Abstract
The transcription factor Nrf2 regulates cellular redox homeostasis. Under basal conditions, Keap1 recruits Nrf2 into the Cul3-containing E3 ubiquitin ligase complex for ubiquitin conjugation and subsequent proteasomal degradation. Oxidative stress triggers activation of Nrf2 through inhibition of E3 ubiquitin ligase activity, resulting in increased levels of Nrf2 and transcriptional activation of Nrf2-dependent genes. In this study, we identify Keap1 as a key postinduction repressor of Nrf2 and demonstrate that a nuclear export sequence (NES) in Keap1 is required for termination of Nrf2-antioxidant response element (ARE) signaling by escorting nuclear export of Nrf2. We provide evidence that ubiquitination of Nrf2 is carried out in the cytosol. Furthermore, we show that Keap1 nuclear translocation is independent of Nrf2 and the Nrf2-Keap1 complex does not bind the ARE. Collectively, our results suggest the following mechanism of postinduction repression: upon recovery of cellular redox homeostasis, Keap1 translocates into the nucleus to dissociate Nrf2 from the ARE. The Nrf2-Keap1 complex is then transported out of the nucleus by the NES in Keap1. Once in the cytoplasm, the Keap1-Nrf2 complex associates with the E3 ubiquitin ligase, resulting in degradation of Nrf2 and termination of the Nrf2 signaling pathway. Hence, postinduction repression of the Nrf2-mediated antioxidant response is controlled by the nuclear export function of Keap1 in alliance with the cytoplasmic ubiquitination and degradation machinery.
Mammalian cells are inevitably exposed to environmental insults, such as pollutants, chemicals, and natural toxins. Many of these compounds exert their biological effects by perturbation of cellular redox homeostasis, a condition defined as oxidative stress. Oxidative stress has been associated with the etiology of many human diseases, including cancer, neurodegenerative diseases, cardiovascular diseases, inflammation, and autoimmune diseases (19, 22, 34, 35, 45). To counteract the detrimental effect of environmental insults, mammalian cells have evolved sensing and signaling mechanisms to turn on or off endogenous antioxidant responses accordingly (6, 32).
One of the major cellular antioxidant responses is mediated by the transcription factor Nrf2. Nrf2 controls transcriptional activation of its downstream target genes by binding to the antioxidant response element (ARE) present in the promoters of many antioxidant and phase II detoxifying genes, including those encoding glutathione S-transferase (GST), NAD(P)H quinone oxidoreductase, γ-glutamylcysteine synthetase, and heme oxygenase 1 (1, 15, 24, 30, 44). Recently a growing body of evidence has suggested that the Nrf2-dependent antioxidant response is a cell survival signal, and activation of the Nrf2 pathway confers cellular protection against detrimental effects from various insults (3, 5, 25, 29). For instance, Nrf2 knockout mice have decreased constitutive and inducible expression of detoxification enzymes and antioxidants. As a consequence, these mice are highly susceptible to chemical carcinogens and toxicants, including benzo[a]pyrene, N-nitrosobutyl(4-hydroxybutyl)amine, pentachlorophenol, acetaminophen, 4-vinyl cyclohexene diepoxide, diesel exhaust, and cigarette smoke (2, 3, 9, 12-14, 33, 37).
The activity of Nrf2 is rigidly regulated by an inhibitor protein named Keap1, a member of the BTB-Kelch family that is rich in cysteine residues (16). The Nrf2-Keap1 signaling pathway is activated by many chemopreventive compounds and antioxidants. Interestingly, the Nrf2 inducers share a common chemical property: they have strong reactivity with sulfhydryl groups, such as those found in cysteine residues. Therefore, Keap1 has been proposed to function as a molecular sensor for cellular redox changes in response to exogenous stimuli (7, 40). In in vivo systems, different cysteine residues in Keap1 display different preferences for alkylating reagents (7, 8, 11). In cultured cells, cysteines 273 and 288 in the linker region of the Keap1 protein have been proven to be indispensable for the inhibition of Nrf2 activity under basal conditions (26, 40, 47), while mutation of a single cysteine residue (C-151) in the BTB domain of Keap1 completely blocks the activation of this pathway by Nrf2 inducers (47). These findings further confirm the role of cysteine residues of Keap1 in sensing intracellular redox conditions. Another recent advance in understanding Nrf2 regulation is the identification of Keap1 as a substrate adaptor protein for Cul3-containing E3 ubiquitin ligase (4, 10, 23, 48). Under basal conditions, Keap1 constantly targets Nrf2 for ubiquitination and degradation. Upon induction, Keap1 inhibits the enzymatic activity of the Keap1-Cul3-Rbx1 E3 ubiquitin ligase, resulting in decreased Nrf2 ubiquitination and degradation. As a consequence, Nrf2 saturates the Keap1 binding sites and free Nrf2 translocates into the nucleus to activate ARE-dependent genes (46).
Clearly, Keap1-mediated ubiquitination and degradation play a central role in regulating the level of Nrf2 and subsequent activation of the cellular antioxidant response. Another level of regulation is the controlled nuclear import and export of Nrf2. For example, a canonical nuclear localization sequence (NLS) containing a short stretch of basic amino acids has been identified in the Nrf2 protein. The RKRK NLS (amino acids 515 to 518 in the human Nrf2 protein) is necessary for nuclear translocation of Nrf2 (17, 46). Similarly, nuclear export of both Nrf2 and Keap1 has been reported to involve a nuclear export factor CRM-dependent process, because leptomycin B (LMB), a specific inhibitor of CRM1, blocks nuclear export of Nrf2 and Keap1(17, 20, 27, 28, 31, 38). In contradiction with the prevailing notion that Keap1 is a cytoplasmic factor, these recent findings clearly classify Keap1 as a shuttle protein and imply that Keap1 must have a functional role not only in the cytoplasm but also in the nucleus. However, the search for the classical leucine-rich nuclear export sequences (NESs) that mediate nuclear export of Nrf2 has given rise to very controversial results. Amino acids 301-LVKIFEELTL-310 in human Keap1 have been demonstrated to be a NES, as evidenced by the fact that a Keap1 mutant with both L308/A and L310/A substitutions localized predominantly in the nucleus (20, 31, 38). Intriguingly, two separate LMB-dependent NESs have also been identified in the Nrf2 protein. The first identified putative NES (552-LLKKQLSTLYLE-563 in hNrf2) is located at the leucine zipper domain and is sufficient to support nuclear export of green fluorescent protein (GFP) or GAL4 DNA binding domain (DBD) when it is fused to them (17, 27). Another putative NES (191-LLSIPELQCLNIEN-204 in hNrf2), in the transactivation domain of Nrf2, has been shown to be a redox-sensitive nuclear export signal when it is fused with GFP (28). Based on the current data, it was uncertain which NES controls nuclear export of Nrf2. In these studies, demonstration of NES was carried out using the singly overexpressed Nrf2 or Keap1 protein or using a NES-containing fragment from Nrf2 or Keap1 fused with GFP (NES-GFP fusion protein) or fused with the GAL4 DBD (NES-GAL4-DBD fusion protein). Since Keap1 plays a pivotal role in controlling the activity of Nrf2 by binding and recruiting Nrf2 into the E3 ubiquitin ligase complex for ubiquitination and degradation, it is essential to identify the authentic NES in a system where both the Nrf2 and Keap1 proteins are present.
In this study, we have identified Keap1 as a key postinduction repressor of Nrf2 and have demonstrated that a NES in Keap1 is required for termination of Nrf2-ARE signaling by escorting nuclear export of Nrf2. Furthermore, we provide evidence that ubiquitination of Nrf2, mediated by the Keap1-Cul3-Rbx1 E3 ubiquitin ligase complex, is carried out in the cytosol and the Nrf2-Keap1 complex does not bind the ARE. Hence, postinduction repression of the Nrf2-mediated antioxidant response is controlled by the nuclear export function of Keap1 in alliance with the cytoplasmic ubiquitination and degradation machinery.
MATERIALS AND METHODS
Construction of recombinant DNA molecules.
Plasmids expressing wild-type Keap1-chitin binding domain (CBD) and hemagglutinin (HA)-tagged Nrf2 (HA-Nrf2) proteins have been previously described (47). The Keap1 and Nrf2 mutants, including Nrf2-NLS1, Nrf2-NLS2, Nrf2-NES1, Nrf2-NES2, and Keap1-NES, were generated by site-directed mutagenesis using the PCR and Dpn1-based method. Briefly, synthetic single-stranded-fragment DNA containing desired mutations was synthesized by integrated DNA technologies and used as primers for PCR amplification. PCR amplification conditions were as follows: one cycle of 95°C for 30 s; and 20 cycles of 95°C for 30 s, 55°C for 30 s, and 68°C for 10 s. After digestion of the parental methylated DNAs, newly unmethylated DNAs were transformed into DH5α. The plasmids were extracted and sequenced to ensure that the correct mutations were introduced. For most of mutants, mutagenesis was performed two to three times for each of the mutant proteins to replace several amino acid residues with alanines.
Cell culture, transfection, induction, and reporter gene assay.
COS-1 and MDA-MB-231 cells were purchased from ATCC. Cells were maintained in either Dulbecco's modified Eagle's medium or Eagle's minimal essential medium in the presence of 10% fetal bovine serum. Transfections were performed with Lipofectamine Plus (Gibco BRL) according to the manufacturer's instructions. DNA amounts in each transfection were kept constant by addition of empty pcDNA3 plasmid. The ARE TATA-Inr luciferase reporter plasmid pARE-Luc was described previously (47). Two Nrf2 inducers, tert-butylhydroquinone (tBHQ) and sulforaphane (SF), were purchased from Sigma. Cells were treated with 50 μM tBHQ and 10 μM SF for 16 h prior to cell lysis for analysis of reporter gene activity. A plasmid encoding Renilla luciferase was included in all samples to control for transfection efficiency. Reporter assays were performed using the Promega dual-luciferase reporter gene assay system.
Antibodies, immunoprecipitation, and immunoblot analysis.
Antibodies against Nrf2 (Santa Cruz), Keap1 (Santa Cruz), Gal4 (Santa Cruz), ubiquitin (Sigma), CBD (New England Biolabs), and the Myc and HA epitopes (Covance) were purchased from commercial sources. For immunoprecipitation or immunoblot analysis, cells were treated with 100 μM tBHQ and 15 μM SF for 4 h prior to cell lysis.
For detection of protein expression in total cell lysates, cells were lysed in sample buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 100 mM dithiothreitol [DTT], 0.1% bromophenol blue) 48 h following transfection. For immunoprecipitation assays, cells were lysed in RIPA buffer (10 mM sodium phosphate [pH 8.0], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor cocktail (Sigma). Cell lysates were precleared with protein A beads and incubated with 2 μg of affinity-purified antibodies for 2 h at 4°C, followed by incubation at 4°C with protein A-agarose beads for 2 h. After four washes with RIPA buffer, immunoprecipitated complexes were eluted in sample buffer by boiling them for 4 min, electrophoresed through SDS-polyacrylamide gels, and subjected to immunoblot analysis.
