998a;148:233–242. doi: 10.1093/genetics/148.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevskaya O.N., Tan C., Wong J., Alibhai M., Pardue M.-L., Tan C., Wong J., Alibhai M., Pardue M.-L., Wong J., Alibhai M., Pardue M.-L., Alibhai M., Pardue M.-L., Pardue M.-L. Unusual features of the Drosophila melanogaster telomere transposable element HeT-A are conserved in Drosophila yakuba telomere elements. Proc. Natl. Acad. Sci. 1998b;95:3770–3775. doi: 10.1073/pnas.95.7.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Osanai M., Matsumoto T., Kojima K.K., Osanai M., Matsumoto T., Kojima K.K., Matsumoto T., Kojima K.K., Kojima K.K. Telomere specific non-LTR retrotransposons and telomere maintenance in the silkworm, Bombyx mori. Chromosome Res. 2005;13:455–467. doi: 10.1007/s10577-005-0990-9. [DOI] [PubMed] [Google Scholar]
- Gilbert D.G. DroSpeGe: Rapid access database for new Drosophila species genomes. Nucleic Acids Res. 2006;35:D480–D485. doi: 10.1093/nar/gk1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey Bee Genome Sequencing Consortium Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins R.A., Smith C.D., Carlson J., Carvalho B.A., Halpern A., Kaminker J.S., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Smith C.D., Carlson J., Carvalho B.A., Halpern A., Kaminker J.S., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Carlson J., Carvalho B.A., Halpern A., Kaminker J.S., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Carvalho B.A., Halpern A., Kaminker J.S., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Halpern A., Kaminker J.S., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Kaminker J.S., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Kennedy C., Mungall C.J., Sullivan B.A., Sutton G.G., Mungall C.J., Sullivan B.A., Sutton G.G., Sullivan B.A., Sutton G.G., Sutton G.G., et al. Heterochromatic sequences in a Drosophila whole genome shotgun assembly. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn T., Savitsky M., Georgiev P., Savitsky M., Georgiev P., Georgiev P. Attachment of HeT-A sequences to chromosomal termini in Drosophila melanogaster may occur by different mechanisms. Mol. Cell. Biol. 2000;20:7634–7642. doi: 10.1128/mcb.20.20.7634-7642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J. 2005. “Molecular cloning and initial characterization of a TART-like sequence in Drosophila ananassae”. M.S. thesis, University of Syracuse, NY. [Google Scholar]
- Kumar S., Tamura K., Jakobsen I.B., Nei M., Tamura K., Jakobsen I.B., Nei M., Jakobsen I.B., Nei M., Nei M. MEGA2: Molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- Levis R.W., Ganesan R., Houtchens K., Tolar L.A., Sheen F.-M., Ganesan R., Houtchens K., Tolar L.A., Sheen F.-M., Houtchens K., Tolar L.A., Sheen F.-M., Tolar L.A., Sheen F.-M., Sheen F.-M. Transposons in place of telomeric repeats at a Drosophila telomere. Cell. 1993;75:1083–1093. doi: 10.1016/0092-8674(93)90318-k. [DOI] [PubMed] [Google Scholar]
- Losada A., Abad J.P., Villasante A., Abad J.P., Villasante A., Villasante A. Organization of DNA sequences near the centromere of the Drosophila melanogaster Y chromosome. Chromosoma. 1997;106:503–512. doi: 10.1007/s004120050272. [DOI] [PubMed] [Google Scholar]
- Losada A., Agudo M., Abad J.P., Villasante A., Agudo M., Abad J.P., Villasante A., Abad J.P., Villasante A., Villasante A. HeT-A telomere-specific retrotransposons in the centric heterochromatin of Drosophila melanogaster chromosome 3. Mol. Gen. Genet. 1999;262:618–622. doi: 10.1007/s004380051124. [DOI] [PubMed] [Google Scholar]
- Malik H.S., Burke W.D., Eickbush T.H., Burke W.D., Eickbush T.H., Eickbush T.H. The age and evolution of non-LTR retrotransposable elements. Mol. Biol. Evol. 1999;16:793–805. doi: 10.1093/oxfordjournals.molbev.a026164. [DOI] [PubMed] [Google Scholar]
