Abstract
Objective
To determine levels of intrauterine infection and transcriptional activity in cord blood mononuclear cells collected at term from fetuses born to HIV-infected pregnant women on highly active anti-retroviral therapy (HAART).
Methods
RNA and DNA were isolated from maternal placental tissues and fetal cord blood specimens obtained at term from HIV-infected pregnant women on HAART. Levels of integrated HIV provirus and mRNA transcripts were determined by real-time PCR.
Results
Detectable levels of transcriptionally active integrated provirus were present in approximately 27% of cord blood samples (n=22) collected from fetuses, born to HIV-positive mothers on HAART. Levels of HIV-p24 antigen in cultures detected in randomly selected cord blood samples confirmed the presence of inducible infectious virus.
Conclusions
These findings suggest that some fetuses from HIV-infected mothers on HAART who may present as HIV-negative infants postpartum, can harbor circulating leukocytes that are productively infected by intrauterine transmission (IUT) of HIV.
Keywords: HIV, Intrauterine Transmission, Cesarean Section, MTCT
Introduction
Perinatal transmission of HIV is a serious problem worldwide, especially in developing countries. Based on statistics gathered through 2002, an estimated 19.2 million women and 3.2 million children (≤15 years old) were infected with HIV globally1. Mother-to-Child-Transmission (MTCT) of HIV occurs generally by transfer of infection in one of three ways: from mother to child in utero before delivery (intrauterine transmission, IUT), intrapartum (during labor), or postpartum (through breast feeding). In the US and other developed countries, effective clinical measures to limit MTCT, including administration of highly active anti-retroviral therapy (HAART) to the mother during the antenatal period, cesarean deliveries, scheduled prior to rupture of membranes or onset of labor (when IUT is most likely to occur) and avoidance of breast feeding have reduced the overall incidence of MTCT to ∼1−2%2. These transmission rates however, are based primarily on measurements of neonatal plasma viral loads determined during the early postpartum period and may not accurately or completely reflect the status of fetal infection during the antenatal period. Recent studies indicate that after the administration of such effective clinical strategies, IUT accounts for ∼50% of MTCT of HIV3. However, the frequency of perinatal transmission, resulting in the presence of cells in fetal circulation harboring potentially infectious integrated proviral DNA, has not been determined for pregnant women undergoing prenatal anti-retroviral therapy. A discordance between the frequency of peripheral blood mononuclear cells harboring integrated provirus in the fetus and the frequency of neonates positive for plasma viral RNA could raise important questions relating to mechanisms of viral clearance and the establishment of either “occult” or “active” infection in the neonate. Without a better understanding of the status of infection (active versus latent or non-productive) of circulating fetal cells, the timing, mechanism(s) and clinical significance of perinatal transmission during HAART remains unclear. In this report we address these issues in a focused study to test the hypothesis that the frequency of evidence of transcriptionally active and potentially infectious, integrated proviral DNA in circulating mononuclear cells in the fetus corresponds with the frequency of evidence of detectable levels of HIV RNA in plasma of infants born to infected women undergoing prenatal anti-retrovial therapy to prevent MTCT.
Material and Methods
Subjects
Subjects from whom blood and tissue samples were collected were identified from parturients who were known to be HIV-positive via a positive enzyme-linked immunosorbent assay and confirmatory West blot and received prenatal care and delivered at Grady Memorial Hospital (GMH) in Atlanta, Georgia. Grady serves a high-risk, indigent population and is a regional referral center for high-risk obstetrics patients in the state of Georgia, including HIV-infected pregnant patients and provides care for 60−75 patients per year in accordance with current guidelines for the care of the HIV-positive parturients4, 5.
Twenty-two patients were recruited for this study over an approximate 2 year period between 2004−2006. Those who consented to participate in the study received standard initial prenatal laboratory tests plus hepatic and renal function tests, total CD4+ T cell counts and HIV plasma viral loads as well as ultrasound examination to determine the estimated gestational ages and date of delivery. Once evidence of HIV infection and plasma viral loads had been determined, HAART was initiated for enrolled patients beyond their 14th week of pregnancy (n=20) after detailed counseling on the risks and benefits. Two patients were already undergoing HAART prior to their most recent pregnancy. HAART consisted of lamivudine and zidovudine plus either nelfinavir or nevirapine, depending on levels of patient plasma viral loads. Plasma viral loads were determined intermittently throughout pregnancy to assess the response to medication and for evidence of resistance. Renal and hepatic functions were followed to ensure maternal tolerance to the antiretrovirals. Third trimester viral loads were assessed for appropriate counseling on route of delivery.