To measure the half-life of a protein, transfected cells were treated with 50 μg/ml cycloheximide. Total cell lysates were collected at different time points and subjected to immunoblot analysis. The relative intensities of bands were quantified by the ChemiDoc XRS gel documentation system from Bio-Rad.
Ubiquitination of Nrf2.
To detect ubiquitinated Nrf2 in vivo, cells were transfected with expression vectors for HA-tagged ubiquitin (HA-ubiquitin), HA-tagged Cul3 (HA-Cul3), Myc-tagged Rbx1 (Myc-Rbx1), Keap1, and Nrf2. The transfected cells were exposed to 10 μM MG132 (Sigma) for 4 h. Cells were lysed by boiling in a buffer containing 2% SDS, 150 mM NaCl, 10 mM Tris-HCl, and 1 mM DTT. This rapid lysis procedure inactivated cellular ubiquitin hydrolases and therefore preserved ubiquitin-Nrf2 conjugates present in cells prior to lysis. Protein-protein interactions, including association of Nrf2 with Keap1, were also disrupted by this lysis procedure. For immunoprecipitation, these lysates were diluted fivefold in buffer lacking SDS and incubated with anti-Nrf2 antibodies. Immunoprecipitated proteins were analyzed by immunoblotting with antibodies directed against the HA epitope.
For ubiquitination of Nrf2 in vitro, COS-1 cells were transfected with expression vectors for HA-Nrf2, Keap1-CBD, HA-Cul3, and Myc-Rbx1. The transfected cells were lysed in buffer B (15 mM Tris-HCl [pH 7.4], 500 mM NaCl, and 0.25% NP-40) containing 1 mM DTT, 1 mM PMSF, and protease inhibitor cocktail. The lysates were precleared with protein A beads prior to incubation with chitin beads (New England Biolabs) for 4 h at 4°C. Chitin beads were washed twice with buffer B, twice with buffer A (25 mM Tris-HCl [pH 7.5], 10% [vol/vol] glycerol, 1 mM EDTA, 0.01% NP-40, and 0.1 M NaCl), and twice with reaction buffer (50 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 2 mM NaF, and 0.6 mM DTT). The pellets were incubated with ubiquitin (300 pmol), E1 (2 pmol), E2-UbcH5a (10 pmol), and ATP (2 mM) in 1× reaction buffer in a total volume of 30 μl for 1 h at 37°C. Ubiquitin, E1, E2-UbcH5a, and ATP were purchased from Boston Biochem. The chitin beads were centrifuged at 3,000 × g, resuspended in 2% SDS, 150 mM NaCl, 10 mM Tris-HCl [pH 8.0], and 1 mM DTT, and boiled for 5 min to release bound proteins and disrupt protein-protein interactions. The supernatant was diluted fivefold with buffer lacking SDS prior to immunoprecipitation with anti-Nrf2 antibodies. Immunoprecipitated proteins were subjected to immunoblot analysis with antiubiquitin antibodies.
Immunofluorescence assays.
NIH 3T3 cells were grown on glass coverslips in 35-mm plates. Cells were transfected with plasmids encoding Nrf2, Keap1, or Cul3 proteins. Cells were fixed with 100% methanol at −20°C for 10 min. Fixed cells were incubated for 40 min with primary antibodies at a 1:200 dilution in PBS (10 mM sodium phosphate pH [8.0] and 150 mM NaCl) containing 10% (vol/vol) fetal bovine serum. Coverslips were washed and incubated with Alexa Fluor 488-conjugated antimouse, Alexa Fluor 593-conjugated antirabbit, or Alexa Fluor 680-conjugated antigoat secondary antibody (Invitrogen) at a 1:200 dilution for another 40 min. Coverslips were washed and mounted on glass slides. At least 100 positive cells were scored for localization of the Nrf2, Keap1, and Cul3 proteins under a microscope. Images were obtained using a Zeiss LSM 510NLO-Meta multiphoton/confocal system. The images were exported from the native Zeiss file format to Tiff files using the Zeiss LSM Image Browser, and then Adobe Photoshop was used to construct the figure. Minimal alterations were performed on the digital images in either Browser or Photoshop.
Subcellular fractionation.
To obtain nuclear and cytoplasmic subcellular fractions, transfected MDA-MB-231 cells in 60-mm dishes were trypsinized first to separate cells. Cell pellets were collected in tubes and washed twice with PBS. Cell pellets were resuspended in hypotonic buffer (10 mM HEPES [pH 8.0], 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 1 mM PMSF, and protease inhibitor cocktail) and incubated on ice for 15 min to allow cells to swell. To the swollen cells in lysis buffer, NP-40 was added to a final concentration of 0.1% and vortexed vigorously for 10 s, followed by immediate centrifugation for 1 min at 6,000 rpm. The supernatant was further purified by centrifugation at 14,000 rpm for 20 min and collected as the cytoplasmic extract. Nuclear extract was prepared by resuspension of the crude nuclei in high-salt buffer (20 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 20% glycerol, 420 mM NaCl, 1 mM DTT, 1 mM PMSF, and protease inhibitor cocktail) at 4°C for 30 min, and the supernatants were collected after centrifugation at 13,000 rpm for 5 min.
EMSA.
Nuclear fractions from transfected MDA-MB-231 cells were preincubated for 10 min at room temperature with poly(dI-dC) in the binding buffer (50 mM HEPES, 60 mM KCl, 2 mM MgCl2, 1 mM EDTA, 0.4% NP-40, 10% glycerol, and 1 mM DTT). 32P-end-labeled, gel-purified double-stranded DNA was added and further incubated for an additional 20 min at room temperature. The samples were electrophoresed through a native acrylamide gel in 0.5× TBE (4.45 mM Tris, 4.45 mM boric acid, and 1 mM EDTA). Gels were dried, and autoradiographs were developed. The double-stranded oligonucleotides containing the following sequences were used for electrophoretic mobility shift assay (EMSA): 5′-AAATCGCAGTCACAGTGACTCAGCAGAATCTGAGCCTAG-3 (human NQO1 ARE) and 5′-GCGCGCGCACCGCCTCCCCGTGACTCAGCGCTTTGTGCG-3′ (human glutamate cysteine ligase catalytic subunit [GCLC] ARE). The mutated hNQO1 ARE used for cold competition was 5′-AAATCGCAGTCACAGactCTCAcgAGAATCTGAGCCTAG-3′.
ChIP assay.
MDA-MB-231 cells (approximately 1 × 106) were cross-linked with formaldehyde, collected in PBS, resuspended in 200 μl SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl [pH 8.0]) with PMSF and protease inhibitors, and sonicated on ice. The lysates were then diluted to 2 ml with chromatin immunoprecipitation (ChIP) dilution buffer (0.01% SDS, 1.1% TritionX-100, 1.2 mM EDTA, 167 mM NaCl, and 16.7 mM Tris-HCl [pH 8.0]), precleared with protein A agarose, and then incubated with appropriate antibodies (4 μg/sample) overnight. The immune complexes were collected with 50 μl protein A agarose, washed with low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 20 mM Tris-HCl [pH 8.0]), high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl, and 20 mM Tris-HCl [pH 8.0]), LiCl buffer (0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid, 1 mM EDTA, and 10 mM Tris-HCl [pH 8.0]), and TE buffer (1 mM EDTA and 10 mM Tris-HCl [pH 7.5]). The complexes were eluted in 500 μl fresh elution buffer (1% SDS and 0.1 M NaHCO3). The cross-links were reversed by heating at 65°C for 5 h after addition of 20 μl of 5 M NaCl. Samples were treated with RNase and proteinase K. DNA was recovered by phenol-chloroform extraction and ethanol precipitation. Relative amounts of DNA in the complex were quantified by the real-time PCR method using the LightCycler 480 DNA SYBR green I kit (Roche). Primers used were as follows: human NQO1 ARE forward, 5′-GCAGTCACAGTGACTCAGC-3′; human NQO1 ARE reverse, 5′-TGTGCCCTGAGGTGCAA-3′; tubulin promoter forward, 5′-GTCGAGCCCTACAACTCTATC-3′; tubulin promoter reverse, 5′-CCGTCAAAGCGCAGAGAA-3′.
PCR cycling was performed as follows: initial denaturation at 95°C for 5 min (1 cycle), 40 cycles of amplification of 95°C for 10 s, 60°C for 10 s, and 72°C for 20 s, with a single fluorescence acquisition. The amplification was followed by a melting curve program (65 to 95°C with a heating rate of 0.1°C per second and a continuous fluorescence measurement) and then a cooling program at 40°C for 30 s. The mean crossing-point values and standard deviations for NQO1 and tubulin were determined for the different samples. The crossing point is defined as the point at which the fluorescence rises appreciably above the background fluorescence. A nontemplate control was run for each primer pair to assess the overall specificity and to ensure that primer dimers were not interfering with amplification detection. Amplification specificity was checked using melting-curve and agarose gel electrophoresis. Melting-curve analysis showed a single sharp peak for all samples, and agarose gel electrophoresis showed a single band at the expected size. Data are presented as n-fold change. The real-time PCR assays were performed two times, each with triplicate samples.
RESULTS
The NES in Keap1 regulates nuclear export of Nrf2.
Recent findings indicate that both Keap1 and Nrf2 are able to travel between the nucleus and the cytosol. To confirm the cytoplasmic-nuclear trafficking of endogenous Nrf2 and Keap1, MDA-MB-231 cells were treated with LMB and subcellular localization of Nrf2 and Keap1 was detected by indirect immunofluorescence staining with the anti-Nrf2 and anti-Keap1 antibodies. As expected, Nrf2 and Keap1 predominantly localized in the cytosol in untreated cells, likely due to the dominant function of nuclear export over nuclear import (Fig. 1A, panels A to D). Blockage of nuclear export by LMB significantly located Nrf2 and Keap1 in the nucleus, demonstrating dynamic trafficking of Nrf2 and Keap1 under the physiological condition (Fig. 1A, panels E to H). We also performed subcellular fractionation analysis in the presence or absence of LMB. LMB treatment diminished cytoplasmic Nrf2 and Keap1 while enriching nuclear Nrf2 and Keap1 (Fig. 1B). Taken together, these results clearly demonstrate that both Nrf2 and Keap1 are shuttle proteins which constantly undergo cytoplasmic-nuclear trafficking even under basal conditions.
FIG. 1.