- Morrish T.A., Garcia-Perez J.L., Stamato T.D., Taccioli G.E., Sekiguchi J., Moran J.V., Garcia-Perez J.L., Stamato T.D., Taccioli G.E., Sekiguchi J., Moran J.V., Stamato T.D., Taccioli G.E., Sekiguchi J., Moran J.V., Taccioli G.E., Sekiguchi J., Moran J.V., Sekiguchi J., Moran J.V., Moran J.V. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature. 2007;446:208–212. doi: 10.1038/nature05560. [DOI] [PubMed] [Google Scholar]
- Osanai M., Kojima K.K., Futahashi R., Yaguchi S., Fujiwara H., Kojima K.K., Futahashi R., Yaguchi S., Fujiwara H., Futahashi R., Yaguchi S., Fujiwara H., Yaguchi S., Fujiwara H., Fujiwara H. Identification and characterization of the telomerase reverse transcriptase of Bombyx mori (silkworm) and Tribolium castaneum (flour beetle) Gene. 2006;376:281–289. doi: 10.1016/j.gene.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Osoegawa K., Vessere G.M., Li Shu C., Hoskins R.A., Abad J.P., de Pablos B., Villasante A., de Jong P.J., Vessere G.M., Li Shu C., Hoskins R.A., Abad J.P., de Pablos B., Villasante A., de Jong P.J., Li Shu C., Hoskins R.A., Abad J.P., de Pablos B., Villasante A., de Jong P.J., Hoskins R.A., Abad J.P., de Pablos B., Villasante A., de Jong P.J., Abad J.P., de Pablos B., Villasante A., de Jong P.J., de Pablos B., Villasante A., de Jong P.J., Villasante A., de Jong P.J., de Jong P.J. BAC clones generated from sheared DNA. Genomics. 2006;89:291–299. doi: 10.1016/j.ygeno.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M.-L., DeBaryshe P.G., DeBaryshe P.G. Telomeres telomerase: More than the end of the line. Chromosoma. 1999;108:73–82. doi: 10.1007/s004120050354. [DOI] [PubMed] [Google Scholar]
- Pardue M.-L., DeBaryshe P.G., DeBaryshe P.G. Telomeres transposable elements. In: Craig N.L., editor. Mobile DNA II. ASM Press; Washington, D.C: 2002. pp. 870–887. [Google Scholar]
- Pardue M.-L., DeBaryshe P.G., DeBaryshe P.G. Retrotransposons provide an evolutionary robust non-telomerase mechanism to maintain telomeres. Annu. Rev. Genet. 2003;37:485–511. doi: 10.1146/annurev.genet.38.072902.093115. [DOI] [PubMed] [Google Scholar]
- Pardue M.-L., Danilevskaya O.N., Lowenhaupt K., Slot F., Traverse K.L., Danilevskaya O.N., Lowenhaupt K., Slot F., Traverse K.L., Lowenhaupt K., Slot F., Traverse K.L., Slot F., Traverse K.L., Traverse K.L. Drosophila telomeres: New views on chromosome evolution. Trends Genet. 1996;12:48–52. doi: 10.1016/0168-9525(96)81399-0. [DOI] [PubMed] [Google Scholar]
- Pardue M.-L., Rashkova S., Casacuberta E., DeBaryshe P.G., George J.A., Traverse K.L., Rashkova S., Casacuberta E., DeBaryshe P.G., George J.A., Traverse K.L., Casacuberta E., DeBaryshe P.G., George J.A., Traverse K.L., DeBaryshe P.G., George J.A., Traverse K.L., George J.A., Traverse K.L., Traverse K.L. Two retrotransposons maintain telomeres in Drosophila. Chromosome Res. 2005;13:443–453. doi: 10.1007/s10577-005-0993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M.F., Pope B.J., Owen D., Wanker E.E., Scherzinger E., Pope B.J., Owen D., Wanker E.E., Scherzinger E., Owen D., Wanker E.E., Scherzinger E., Wanker E.E., Scherzinger E., Scherzinger E. Aggregation of proteins with expanded glutamine and alanine repeats of the glutamine-rich and asparagine-rich domains of Sup35 and of the amyloid beta-peptide of amyloid plaques. Proc. Natl. Acad. Sci. 2002;99:5596–5600. doi: 10.1073/pnas.042681599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpinelli S., Bonaccorsi S., Fanti L., Gatti M., Bonaccorsi S., Fanti L., Gatti M., Fanti L., Gatti M., Gatti M. Preparation and analysis of Drosophila mitotic chromosomes. In: Sullivan W., et al., editors. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. pp. 1–24. [Google Scholar]