Patients were screened during their antenatal course for evidence of genital tract infections including bacterial vaginosis, group B streptococcus (GBS) and sexually transmitted diseases other than HIV. None of the patients from whom tissue sample were collected demonstrated evidence of co-existing allergic diseases, chorioamnionitis or other untreated genital tract infections at the time of delivery.
Routinely, cesarean deliveries are performed on HIV+ mothers at Grady when their plasma viral loads at term is > 1000 copies/ml or there is an obstetric indication, e.g. previous cesarean section, or mal-presentation. Elective Cesarean deliveries, however, were scheduled for all patients enrolled in this study at 38 weeks gestation with three hours of intravenous zidovudine immediately prior to surgery. Maternal blood samples were obtained when the patient presented for her pre-operative evaluation the day prior to her scheduled surgery. Cord blood and placental tissues were meticulously collected in a sterile fashion during delivery. Portions of placental tissue were subjected to routine post-operative pathological examination. No clinical or pathological evidence of abruption was observed in any of the 22 patients. All blood and tissue specimens were collected with informed consent under Emory University IRB approved protocols. Plasma levels of HIV RNA in infants were routinely performed with the first week postpartum using the Amplicor HIV-1 Monitor test (Roche Molecular Systems, Inc,. Somerville, NJ).
Collection and Preparation of Human tissue samples
Single cell suspensions of maternal cells from collagenase digested placental tissues and fetal mononuclear cells from human umbilical cord blood, collected following delivery of term fetuses, were separated on density gradient medium to isolate cells of hematopietic origin ad previously described6, 7.
Real-time PCR amplification
Determining host cell nuclear integration of HIV
Infection of maternal and fetal tissues was determined at the level of HIV proviral nuclear integration into the host cell genome by measuring the mean number of copies of integrated proviral DNA per 106 cells. DNA was prepared from 2 × 106 − 1 × 107 CBMCs and real time PCR amplification and measurements were performed using appropriate β-globin and HIV proviral cDNA copy-number standards as previously described7 and using real-time PCR primers and conditions listed in Table 1. Briefly, 100 nM each of Alu 1 and a compound primer, LM667 were incubated with 200 ng of sample DNA. PCR was performed (Apollo ATC 401 thermocycler; 95 °C for 3 min, then 23 cycles at 94 °C, 1 min; 55 °C, 1 min; 72 °C, 1 min). Integrated provirus or HIV cDNA standard concentrations were determined by a modified absolute concentration Nested Real-Time qPCR assay (2). Pre-amplified samples and standards (2 uL/well) were subjected to qPCR with 200 nM each of a quasi-nested primer pair (Lambda-T; AA55M; 5') with SYBR Green on a BioRad iCycler (95 °C for 3 min, then 50 cycles at 93 °C, 1 min; 55 °C, 1 min; 72 °C, 1 min). Efficiencies of the qPCR assays were calculated from the slopes of the standard curves, and inverse natural logarithms of the Cts (adjusted for efficiency) used to calculate starting copy numbers of integrated HIV provirus over a linear range between 6 and 6000 copies per 106 cell equivalents.
Table 1.