The cytoplasmic-nuclear trafficking of Nrf2 and Keap1. (A) MDA-MB-231 cells were treated with 10 nM of LMB for 3 h. Subcellular localization of Nrf2 and Keap1 was detected by indirect immunofluorescence staining with anti-Nrf2 and anti-Keap1 antibodies. (B) Nuclear and cytoplasmic fractions of mock- or LMB-treated MDA-MB-231 cells were subjected to immunoblot analysis with anti-Nrf2 and anti-Keap1 antibodies (α-Nrf2 and α-Keap1) α-Lamin A, anti-lamin A antibody; α-tubulin, antitubulin antibody. (C) Upper panel, six discrete domains of the Nrf2 proteins are designated Neh2, Neh4, Neh5, Neh6, Neh1, and Neh3. The two putative NESs in Nrf2 are located in the Neh5 and Neh1 domains. The amino acid sequences of the two NESs are indicated in the single-letter code below the Nrf2 drawing. The residues relevant to the present work are in boldface, and the mutations introduced into the Nrf2 protein are indicated. Lower panel, Keap1 contains five domains: N-terminal, BTB, Linker, Kelch, and C-terminal. The NES in Keap1 is in the Linker domain. The NES sequence with a cluster of hydrophobic residues is shown, and the four hydrophobic residues are replaced by alanines. (D) COS1 cells were cotransfected with expression vectors for the indicated Nrf2 and Keap1 proteins. Total lysates were analyzed by immunoblotting with anti-HA and anti-CBD antibodies (α-HA and α-CBD) for detection of Nrf2 and Keap1 proteins (upper two panels). The lysates were incubated with chitin beads. Following washing, protein complexes bound to chitin beads were eluted by heating in sample buffer and subjected to immunoblot analysis with anti-HA and anti-CBD antibodies (lower two panels).
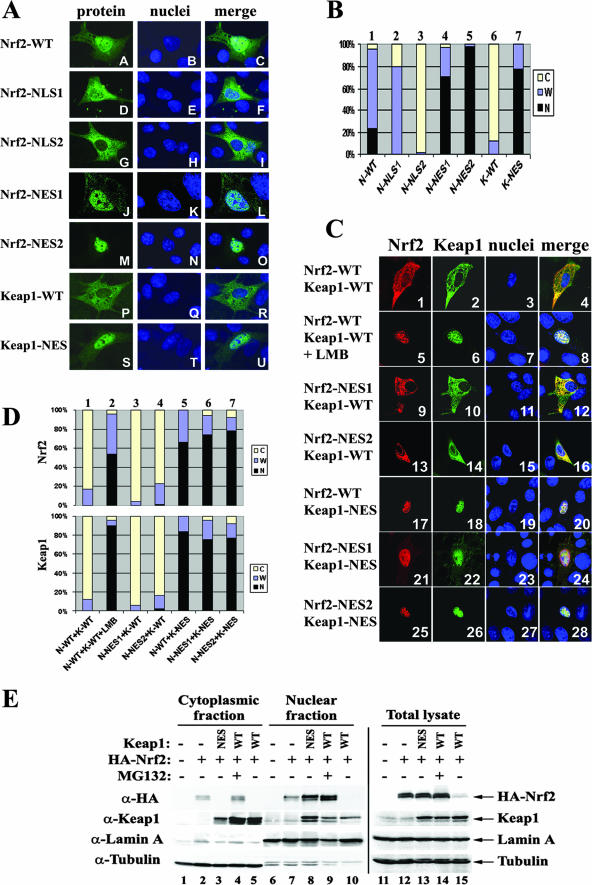
Three putative NESs have been found in Nrf2 and Keap1 that have some degree of nuclear export activity when either Nrf2, Keap1, or a fusion protein containing any of the NESs is overexpressed alone. The two reported NESs in human Nrf2 are 191-LLSIPELQCLNIEN-204 and 552-LLKKQLSTLYLE-563, while the NES in human Keap1 is 301-LVKIFEELTL-310 (Fig. 1C). To address the current controversial issue regarding NESs and to identify the authentic NES that controls the subcellular localization of Nrf2 under physiological conditions, we have made alanine substitutions for the hydrophobic leucine or isoleucine residues in all three NESs and named them Nrf2-NES1, Nrf2-NES2, and Keap1-NES (Fig. 1C). The other two Nrf2 mutants, Nrf2-NLS1 and Nrf2-NLS2 (for NLS1, 502-RRR-504 was replaced with AAA, and for NLS2, 515-RKRK-518 with AAAA), were also included as controls for the subcellular localization experiment. As reported previously, Nrf2-NLS1 behaved as did wild-type Nrf2 (Nrf2-WT) while Nrf2-NLS2 localized exclusively in the cytosol, indicating 515-RKRK-518 is the primary NLS that controls the nuclear import of Nrf2. All of the Nrf2 mutants were expressed; however, their expression levels varied slightly, while there was no difference in the expression levels of wild-type Keap1 (Keap1-WT) and Keap1-NES (Fig. 1D, upper two panels). To ensure that the mutations introduced do not compromise the interaction between Keap1 and Nrf2, a pull-down assay was performed with COS-1 cells cotransfected with the appropriate Nrf2 and Keap1 proteins. As shown in the lower two panels of Fig. 1D, chitin beads pulled down proportional amounts of Keap1-associated Nrf2 proteins, indicating that the mutations introduced into Nrf2 or Keap1 have no effect on the binding of these two proteins.
To verify that the point mutations we introduced in the NESs in Nrf2 or Keap1 are sufficient to impair the nuclear export function of the individual protein, each of the Nrf2 or Keap1 proteins was transfected into NIH 3T3 cells and subcellular localization was detected by an indirect immunofluorescence analysis. As shown in Fig. 2A and B, when Nrf2-WT was overexpressed, approximately 20% of the cells showed predominant nuclear localization and 80% of the cells displayed even staining throughout the entire cell (Fig. 2A, panels A to C, and 2B, bar 1). Mutation in each of the NESs in Nrf2 significantly increased the nuclear localization (Fig. 2A, panels J to O, and Fig. 2B, bars 4 and 5), indicating these two reported NESs in Nrf2 have some degree of nuclear export activities when they are overexpressed alone. Mutation in the NES in Keap1 dramatically shifted Keap1 to the nucleus (approximate 80% nuclear localization and 20% even staining throughout the entire cell in bar 7), compared to results for wild-type Keap1 (10% even staining throughout the entire cells and 90% cytosolic localization in bar 6). Collectively, these results demonstrate that all three NESs identified in Nrf2 or Keap1 proteins have nuclear export activities when each of the proteins is individually overexpressed. To identify the authentic NES that controls the subcellular localization of Nrf2 under physiological conditions, we compared the subcellular localization of different pairs of coexpressed Nrf2 and Keap1 proteins in the same system. Nrf2-WT was localized primarily in the cytoplasm in the presence of Keap1-WT (>80% cytosolic localization; Fig. 2D, upper panel, bar 1, and 2C, panels 1 to 4). In contrast, localization of Nrf2-WT shifted to the whole cell or even was confined to the nucleus when Keap1-NES was coexpressed (35% whole-cell localization and 65% nuclear localization in Fig. 2D, upper panel, bar 5 and 2C, panels 17 to 20). In contrast, there was only a slight change in the subcellular localization of Nrf2 in cells coexpressing Nrf2-NES1 and Keap1-WT (Fig. 2D, upper panel, bar 3 and 2C, panels 9 to 12) or coexpressing Nrf2-NES2 and Keap1-WT (Fig. 2D, upper panel, bar 4 and 2C, panels 13 to 16), indicating that the two NESs in Nrf2 are weaker than the NES in Keap1 under the cotransfected conditions. As expected, in cells coexpressing Nrf2-NES1/Keap1-NES or Nrf2-NES2/Keap1-NES, there was a further increase in the percentage of cells that have predominantly nuclear Nrf2, although there was also a slight increase in the cytoplasmic staining (Fig. 2D, upper panel, compare bar 6 and bar 7 with bar 5, and 2C, panels 17 to 28). Blockage of nuclear export by 10 nM of LMB treatment for 3 h shifted both Nrf2 and Keap1 to the nucleus (Fig. 2D, upper panel, bar 2, and 2C, panels 5 to 8). The localization profile of Keap1 was similar to that of Nrf2 in the cotransfected cells (Fig. 2C and D, lower panel). The results of the immunofluorescence staining indicate that the NES in Keap1 is much stronger than any of the two NESs in Nrf2, although it is clear that both of the NESs in Nrf2 have nuclear export functions when they are singly overexpressed. Nevertheless, the drastically stronger nuclear export activity of the NES in Keap1 than in Nrf2 suggests that the NES in Keap1 is the nuclear export signal utilized by cells to control the nuclear export of Nrf2 under physiological conditions.
FIG. 2.
The NES in Keap1 controls nuclear export of Nrf2. (A) NIH 3T3 cells were singly transfected with an expression vector for the indicated Nrf2 or Keap1 protein. Subcellular localization of Nrf2 or Keap1 was determined by indirect immunofluorescence analysis using anti-HA for Nrf2 (panels A, D, G, J, and M) or anti-CBD for Keap1 (panels P and S). The nucleus was visualized by Hoechst staining (panels B, E, H, K, N, Q, and T). (B) Subcellular distribution of the Nrf2 or Keap1 protein in singly transfected cells (the representative image is shown in A) was determined by examining at least 100 positive cells. Percentages of cells that localized predominantly in the cytosol (C), the whole cell (W), or the nucleus (N) were presented as a bar graph. (C) NIH 3T3 cells were cotransfected with expression vectors for the indicated Nrf2 and Keap1 proteins. Subcellular localization of the Nrf2 and Keap1 proteins was determined by double-labeling indirect immunofluorescence assay using anti-HA (panels 1, 5, 9, 13, 17, 21, and 25) and anti-CBD antibodies (panels 2, 6, 10, 14, 18, 22, and 26). The nucleus was visualized by Hoechst staining (panels 3, 7, 11, 15, 19, 23, and 27). Colocalization of the Nrf2 and Keap1 proteins is indicated by the presence of yellow in the merged images (panels 4, 8, 12, 16, 20, 24, and 28). (D) Subcellular localization of the Nrf2 and Keap1 proteins in double-transfected cells (the same slides were used for C) were examined and counted in the same way as in B except that the data were collected from 100 cells that are positive for both Nrf2 and Keap1 proteins. (E) Nuclear and cytoplasmic proteins were isolated from MDA-231 cells cotransfected with expression vectors for Nrf2 and either Keap1-WT or Keap1-NES. The transfected cells were either left untreated or treated with 10 μM MG132 for 4 h prior to subcellular fractionation. Nuclear and cytoplasmic proteins derived from equal numbers of cells were electrophoresed through a 7.5% SDS-polyacrylamide gel and subjected to immunoblot analysis using anti-HA, anti-Keap1, antitubulin, or anti-lamin A antibodies. α, anti.