- Pirrotta V., Hadfield C., Pretorius G.H.J., Hadfield C., Pretorius G.H.J., Pretorius G.H.J. Microdissection and cloning of the white locus and the 3B1–3C2 region of the Drosophila X chromosome. EMBO J. 1983;2:927–934. doi: 10.1002/j.1460-2075.1983.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashkova S., Athanasiadis A., Pardue M.-L., Athanasiadis A., Pardue M.-L., Pardue M.-L. Intracellular targeting of Gag proteins of the Drosophila telomeric retrotransposons. J. Virol. 2003;77:6376–6384. doi: 10.1128/JVI.77.11.6376-6384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat Genome Sequencing Project Consortium Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Robertson H.M., Gordon K.H., Gordon K.H. Canonical TTAGG-repeat telomeres and telomerase in the honey bee, Apis mellifera. Genome Res. 2006;16:1345–1351. doi: 10.1101/gr.5085606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G.M. Isolation of a telomeric DNA sequence from Drosophila melanogaster. Cold Spring Harb. Symp. Quant. Biol. 1978;42:1041–1046. doi: 10.1101/sqb.1978.042.01.104. [DOI] [PubMed] [Google Scholar]
- Rudin C.M., Thompson C.B., Thompson C.B. Transcriptional activation of short interspersed elements by DNA-damaging agents. Genes Chromosomes Cancer. 2001;30:64–71. [PubMed] [Google Scholar]
- Sheen F.-M., Levis R.W., Levis R.W. Transposition of the LINE-like retrotransposon TART to Drosophila chromosome termini. Proc. Natl. Acad. Sci. 1994;91:12510–12514. doi: 10.1073/pnas.91.26.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A.F. Interspersed repeats and other mementos of transposable elements in mammalian genomes. Curr. Opin. Genet. Dev. 1999;9:657–663. doi: 10.1016/s0959-437x(99)00031-3. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G., Plewniak F., Jeanmougin F., Higgins D.G., Jeanmougin F., Higgins D.G., Higgins D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse K.L., Pardue M.-L., Pardue M.-L. A spontaneously opened ring chromosome of Drosophila melanogaster has acquired He-T DNA sequences at both new telomeres. Proc. Natl. Acad. Sci. 1988;85:8116–8120. doi: 10.1073/pnas.85.21.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov E.N. The tuning function of tandemly repeating sequences: A molecular device for fast adaptation. In: Wasser S.P., editor. Evolutionary theory and processes: Modern horizons. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2004. pp. 115–138. [Google Scholar]
- Wei W., Gilbert N., Ooi S.L., Lawler J.F., Ostertag E.M., Kazazian H.H., Boeke J.D., Moran J.V., Gilbert N., Ooi S.L., Lawler J.F., Ostertag E.M., Kazazian H.H., Boeke J.D., Moran J.V., Ooi S.L., Lawler J.F., Ostertag E.M., Kazazian H.H., Boeke J.D., Moran J.V., Lawler J.F., Ostertag E.M., Kazazian H.H., Boeke J.D., Moran J.V., Ostertag E.M., Kazazian H.H., Boeke J.D., Moran J.V., Kazazian H.H., Boeke J.D., Moran J.V., Boeke J.D., Moran J.V., Moran J.V. Human L1 retrotransposition: Cis preference versus trans complementation. Mol. Cell. Biol. 2001;21:1429–1439. doi: 10.1128/MCB.21.4.1429-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Fujimoto Y., Arai R., Fujie M., Usami S., Yamada T., Fujimoto Y., Arai R., Fujie M., Usami S., Yamada T., Arai R., Fujie M., Usami S., Yamada T., Fujie M., Usami S., Yamada T., Usami S., Yamada T., Yamada T. Retrotransposon-mediated restoration of Chlorella telomeres: Accumulation of Zepp retrotransposons at termini of newly formed minichromosomes. Nucleic Acids Res. 2003;31:4646–4653. doi: 10.1093/nar/gkg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.S., Pession A., Traverse K.L., French C., Pardue M.L., Pession A., Traverse K.L., French C., Pardue M.L., Traverse K.L., French C., Pardue M.L., French C., Pardue M.L., Pardue M.L. Telomere regions in Drosophila share complex DNA sequences with pericentric heterochromatin. Cell. 1983;34:85–94. doi: 10.1016/0092-8674(83)90138-1. [DOI] [PubMed] [Google Scholar]