Next, we used a subcellular fractionation assay to analyze the protein levels of Nrf2 and Keap1 in the cytoplasmic and nuclear fractions of MDA-MB-231 cells when Nrf2 was cotransfected with either Keap1-WT or Keap1-NES. Keap1-WT and Keap1-NES were expressed at the same level (Fig. 2E, second panel, lanes 13 to 15). As expected, the expression of Nrf2 in the presence of Keap1-WT was significantly reduced (Fig. 2E, top panel, compare lane 12 with lane 15). MG132, a 26S proteasome inhibitor, markedly increased the levels of Nrf2 (compare lane 14 with lane 15). Interestingly, expression of Nrf2 was significantly high in the presence of Keap1-NES (compare lane 13 with lane 15), which is approximately equivalent to the expression of Nrf2 in cells transfected with Nrf2 alone (compare lane 13 with lane 12) or in double-transfected cells treated with MG132 (compare lane 13 with lane 14). These results indicate that Nrf2 degradation in the presence of Keap1-NES is likely impaired. Interestingly, the cytoplasmic expression profile was very different. Regardless of the high expression level of Nrf2 in Keap1-NES-cotransfected cells, the cytoplasmic level of Nrf2 was also below detection, similar to the Nrf2 expression in the cells cotransfected with Keap1-WT (compare lane 3 and lane 5), whereas there was a significant amount of cytoplasmic Nrf2 in cells transfected with Nrf2 alone (lane 2) or in doubly transfected cells treated with MG132 (lane 4). In the nucleus, the Nrf2 level was high in cells cotransfected with Keap1-NES (lane 8). Likewise, there was minimal expression of Keap1-WT in the nucleus (second panel, lanes 9 and 10) and high expression in the cytosol (second panel, lane 4 and 5). In contrast, there was more Keap1-NES in the nucleus than in the cytosol (compare lane 8 with lane 3). The result obtained from the fractionation analysis is consistent with the finding from the immunostaining assay, indicating that the NES in Keap1 regulates nuclear export of the Nrf2-Keap1 complex. In addition, the result also implies the possibility of impaired Nrf2 degradation in the presence of Keap1-NES. For the subcellular fractionation assay, lamin A and α-tubulin were used as nuclear and cytoplasmic markers for the indication of good separation between the nucleus and the cytosol.
Ubiquitination of Nrf2, mediated by the Keap1-Cul3-Rbx1 E3 ubiquitin ligase, occurs in the cytosol.
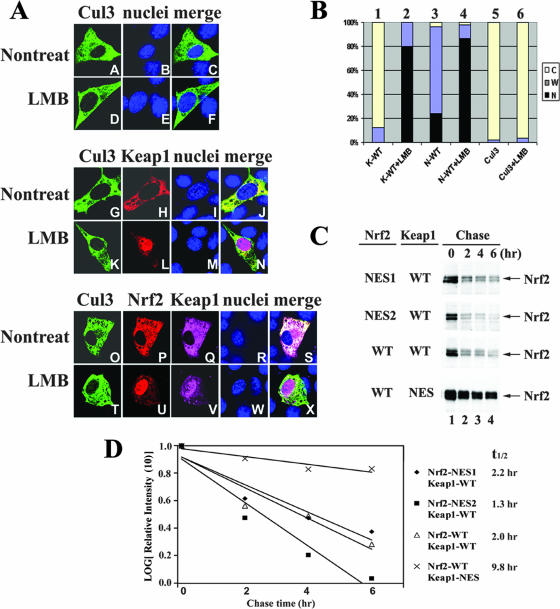
Recently Keap1 was identified as a substrate adaptor protein for the Cul3-Rbx1-containing E3 ubiquitin ligase by several independent groups. Oxidative stress and chemopreventive compounds inhibit the enzymatic activity of this E3 ubiquitin ligase, resulting in stabilization of Nrf2 and activation of Nrf2-dependent genes. However, it is not clear in which compartment ubiquitination and degradation of Nrf2 occur. Given that the Keap1-Cul3-Rbx1 complex is the specific E3 ubiquitin ligase for Nrf2, we tested subcellular localization of Cul3. Transiently expressed Cul3 localized exclusively in the cytosol and remained in the cytosol even after LMB treatment (Fig. 3A, panels A to F), indicating that Cul3 is a cytoplasmic protein that does not shuttle between the cytosol and the nucleus. Localization of Cul3 in the presence of Keap1 or in the presence of both Nrf2 and Keap1 remained the same (Fig. 3A, panels G to J and O to S). LMB-treated, Cul3- and Keap1-coexpressed cells showed predominantly nuclear localization of Keap1 but cytoplasmic localization of Cul3 (Fig. 3A, panels K to N). Even in Nrf2-, Keap1-, and Cul3-coexpressed cells, Cul3 remained in the cytosol after blockage of nuclear export while Nrf2 and Keap1 were shifted to the nucleus (Fig. 3A, panels T to X). This result clearly demonstrates that the ubiquitination machinery for Nrf2 is located exclusively in the cytosol. Figure 3B represents a subcellular localization analysis of each indicated protein in singly transfected cells with or without LMB treatment. LMB significantly shifted subcellular localization of Nrf2 and Keap1 (compare bar 2 with bar 1 and bar 4 with bar 3), while having no effect on Cul3 localization (compare bar 6 with bar 5).
FIG. 3.
Ubiquitination of Nrf2, mediated by the Keap1-Cul3-Rbx1 E3 ubiquitin ligase, occurs in the cytosol. (A) NIH 3T3 cells were singly transfected with an expression vector for Flag-Cul3 (upper panel), cotransected with expression vectors for both Flag-Cul3 and Keap1 (middle panel), or cotransfected with expression vectors for Flag-Cul3, Keap1, and HA-Nrf2 (lower panel). Subcellular localization of Cul3, Nrf2, or Keap1 was determined by indirect immunofluorescence analysis using anti-Flag for Cul3 (panels A, D, G, K, O, and T), anti-HA for Nrf2 (panels P and U), or anti-Keap1 for Keap1 (panels H, L, Q, and V). Nontreat, nontreated; LMB, LMB treatment. (B) Subcellular distribution of Cul3, Nrf2, and Keap1 in singly transfected cells in the absence or presence of LMB was determined by indirect immunofluorescence staining. At least 100 positive cells were examined. Percentages of cells that localized predominantly in the cytosol (C), the whole cell (W), or the nucleus (N) were presented as a bar graph. K-WT, Keap1-WT; N-WT, Nrf2-WT; +LMB, LMB treatment. (C) MDA-MB-231 cells were cotransfected with expression vectors for the indicted Nrf2 and Keap1 proteins. Fifty micrograms per milliliter cycloheximide was added 36 h after transfection. Total cell lysates were collected at the indicated time points following cycloheximide treatment and subjected to immunoblot analysis with anti-HA antibodies. (D) The relative intensities of the Nrf2 bands were quantified by the ChemiDoc XRS gel documentation system from Bio-Rad and plotted on a semilog scale. The amount of Nrf2 present at the beginning of cycloheximide treatment was set at 1. The half-life of Nrf2 in each group was indicated.
Based on our findings, (i) Keap1-NES blocks the nuclear export of Nrf2, as indicated in the immunostaining analysis (Fig. 2C and D); (ii) ubiquitination and degradation of Nrf2 occur in the cytosol (Fig. 3A and B); and (iii) there is greater expression of Nrf2 in the presence of Keap1-NES than in the presence of Keap1-WT; it is conceivable that Nrf2 is more stable in cells cotransfected with Keap1-NES than in cells cotransfected with Keap1-WT. To test this, the stability of each of the Nrf2 proteins was measured in MDA-MB-231 cells in the presence of Keap1-WT or Keap1-NES. Fifty micrograms per milliliter cycloheximide was used to block de novo protein synthesis, and the levels of the Nrf2 proteins were determined at different time points following cycloheximide treatment. Nrf2 in the presence of Keap1-WT had a half-life of 2.0 h, while the half-life of Nrf2 increased to 9.8 h when Keap1-NES was coexpressed (Fig. 3C and D). Nrf2-NES1 or Nrf2-NES2 in the presence of Keap1-WT had a half-life of 2.2 h or 1.3 h, respectively, similar to Nrf2-WT (Fig. 3C and D).
To determine if the increased half-life of Nrf2 in the presence of Keap1-NES is due to reduced ubiquitination of Nrf2 as a consequence of increased nuclear retention of Nrf2, in vivo ubiquitination of Nrf2 in the presence of either Keap1-WT or Keap1-NES was measured. MDA-MB-231 cells were cotransfected with expression vectors for HA-ubiquitin, Nrf2, and either Keap1-WT or Keap1-NES. The transfected cells were treated with MG132 for 4 h and lysed under strong denaturing conditions to destroy noncovalent protein-protein interactions. Aliquots of cell lysates were used for immunoblot analysis with anti Nrf2, anti-CBD, and antitubulin antibodies for detection of Nrf2, Keap1, and tubulin. The rest of the cell lysates were then diluted and immunoprecipitated with anti-Nrf2 antibodies. The immunoprecipitated proteins were analyzed by immunoblotting with anti-HA antibodies for the detection of ubiquitinated Nrf2. Since we observed a higher level of Nrf2 expressed in the presence of Keap1-NES than in the presence of Keap1-WT (Fig. 2E, compare lane 13 with lane 15), we measured the levels of Nrf2 in the presence of either Keap1-WT or Keap1-NES following MG132 treatment in the first experiment. Then, we equalized the total amounts of Nrf2 expressed in different samples by transfecting less Nrf2 vector DNA in Keap1-NES-cotransfected groups. As shown in the lower three panels of Fig. 4A, expression levels of the Nrf2, Keap1, and tubulin proteins were similar in different samples. Nevertheless, ubiquitination of Nrf2 was markedly reduced in cells coexpressing Keap1-NES compared to that in Keap1-WT-cotransfected cells (Fig. 4A, upper panel, compare lane 4 with lane 2). As expected, tBHQ blocked ubiquitination of Nrf2 in both Keap1-WT and Keap1-NES-cotransfected cells (Fig. 4A, compare lane 3 with lane 2 and lane 5 with lane 4).
FIG. 4.

Keap1-mediated nuclear export of Nrf2 is required for ubiquitination of Nrf2. (A) In vivo ubiquitination of Nrf2 in the presence of Keap1-WT or Keap1-NES was measured in MDA-MB-231 cells cotransfected with expression vectors for HA-ubiquitin, Nrf2, and the indicated Keap1 protein. The transfected cells were left untreated or treated with tBHQ for 4 h. Cells were lysed under denaturing conditions, and small aliquots of total lysates were used for immunoblot analysis with anti-Nrf2 (α-Nrf2), anti-CBD (α-CBD), and antitubulin (α-Tub) antibodies (lower panel). Anti-Nrf2 immunoprecipitates (IP) were analyzed by immunoblotting with anti-HA antibodies for detection of ubiquitinated Nrf2 (upper panel). (B) In vitro ubiquitination of Nrf2 in the presence of Keap1-WT and Keap1-NES was measured in COS-1 cells transfected with expression vectors for Nrf2, HA-Cul3, Myc-Rbx1, and the indicated Keap1 protein. Lysates from one 100-mm dish were incubated with chitin beads, and the bound proteins were eluted in sample buffer by boiling and subjected to immunoblot analysis with anti-HA, anti-CBD, and anti-Myc antibodies (α-HA, α-CBD, and α-Myc) (lower panel). Chitin bead-bound proteins from another 100-mm dish were incubated with purified E1, E2-UbcH5a, ubiquitin, and ATP. The chitin beads were pelleted and washed. Proteins that were eluted from the beads after boiling were immunoprecipitated with anti-Nrf2 antibodies (α-Nrf2), and Nrf2 immunoprecipitates were analyzed by immunoblotting with antiubiquitin antibodies (α-Ub) for detection of ubiquitin-conjugated Nrf2 (upper panel).
To exclude the possibility that mutations introduced in the NES in Keap1 (i) impair the Nrf2-Keap1-Cul3-Rbx1 complex formation or (ii) reduce the enzymatic activity of the Keap1-Cul3-Rbx1 complex, an in vitro ubiquitination assay was performed. Expression vectors for HA-Nrf2, Keap1-CBD (either Keap1-WT or Keap1-NES), HA-Cul3, and Myc-Rbx were cotransfected into COS-1 cells. Cells were treated with 10 μM MG132 for 4 h to block degradation of Nrf2. Keap1-associated proteins were pulled down by chitin beads and immunoblotted with anti-HA for detection of Nrf2 and Cul3, anti-CBD for detection of Keap1, and anti-Myc for detection of Rbx1. Equal amounts of Nrf2, Cul3, and Rbx1 were associated with either Keap1-WT or Keap1-NES (Fig. 4B, lower four panels), indicating that mutations introduced in the NES in Keap1 do not interfere with complex formation. In a parallel experiment, the Nrf2-Keap1-Cul3-Rbx1 complex pulled down by chitin beads was used for an in vitro ubiquitination assay. The chitin bead-bound proteins were incubated with purified E1, E2-UbcH5a, and ubiquitin in the presence of ATP. The ubiquitination reactions were terminated by boiling to release proteins from the chitin beads. The Nrf2 protein was immunoprecipitated with anti-Nrf2 antibodies and immunoblotted with antiubiquitin antibodies for detection of ubiquitinated Nrf2. In the absence of Cul3/Rbx during transfection or with omission of the purified E1 during the in vitro reaction, there were very low levels of ubiquitinated Nrf2 detected, indicating that ubiquitin was added during the in vitro reaction and both Cul3 and Rbx were required for the reaction (Fig. 4B, lanes 1 and 2). Significantly, there was a similar degree of ubiquitinated Nrf2 in the presence of either Keap1-WT or Keap1-NES (Fig. 4B, compare lane 3 with lane 4), demonstrating that the Keap1-NES-containing E3 ubiquitin ligase has enzymatic activity similar to that of the Keap1-WT-containing E3 ubiquitin ligase. Taken together, these data further verify that ubiquitination and degradation of Nrf2 are carried out in the cytosol. Keap1-NES is able to sequester Nrf2 in the nucleus to separate Nrf2 from the cytoplasmic ubiquitination and degradation machinery, resulting in stabilization of Nrf2.
Since Keap1-NES elevates the levels of Nrf2 in the nucleus, it is conceivable that there is increased activity of Nrf2 in the presence of Keap1-NES compared to that in the presence of Keap1-WT. To assess the abilities of Keap1-WT and Keap1-NES in regulating Nrf2-dependent transcriptional activity, a luciferase reporter gene assay was performed with transiently transfected MDA-MB-231 cells (Fig. 5A). An ARE (41 bp from mouse GST-Ya gene)-dependent firefly luciferase reporter gene was used to assess the transcriptional activity of Nrf2. The Renilla luciferase reporter gene was used as an internal control for transfection efficiency. In the presence of Keap1-WT, the activity of Nrf2-WT was low but was induced by tBHQ or SF treatment, as reported previously (Fig. 5A). All other Nrf2 mutants had impaired basal and inducible activities (Fig. 5A). The decreased activity of Nrf2-NLS2 was expected, since Nrf2-NLS2 is localized primarily in the cytosol. However, we are uncertain why Nrf2-NES1 lost its activity. It is likely due to the fact that NES1 is within Neh5, a transactivation domain (Fig. 1A). Nrf2-NES2 lost its activity because it could no longer bind the ARE as seen in the EMSA (Fig. 6A, lane 6). In contrast, the Nrf2 activity was greatly enhanced (>30-fold) in the presence of Keap1-NES compared to that in Keap1-WT-cotransfected cells (Fig. 5B). Two possible explanations can be proposed: (i) the blockage of nuclear export of Nrf2 by Keap1-NES increases the nuclear Nrf2-Keap complex, which is active in inducing ARE-dependent transcription, or (ii) the blockage of Keap1 export reduces trafficking of Keap1 between the cytosol and the nucleus, resulting in impaired nuclear export of Nrf2 and inefficient removal of Nrf2 from ARE.
FIG. 5.
Nuclear export of Nrf2 is required for repression of Nrf2-dependent transcriptional activity. (A and B) MDA-MB-231 cells were cotransfected with plasmids containing an ARE-dependent firefly luciferase reporter gene and expression plasmids for the indicated Nrf2 and Keap1 proteins. A plasmid encoding Renilla luciferase driven by the herpes simplex virus thymidine kinase promoter was included in all transfections to normalize transfection efficiency. The transfected cells were exposed to dimethyl sulfoxide (−), 50 μM tBHQ (t), or 10 μM SF (SF) for 16 h prior to analysis of firefly and Renilla luciferase activities in cell lysates. All samples were duplicated for each experiment, and the data shown represent the means for three independent experiments. The error bars indicate the standard deviations for the three experiments. Please note the difference in the scale of relative units between panels A and B.
FIG. 6.
Nrf2, not Keap1, associates with ARE. (A) In vitro interaction of Nrf2 or Keap1 with the ARE was analyzed by EMSA. MDA-MB-231 cells cotransfected with either an empty vector or expression vectors for the indicated Nrf2 and Keap1 proteins were either left untreated, treated with 10 μM SF, or treated with 100 μM tBHQ. Nuclear fractions were extracted and incubated with a 32P-labeled ARE-containing oligonucleotide in the absence or presence of either the unlabeled competing oligonucleotides or antibodies. The protein-DNA complexes were size separated on a nondenaturing polyacrylamide gel. The arrow indicates the position of the ARE-Nrf2 complexes. The asterisk indicates an ARE binding complex that does not contain Nrf2. Two different types of Nrf2 antibodies were used. The one labeled with an asterisk has a higher concentration. α-Nrf2, α-Keap, α-p300, and α-Gal4, anti-Nrf2, -Keap1, -p300, and -Gal4 antibodies. (B and C) In vivo interaction of Nrf2 or Keap1 with the ARE was determined by a ChIP assay. MDA-MB-231 cells were left untreated, treated with tBHQ, or treated with SF. DNA-protein complexes were cross-linked and immunoprecipitated with the indicated antibodies. Amounts of DNA containing the NQO1-ARE or the tubulin promoter were semiquantified by real-time PCR amplification with a primer pair flanking the human NQO1 ARE sequence (upper panel) or a primer pair specific for the human tubulin promoter (middle panel). No Ab, no antibody. A 0.8% proportion of total input DNA for immunoprecipitation was included as positive controls for real-time PCR amplification (lower panel). (C) Amounts of immunoprecipitated NQO1 ARE (upper panel) and the tubulin promoter (lower panel) were semiquantified by real-time PCR amplification and presented as a bar graph using the LightCycler 480 software.
Nrf2, not the Nrf2-Keap1 complex, binds to the ARE DNA regulatory sequence.
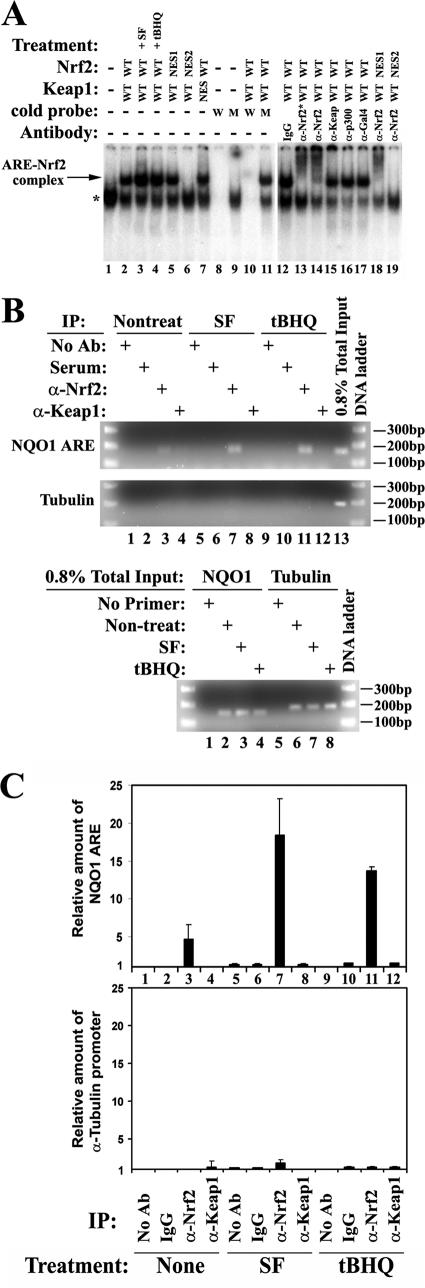
To determine whether the Nrf2-Keap1 complex binds the ARE DNA sequence, we used both EMSAs and ChIP assays to test the coexistence of Nrf2 and Keap1 on the ARE. In EMSA, the following two probes were designed and tested: (i) an ARE-containing DNA fragment from the human NQO1 gene and (ii) an ARE-containing DNA fragment from the gene encoding the human GCLC. Similar results were obtained, and the representative data using the probe from the human NQO1 ARE sequence are presented in Fig. 6A. Nuclear extracts from COS-1 cells, either mock transfected or cotransfected with expression vectors encoding the indicated Nrf2 and Keap1 proteins, were used for probe binding. Only one band appeared in the mock-transfected sample, while cotransfection of Nrf2 and Keap1 resulted in an additional band (Fig. 6A, lanes 1 and 2). SF and tBHQ increased the intensity of the upper band but had no effect on the lower band (Fig. 6A, compare lanes 3 and 4 with lanes 1 and 2), implying that the upper band contains Nrf2. Nrf2-NES1 behaved in the same way as Nrf2-WT, while Nrf2-NES2 lost its association with the ARE probe (lanes 5 and 6). Cotransfection of Nrf2-WT and Keap1-NES gave rise to two similar bands (lane 7). To test the binding specificity to the ARE-containing DNA probe, a competition assay was performed with a 100-fold excess of unlabeled DNA fragments: (i) a wild-type ARE core containing DNA fragment, the same one used for radiolabeling (Fig. 6A, lanes 8 and 10), and (ii) the mutated DNA fragment with the ARE core sequence GTGACTCAGC changed to GactCTCAcg (lowercase indicates the mutated nucleotides) (Fig. 6A, lanes 9 and 11). Only the wild-type DNA fragment abolished the formation of both bands, while the mutated one had no effect on the formation of either band (lanes 8 to 11), indicating that the protein complexes in these two bands bind the ARE core sequence specifically. We also tested the existence of Nrf2 and Keap1 in the two bands by a supershift assay. Two types of Nrf2 antibodies were used (lane 13 and 14). Only anti-Nrf2 antibodies abolished the upper band (Fig. 6A, compare lanes 13 and 14 with lane 2 and lane 18 with lane 5). In contrast, addition of the same amount of Keap1 antibodies had no effect on either band (compare lane 15 with lane 2). Immunoglobulin G (IgG), anti-p300, and anti-Gal4 had no effect (lanes 12, 16, and 17). These results demonstrate that Nrf2 binds the ARE sequence specifically and Keap1 does not exist in the ARE-binding protein complex, indicating that the Nrf2-Keap1 complex does not bind the ARE.
Next, we used a ChIP assay to confirm our conclusion from EMSA that Nrf2 but not Keap1 associates with ARE. Quantification of the immunoprecipitated DNA fragments was performed using a real-time PCR method. MDA-MB-231 cells were left untreated, treated with SF, or treated with tBHQ for 4 h. Chromatin DNA bound by Nrf2 or Keap1 was immunoprecipitated with anti-Nrf2 or anti-Keap1 antibody, respectively. The absence of antibodies and IgG incubation during chromatin immunoprecipitation were included as negative controls. The precipitated DNAs were recovered and used as templates for amplification of the ARE core-containing NQO1 promoter region or the promoter region of the α-tubulin gene using sequence-specific primer pairs. As shown in Fig. 6B and C, the NQO1 ARE was immunoprecipitated with the anti-Nrf2 antibody in the untreated cells (Fig. 6B and C, upper panels, lane 3 and bar 3), indicating the constitutive level of Nrf2 activity. SF or tBHQ increased the association of the ARE with Nrf2 approximately fourfold or threefold, respectively (Fig. 6B and C, upper panels, lanes 7 and 11 or bars 7 and 11). In contrast, the anti-Keap1 antibody did not immunoprecipitate any measurable amount of the NQO1 ARE (Fig. 6B and C, upper panels, lanes or bars 4, 8, and 12). The specific binding of Nrf2 to the ARE was further verified by the absence of DNA amplification in the negative control samples (no antibody, lanes and bars 1, 5, and 9, or IgG immunoprecipitation, lanes and bars 2, 6, and 10). To test the binding specificity of Nrf2 for the ARE sequence, aliquots of the same samples were used for amplification of the promoter region of a non-Nrf2 downstream gene, that for α-tubulin. There was no amplification of the α-tubulin promoter in any sample (Fig. 6B, middle panel, and 6C, lower panel), although the total input amounts of DNA were similar among the samples (Fig. 6B, lower panel). These results indicate that endogenous Nrf2 binds specifically to the ARE sequence under both basal and induced conditions. More significantly, there is no Keap1 in the ARE binding complex in vivo. Collectively, data from both EMSA and ChIP assays demonstrate that the Nrf2-Keap complex does not bind the ARE, indicating that the interaction of Nrf2 with the ARE or with Keap1 is mutually exclusive. Therefore, we conclude that the primary function of Keap1 nuclear translocation is to dissociate Nrf2 from the ARE during the postinduction stage.
Nuclear localization of Keap1 is independent of Nrf2.
Since the Nrf2-Keap1 complex does not bind to the ARE, in order to play a role in turning off the Nrf2 activation after induction, nuclear transport of Keap1 is likely independent of Nrf2. To confirm that Keap1 travels into the nucleus without the assistance of Nrf2, a mouse embryonic fibroblast cell line derived from an Nrf2 knockout mouse was used for an indirect immunofluorescence assay. Nrf2−/− cells were singly transfected with a plasmid containing either Keap1-WT or Keap1-NES for 36 h. Cells were left untreated or treated with LMB for 3 h before fixing and staining were done. Keap1-WT was located in the cytosol in untreated cells (Fig. 7A, panels A to C, and F7B, bar 1). It localized in the nucleus when nuclear export was blocked by LMB (Fig. 7A, panels D to F, and 7B, bar 2). In contrast, even in the absence of LMB, Keap1-NES was localized in the nucleus due to its impaired nuclear export function (Fig. 7A, panels G to I, and 7B, bar 3). These results clearly demonstrate that Keap1 is able to travel into the nucleus even in the absence of Nrf2.
FIG. 7.

Nuclear import of Keap1 is independent of Nrf2. (A) Nrf2−/− mouse embryonic fibroblast cells were singly transfected with an expression vector for either Keap1-WT (panels A to F) or Keap1-NES (panels G to I). Cells were left untreated (Nontreat) or treated with LMB (panels D to F) for 3 h. Subcellular localization of the indicated Keap1 protein was determined by an indirect immunofluorescence analysis using anti-CBD antibodies. K-WT, Keap1-WT. (B) The same slides from panel A were used to count at least 100 positive cells. Percentages of cells that localized predominantly in the cytosol (C), the whole cell (W), or the nucleus (N) were presented as a bar graph.
Previously Yamamoto's group identified two Keap1 binding motifs in the N terminus of Nrf2, ETGE and LWRQDIDLG, which are highly conserved in another member of the CNC-bZIP family called Nrf1 (36, 42). It is reasonable to assume that the observed Keap1 nuclear transport in Nrf2 knockout cells may be assisted by Nrf1. Nevertheless, our recent result indicates that Keap1 contains an NLS and mutation of the NLS impairs nuclear translocation of Keap1 in MDA-MB-231 cells treated with LMB (unpublished data). In accordance with our data, Zhang and colleagues have recently demonstrated that Keap1 does not regulate Nrf1, although the Keap1 binding motif is conserved in Nrf1(49).
Keap1 regulates postinduction repression of Nrf2.
In order to fulfill its duty during the postinduction stage, Keap1 must travel into the nucleus to dissociate Nrf2 from the ARE and transport Nrf2 out of the nucleus. Therefore, it is reasonable to assume that any change in Keap1 shuttling will interfere with the postinduction repression process. To test this, the levels of Nrf2 at different postinduction time points were measured by immunoblot analysis using total lysates from 231 cells cotransfected with an expression vector for Nrf2 and an expression vector for either Keap1-WT or Keap1-NES. Following tBHQ treatment for 4 h, cells were washed and incubated in normal medium for the indicated periods of time. When Keap1-WT was cotransfected, Nrf2 levels decreased sharply after removal of tBHQ, and they reached the same level as that for the untreated control within 24 h (Fig. 8A, upper panel, lanes 1 to 6). In contrast, there was a very high basal level of Nrf2 in the presence of Keap1-NES even in the untreated sample (Fig. 8A, upper panel, lane 12). tBHQ treatment did not significantly enhance levels of Nrf2 in the presence of Keap1 NES (Fig. 8A, upper panel, lanes 7 to 11). The postinduction repression curve in Fig. 8B shows a dramatic difference in the inhibition of Nrf2 in the presence of Keap1-WT or Keap1-NES. Hence, the NES in Keap1 controls postinduction repression by removing Nrf2 from the nucleus and targeting Nrf2 to the cytoplasmic ubiquitination machinery for ubiquitin conjugation and subsequent proteasomal degradation.
FIG. 8.
Keap1 confers postinduction repression of Nrf2 by escorting nuclear export of Nrf2. (A and B) Postinduction repression of the steady-state levels of Nrf2 was accessed in MDA-MB-231 cells cotransfected with an expression vector for Nrf2 and an expression vector for either Keap1-WT or Keap1-NES. Cells were treated with 100 μM tBHQ for 4 h. After removal of tBHQ by washing, cells were further incubated in normal medium for the indicated time periods. Total cell lysates were subjected to immunoblot analysis with anti-HA, anti-CBD, and anti-lamin A antibodies. (B) The relative intensities of the Nrf2 bands were quantified by the ChemiDoc XRS gel documentation system from Bio-Rad and plotted on a linear graph. (C) Postinduction repression of the Nrf2-dependent transcriptional activity was determined in MDA-MB-231 cells cotransfected with plasmids encoding an ARE-firefly luciferase, thymidine kinase-Renilla luciferase, Nrf2, and the indicated Keap1 protein. The transfected cells were exposed to 50 μM tBHQ for 16 h. Following removal of tBHQ, cells were further incubated in normal medium for the indicated time periods prior to measurement of firefly and Renilla luciferase activities. The experiment was repeated three times, and standard deviations are shown as error bars. Untreat, untreated.
To circumvent sensitivity problems associated with EMSA methodology, we used the ARE-firefly luciferase reporter gene assay to measure the activity of Nrf2, rather than ARE-bound Nrf2, during the postinduction stage. Cells were transfected with expression vectors for ARE-luciferase, Renilla luciferase, Nrf2, and an expression vector for Keap1-WT or Keap1-NES. Following tBHQ treatment for 16 h, cells were washed and further incubated for different periods of time prior to measurement of dual luciferase activities. The basal activity of Nrf2 was significantly high in cells cotransfected with Keap1-NES compared to that in cells cotransfected with Keap1-WT (Fig. 8C, untreated samples). tBHQ increased the activity of Nrf2 roughly 10-fold in Keap1-WT-cotransfected cells, and the tBHQ-induced Nrf2 activity was quickly reduced in a time-dependent manner, with a half-life of approximately 32 h (Fig. 8C). In contrast, the postinduction repression of Nrf2 activity in the presence of Keap1-NES was minimal, with comparable Nrf2 activity at all postinduction time points tested (Fig. 8C). These data further confirm that disturbance of nuclear export activity of Keap1 results in altered activation of Nrf2-mediated genes with a pronounced increase in protein levels and duration. Collectively, these results clearly illustrate the crucial role of Keap1 trafficking in controlling the postinduction repression of Nrf2 activity.
DISCUSSION
Keap1 has emerged as a key regulator of the Nrf2-mediated antioxidant response pathway. Previously Keap1 was identified as part of the E3 ubiquitin ligase complex, which mediates ubiquitination and subsequent proteasomal degradation to control the low constitutive level of Nrf2 in unstressed cells (4, 10, 23, 48). Keap1 is capable of sensing a change in intercellular redox conditions through cysteine-dependent posttranslational modification, resulting in decreased Nrf2 ubiquitination, increased levels of Nrf2, and ultimately activation of Nrf2-dependent gene expression (46). How the Nrf2-mediated antioxidant response is turned on by oxidative stress or chemopreventive compounds is relatively well studied. However, many questions remain to be answered. For instance, the mechanism of nuclear import and export of Nrf2, Keap1, and their complex is still controversial. More significantly, there is no study regarding how the Nrf2 signal is turned off during the postinduction period, when intracellular redox conditions are gradually recalibrated to homeostasis. Here we report that it is Keap1 that controls postinduction repression of the activity of Nrf2. There are five important findings demonstrated in this study. (i) The NES in Keap1 is the nuclear export sequence that transports the Nrf2-Keap1 complex out of the nucleus during the postinduction stage. (ii) The Keap1-Cul3-Rbx1 E3 ubiquitin ligase complex ubiquitinates Nrf2 in the cytosol, demonstrating that Nrf2 degradation occurs in the cytosol, since ubiquitination and degradation are coupled reactions. (iii) The Keap1-Nrf2 complex does not bind the ARE, indicating that the purpose of Keap1 nuclear translocation is to dissociate Nrf2 from the ARE to turn off the antioxidant response during the postinduction stage. (iv) Keap1 is able to travel into the nucleus independently, meaning that Keap1 most likely possesses its own nonclassical nuclear import sequence that may be controlled by cytoplasmic redox conditions. (v) Nuclear export of Keap1 plays a key role in controlling the postinduction repression of the activity of Nrf2, and impairment of Keap1 trafficking results in prolonged recovery time needed for attenuating the Nrf2-mediated antioxidant response.
In this study, we expand the importance of Keap1 in regulating the Nrf2-dependent antioxidant response to a new horizon. In addition to the previous finding that Keap1 controls the Nrf2-dependent antioxidant response during induction by regulating levels of Nrf2, we show that Keap1 is also critical for postinduction repression. Keap1 has dual roles by functioning as follows: (i) as a molecular sensor; cysteine residues in Keap1 sense redox imbalance; (ii) as a molecular switch; Keap1 turns the Nrf2 signaling pathway on and off according to intracellular redox conditions. Based on these studies, we propose a model that explains how Keap1 fulfills its dual roles (Fig. 9). In unstressed cells, Keap1 functions as an E3 ubiquitin ligase and constantly targets Nrf2 for ubiquitination and proteasomal degradation to maintain low levels of Nrf2 that mediate the constitutive expression of Nrf2 downstream genes. Upon disturbance of redox balance, Keap1 is able to sense a change in intercellular redox conditions through cysteine-dependent posttranslational modification, resulting in decreased Nrf2 ubiquitination and degradation. As a consequence, Nrf2 saturates the binding capacity of Keap1, leading to nuclear translocation of Keap1-unbound Nrf2. In the nucleus, Nrf2 binds to the ARE to activate Nrf2 downstream genes. During the postinduction period, expression of Nrf2 downstream genes, including those encoding γ-glutamylcysteine synthetase, heme oxygenase 1, ferritin H, and thioredoxin, restores intracellular redox homeostasis. Keap1 travels into the nucleus to dissociate Nrf2 from the ARE and subsequently exports the Nrf2-Keap1 complex out of the nucleus. Once in the cytosol, the Keap1-Nrf2 complex binds to the core Cul3-Rbx1 ubiquitin ligase complex, resulting in ubiquitination and degradation of Nrf2. Hence, the Nrf2 pathway is turned off.
FIG. 9.
Schematic model of Nrf2 regulation by Keap1. Keap1 is a key regulator of the Nrf2 signaling pathway and serves as a molecular switch to turn on and off the Nrf2-mediated antioxidant response. (i) The switch is in off position: under basal conditions, Keap1, functioning as an E3 ubiquitin ligase, constantly targets Nrf2 for ubiquitination and degradation. As a consequence, there are minimal levels of Nrf2. (ii) The switch is turned on: oxidative stress or chemopreventive compounds inhibit activity of the Keap1-Cul3-Rbx1 E3 ubiquitin ligase, resulting in increased levels of Nrf2 and activation of its downstream target genes. (iii) The switch is turned off again: Upon recovery of cellular redox homeostasis, Keap1 travels into the nucleus to remove Nrf2 from the ARE. The Nrf2-Keap1 complex is then transported out of the nucleus by the NES in Keap1. In the cytosol, the Nrf2-Keap1 complex associates with the Cul3-Rbx1 core ubiquitin machinery, leading to degradation of Nrf2. For clarity, the constitutive cytoplasmic-nuclear shuttling of Nrf2, Keap1, and the complex is omitted.
Noticeably, there were higher basal levels of Nrf2 in the presence of Keap1-NES than in the presence of Keap1-WT, as shown in Fig. 8 (compare lane 12 with lane 6) and Fig. 2E (lane 13 and lane 15). We believe that this reflects the constitutive Nrf2 and Keap1 shuttling even under basal conditions (without treatment), as demonstrated in Fig. 1A and B. The low level of constitutive Nrf2 is trapped by Keap1-NES in the nucleus to block its access to the cytoplasmic degradation machinery, resulting in increased basal levels of Nrf2 in cells cotransfected with Keap-NES. The constant shuttling of Keap1 and Nrf2 seen in cultured cells is likely due to the low levels of oxidative stress constantly produced by normal metabolic processes. As a result, the Nrf2 signaling pathway is minimally activated with an attempt to restore intracellular redox conditions. This may explain why some of the Nrf2 downstream target genes are constitutively expressed even in untreated cells. Hence, the association and dissociation of Nrf2 and Keap1, as well as their shuttling, are dynamic processes. Any interference in the Nrf2-Keap1 complex formation or trafficking of Keap1 or Nrf2 should affect basal levels of Nrf2 and the Nrf2-mediated antioxidant responses.
It is intriguing that two NESs in Nrf2 and one in Keap1 have been reported to regulate the export of Nrf2 (17, 20, 27, 28, 31, 38). All three NESs have a consensus sequence defined as a classical NES with a cluster of hydrophobic residues. In this study, we made point mutations in each of the three putative NESs in the context of full-length Nrf2 or Keap1, in that the hydrophobic residues in NES are replaced with alanine residues, to minimize possible artifacts seen with deletion mutants or with fusion proteins. When each of these mutated proteins was singly transfected, all three proteins were predominantly localized in the nucleus, consistent with the previous finding that these NESs from Nrf2 and Keap1 have characteristic nuclear export functions. However, in the presence of both Nrf2 and Keap1, the two NESs in Nrf2 lost their nuclear export functions and had subcellular localization profiles similar to that of Nrf2-WT (Fig. 2D, top panel). In sharp contrast, cotransfection of Keap1-NES and Nrf2-WT significantly redistributed Nrf2 into the nucleus (Fig. 2D, top panel), indicating that the NES in Keap1 is the NES that regulates the export of the Keap1-Nrf2 complex under more physiologically relevant conditions. At present, it is not clear whether the NESs in Nrf2 have any endogenous functions or the nuclear export activities observed in the NESs in Nrf2 are just artifacts in Nrf2-singly overexpressed systems.
A striking finding in this report is that Nrf2 ubiquitination and degradation occur in the cytosol. We have provided two pieces of evidence to support this: (i) Cul3 is a cytoplasmic factor that does not shuttle between the cytosol and the nucleus, and (ii) the stability of Nrf2 in the presence of Keap1-NES is dramatically increased over that in the presence of Keap1-WT. Therefore, nuclear export of Nrf2 is required for its degradation. In this regard, our data are in agreement with the conclusion from Jaiswal's group. They showed that an Nrf2 deletion mutant with the C-terminal NES removed had a longer half-life than Nrf2-WT in Hepa-1 cells singly expressing each of the Nrf2 proteins (17). As shown in Fig. 2A and B, the Nrf2-NES2 mutant was primarily localized in the nucleus when it was expressed alone. Together, these two observations demonstrate that blocking of the nuclear export of Nrf2 enhances the stability of Nrf2 due to the subcellular separation of Nrf2 from the ubiquitination and degradation machinery. Surprisingly, Pickett's group has proposed an alternative model in which Nrf2 is considered as a nuclear protein that drives constitutive expression of Nrf2 downstream genes (31). They have concluded that ubiquitination and degradation are carried out in the nucleus following nuclear translocation of Keap1 based on their observation that the ubiquitinated Nrf2 protein was detected only in the nuclear fraction but not in the cytoplasmic fraction. However, there was a significantly smaller amount of Nrf2 in the cytoplasmic fraction, as shown in their immunoprecipitation input blot (31). In addition, they showed that there were higher levels of Nrf2 in cells cotransfected with Keap1ΔNES than in cells cotransfected with Keap1-WT (31). This observation is in contradiction with their conclusion that Keap1-mediated degradation of Nrf2 is carried out in the nucleus, since Keap1ΔNES retains Nrf2 in the nucleus and there was no defect in the interaction of Keap1ΔNES with Nrf2 or Cul3 or in Keap1ΔNES-dependent E3 ubiquitin ligase activity as demonstrated in this study. Our finding that Cul3 is a nonshuttling cytoplasmic factor provides strong evidence that ubiquitination and degradation of Nrf2 are carried out in the cytosol. Furthermore, the notion that the degradation of Nrf2 occurs in the cytosol fits nicely with our finding and others’ that the nuclear export of Nrf2 is required for the degradation of Nrf2 (17).
Interestingly, we consistently observed two bands in EMSA when two different probes, AREs from human GCLC and human NQO1, were used. The EMSA data from human NQO1 have been presented in Fig. 6A. Obviously, both bands resulted from proteins specifically bound to the core ARE sequence, since the unlabeled ARE-core mutated probe had no effect, while both bands disappeared when the cold wild-type ARE was preincubated. In addition, anti-Nrf2 caused the disappearance only of the upper band but not the lower band, indicating that the lower band does not contain Nrf2. The ARE core sequence, also termed the electrophile response element, was first identified in the promoters of Ya subunits of rat and mouse GST. The ARE core consensus sequence has since been defined as 5′-RTGACNNNGC-3′ (43). The AP-1 recognition site TRE (5′-TGACTCA-3′) and the ATF/CREB binding sequence (5′-TGACGTCA-3′) partially overlap with the ARE core sequence (6, 32). Although increasing lines of evidence identify the Nrf2-Maf heterodimer as the functional complex regulating ARE-dependent gene expression, transcription factors, such as Jun, c-Fos, FRA-1, FRA-2, and Nrf1, have been reported to interact with the ARE (18, 21, 39). It is likely that the lower band in our EMSA contains a dimer of the two members of the AP-1 family.
Protein transport between the nucleus and the cytoplasm provides an elegant way to control gene expression in general. In addition to regulating access to DNAs, controlling subcellular localization of Nrf2 adds another dimension of regulation, since degradation of Nrf2 occurs only in the cytosol. We have demonstrated that both Nrf2 and Keap1 are shuttle proteins that are constantly undergoing cytoplasmic-nuclear trafficking. Nrf2 contains a very strong classical NLS, and Keap1 is able to translocate into the nucleus without assistant from Nrf2. Currently we still do not know when and how Keap1 travels into the nucleus. It is possible that Keap1 possesses a redox-sensitive NLS that is activated upon recovery of intracellular redox homeostasis during the postinduction stage, in addition to its low rate of constitutive trafficking. Alternatively, the rate of Keap1 shuttling between the nucleus and the cytosol is constant, regardless of the intracellular redox conditions. In this scenario, the activity of Keap1-Cul3-Rbx1 E3 ubiquitin ligase is the only step that is controlled by intracellular redox conditions. Currently there are no data in favor of either hypothesis. Clearly, understanding the nuclear import mechanism of Keap1 will greatly aid our knowledge of how Keap1 regulates the Nrf2-dependent antioxidant response.
In conclusion, we have identified Keap1 as a postinduction repressor of Nrf2 and have demonstrated that impairment of Keap1 trafficking results in (i) high basal levels of Nrf2 and (ii) prolonged recovery time needed for attenuating the Nrf2-mediated antioxidant response. Although activation of the Nrf2 signaling pathway provides cellular protection against deleterious environmental insults, constitutive activation of Nrf2 is lethal, as seen with Keap1 knockout mice (41). Therefore, it is essential to promptly turn the Nrf2 signaling pathway on and off according to intracellular redox conditions. Disregulation of either the turning-on (induction) or the turning-off (postinduction repression) process will inevitably lead to disease states due to disturbance of the redox balance.
Acknowledgments
We thank S. E. Purdom-Dickinson for critical review of the manuscript.
This work was supported by a research grant from NIEHS to D. D. Zhang (1 R01 ES015010-01).
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Alam, J., D. Stewart, C. Touchard, S. Boinapally, A. M. Choi, and J. L. Cook. 1999. Nrf2, a Cap′n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 274:26071-26078. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, Y., H. Sato, N. Nishimura, S. Takahashi, K. Itoh, and M. Yamamoto. 2001. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 173:154-160. [DOI] [PubMed] [Google Scholar]
- 3.Chan, K., X. D. Han, and Y. W. Kan. 2001. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 98:4611-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullinan, S. B., J. D. Gordan, J. Jin, J. W. Harper, and J. A. Diehl. 2004. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24:8477-8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullinan, S. B., D. D. Zhang, M. Hannink, E. Arvisais, R. J. Kaufman, and J. A. Diehl. 2003. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23:7198-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton, T. P., H. G. Shertzer, and A. Puga. 1999. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 39:67-101. [DOI] [PubMed] [Google Scholar]
- 7.Dinkova-Kostova, A. T., W. D. Holtzclaw, R. N. Cole, K. Itoh, N. Wakabayashi, Y. Katoh, M. Yamamoto, and P. Talalay. 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 99:11908-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggler, A. L., G. Liu, J. M. Pezzuto, R. B. van Breemen, and A. D. Mesecar. 2005. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 102:10070-10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enomoto, A., K. Itoh, E. Nagayoshi, J. Haruta, T. Kimura, T. O'Connor, T. Harada, and M. Yamamoto. 2001. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59:169-177. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa, M., and Y. Xiong. 2005. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol. Cell. Biol. 25:162-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong, F., K. R. Sekhar, M. L. Freeman, and D. C. Liebler. 2005. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J. Biol. Chem. 280:31768-31775. [DOI] [PubMed] [Google Scholar]
- 12.Hu, X., J. R. Roberts, P. L. Apopa, Y. W. Kan, and Q. Ma. 2006. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol. Cell. Biol. 26:940-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iida, K., K. Itoh, Y. Kumagai, R. Oyasu, K. Hattori, K. Kawai, T. Shimazui, H. Akaza, and M. Yamamoto. 2004. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 64:6424-6431. [DOI] [PubMed] [Google Scholar]
- 14.Iizuka, T., Y. Ishii, K. Itoh, T. Kiwamoto, T. Kimura, Y. Matsuno, Y. Morishima, A. E. Hegab, S. Homma, A. Nomura, T. Sakamoto, M. Shimura, A. Yoshida, M. Yamamoto, and K. Sekizawa. 2005. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 10:1113-1125. [DOI] [PubMed] [Google Scholar]
- 15.Itoh, K., T. Ishii, N. Wakabayashi, and M. Yamamoto. 1999. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 31:319-324. [DOI] [PubMed] [Google Scholar]
- 16.Itoh, K., N. Wakabayashi, Y. Katoh, T. Ishii, K. Igarashi, J. D. Engel, and M. Yamamoto. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13:76-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain, A. K., D. A. Bloom, and A. K. Jaiswal. 2005. Nuclear import and export signals in control of Nrf2. J. Biol. Chem. 280:29158-29168. [DOI] [PubMed] [Google Scholar]
- 18.Jeyapaul, J., and A. K. Jaiswal. 2000. Nrf2 and c-Jun regulation of antioxidant response element (ARE)-mediated expression and induction of gamma-glutamylcysteine synthetase heavy subunit gene. Biochem. Pharmacol. 59:1433-1439. [DOI] [PubMed] [Google Scholar]
- 19.Kanwar, J. R. 2005. Anti-inflammatory immunotherapy for multiple sclerosis/experimental autoimmune encephalomyelitis (EAE) disease. Curr. Med. Chem. 12:2947-2962. [DOI] [PubMed] [Google Scholar]
- 20.Karapetian, R. N., A. G. Evstafieva, I. S. Abaeva, N. V. Chichkova, G. S. Filonov, Y. P. Rubtsov, E. A. Sukhacheva, S. V. Melnikov, U. Schneider, E. E. Wanker, and A. B. Vartapetian. 2005. Nuclear oncoprotein prothymosin alpha is a partner of Keap1: implications for expression of oxidative stress-protecting genes. Mol. Cell. Biol. 25:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsuoka, F., H. Motohashi, T. Ishii, H. Aburatani, J. D. Engel, and M. Yamamoto. 2005. Genetic evidence that small Maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol. Cell. Biol. 25:8044-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaunig, J. E., and L. M. Kamendulis. 2004. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 44:239-267. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, A., M. I. Kang, H. Okawa, M. Ohtsuji, Y. Zenke, T. Chiba, K. Igarashi, and M. Yamamoto. 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24:7130-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi, M., and M. Yamamoto. 2005. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 7:385-394. [DOI] [PubMed] [Google Scholar]
- 25.Kwak, M. K., N. Wakabayashi, K. Itoh, H. Motohashi, M. Yamamoto, and T. W. Kensler. 2003. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 278:8135-8145. [DOI] [PubMed] [Google Scholar]
- 26.Levonen, A. L., A. Landar, A. Ramachandran, E. K. Ceaser, D. A. Dickinson, G. Zanoni, J. D. Morrow, and V. M. Darley-Usmar. 2004. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 378:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, W., M. R. Jain, C. Chen, X. Yue, V. Hebbar, R. Zhou, and A. N. Kong. 2005. Nrf2 possesses a redox-insensitive nuclear export signal overlapping with the leucine zipper motif. J. Biol. Chem. 280:28430-28438. [DOI] [PubMed] [Google Scholar]
- 28.Li, W., S. W. Yu, and A. N. Kong. 2006. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J. Biol. Chem. 281:27251-27263. [DOI] [PubMed] [Google Scholar]
- 29.Liu, J., H. Chen, D. S. Miller, J. E. Saavedra, L. K. Keefer, D. R. Johnson, C. D. Klaassen, and M. P. Waalkes. 2001. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol. Pharmacol. 60:302-309. [DOI] [PubMed] [Google Scholar]
- 30.Moinova, H. R., and R. T. Mulcahy. 1999. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem. Biophys. Res. Commun. 261:661-668. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, T., P. J. Sherratt, P. Nioi, C. S. Yang, and C. B. Pickett. 2005. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J. Biol. Chem. 280:32485-32492. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, T., P. J. Sherratt, and C. B. Pickett. 2003. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43:233-260. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Gomez, M., M. K. Kwak, P. M. Dolan, K. Itoh, M. Yamamoto, P. Talalay, and T. W. Kensler. 2001. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 98:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruef, J., K. Peter, T. K. Nordt, M. S. Runge, W. Kubler, and C. Bode. 1999. Oxidative stress and atherosclerosis: its relationship to growth factors, thrombus formation and therapeutic approaches. Thromb. Haemostasis 82(Suppl. 1):32-37. [PubMed] [Google Scholar]
- 35.Simonian, N. A., and J. T. Coyle. 1996. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 36:83-106. [DOI] [PubMed] [Google Scholar]
- 36.Tong, K. I., Y. Katoh, H. Kusunoki, K. Itoh, T. Tanaka, and M. Yamamoto. 2006. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol. Cell. Biol. 26:2887-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umemura, T., Y. Kuroiwa, Y. Kitamura, Y. Ishii, K. Kanki, Y. Kodama, K. Itoh, M. Yamamoto, A. Nishikawa, and M. Hirose. 2006. A crucial role of Nrf2 in in vivo defense against oxidative damage by an environmental pollutant, pentachlorophenol. Toxicol. Sci. 90:111-119. [DOI] [PubMed] [Google Scholar]
- 38.Velichkova, M., and T. Hasson. 2005. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 25:4501-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venugopal, R., and A. K. Jaiswal. 1996. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 93:14960-14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakabayashi, N., A. T. Dinkova-Kostova, W. D. Holtzclaw, M. I. Kang, A. Kobayashi, M. Yamamoto, T. W. Kensler, and P. Talalay. 2004. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA 101:2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakabayashi, N., K. Itoh, J. Wakabayashi, H. Motohashi, S. Noda, S. Takahashi, S. Imakado, T. Kotsuji, F. Otsuka, D. R. Roop, T. Harada, J. D. Engel, and M. Yamamoto. 2003. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 35:238-245. [DOI] [PubMed] [Google Scholar]
- 42.Wang, W., and J. Y. Chan. 2006. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. Inhibition of nuclear translocation and transacting function. J. Biol. Chem. 281:19676-19687. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman, W. W., and W. E. Fahl. 1997. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA 94:5361-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wild, A. C., H. R. Moinova, and R. T. Mulcahy. 1999. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 274:33627-33636. [DOI] [PubMed] [Google Scholar]
- 45.Yang, C. S., J. M. Landau, M. T. Huang, and H. L. Newmark. 2001. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 21:381-406. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, D. D. 2006. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 38:769-789. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, D. D., and M. Hannink. 2003. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 23:8137-8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, D. D., S. C. Lo, J. V. Cross, D. J. Templeton, and M. Hannink. 2004. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 24:10941-10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, Y., D. H. Crouch, M. Yamamoto, and J. D. Hayes. 2006. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem. J. 399:373-385. [DOI] [PMC free article] [PubMed] [Google Scholar]