Real Time PCR Primers and Reaction Conditions
| NA Assessed | Primers | Sequence | Conc. | Conditions |
|---|---|---|---|---|
| Strong-Stop cDNA | AA55 | 5′-CTGCTAGAGATTTTCCACACTGAC-3′ | 200 nM | 95°C, 3 min; 50X (93°C, 1 min; |
| M667 | 5′-GGCTAACTAGGGAACCCACTG-3′ | 57°C, 1 min; 72°C, 1 min) | ||
| Y Chromosome DNA or Purified Standards | DYS14F | 5′-CATCCAGAGCGTCCCTGGCTTCTGG-3′ | 200 nM | 95°C, 3 min; 50X (93°C, 1 min; |
| DYS14R | 5′-TTCCCCTTTGTTCCCCAAA-3′ | 57°C, 1 min; 72°C, 1 min) | ||
| Genomic DNA w/wo | Alu2 (F) | 5′-GCCTCCCAAAGTGCTGGGATTACAG-3′ | 100 nM | 95°C, 3 min; 50X (93°C, 1 min; 55°C, 1 min; 72°C, 1 min) |
| Integrated Provirus or Purified Standards | Alu1 (R) | 5′-TCCCAGCTACTGGGGAGGCTGAGG-3′ | ||
| LM667 | 5′-ATGCCACGTAAGCGAAACTCTGGCTAACTAGGGAACCCACTG-3′ | |||
| Pre-amplified cDNA | AA55M | 5′-GCTAGAGATTTTCCACACTGACTAA-3′ | 200 nM | 95°C, 3 min; 50X (93°C, 1 min; |
| From Above | Lambda T | 5′-ATGCCACGTAAGCGAAACTCT-3′ | 57°C, 1 min; 72°C, 1 min) | |
| HIV mRNA Full Length | La 8.1 | 5′- CTGAAGCGCGCACGGCAA-3′ | 200 nM | 50°C 10 min, 95°C 3 min, (94°C 1min, 56°C 1 min, 72°C 1 min) |
| Transcript | La 9 | 5′- GACGCTCTCGCACCCATCTC-3′ | × 40 cycles, 4°C 10 min | |
| HIV mRNA Multiply-Spliced Transcripts | P659 | 5′- GACTCATCAAGTTTCTCTATCAAA-3′ | 200 nM | 50°C 10 min, 95°C 3 min, (94°C 1min, 56°C 1 min, 72°C 1 min) |
| P413Mod | 5′- AGTCTCTCAAGCGGTGGT-3′ | × 40 cycles, 4°C 10 min | ||
| Genomic β-Globin or Purified Standards | β-GlobinF | 5′CCCTTGGACCCAGAGGTTCT-3′ | 200 nM | 95°C, 3 min; 50X (93°C, 1 min; |
| β-GlobinR | 5′-CGAGCACTTTCTTGCCATGA-3′ | 55/57 °C, 1 min; 72°C, 1 min) |
Maternal microchimerism estimation in CBMC samples; Measurements of Y chromosomal DNA
To rule out microtransfusion from mother to fetal cord blood in determining HIV transfer, male cord blood (n=10) was examined for percentages of the Y chromosomal Marker DYS 14 (fetal alone) compared to β-globin housekeeping standards (fetal + maternal)8. DNA was prepared from 2 × 106 − 1 × 107 CBMCs by standard methods as described7. The PCR conditions in Table 1 were used to quantify DYS14 DNA by an absolute concentration assay. The conversion factor of 2 copies of β-globin and one copy of DYS14 product per cell was used to compute the reference DYS14/β-globin ratio. This ratio (0.5) was then determined from real-time PCR results for male CBMC samples (n=10) and calculated to be 0.5 (p<0.05).
Determining proviral transcription of full–length and multiply-spliced HIV mRNA
RNA was prepared from 1−2 × 106 cells using the RNAeasy Mini Kit and protocol (Qiagen, Valencia, CA). Full length (FL) and multiply-spliced (MS) HIV mRNA transcript levels were determined by a modification of methods described by Brussels et al as previously described7 using FL and MS sense and antisense primers described in outlined in Table 1.
Statistical methods
For this study the primary outcome was the determination of transcriptionally active integrated proviral DNA in fetal CBMCs by real-time PCR. In this case, no arbitrary divisions were employed for subcategorizing outcomes except for the quantitative limits of the “pseudo-nested” RT-qPCR assay employed. Both linear regression and ANOVA were used along with multiple sample repeats, standard curves, as well as know positive and negative samples to test the validity of the PCR measures of primary outcomes. For each result, data from at least three wells from a representative experiment were used to calculate the mean ± standard deviation. For standard curves, data from at least three wells from a representative experiment were used to calculate a single data point. Linear regression analyses (Exel, Microsoft , Seattle, WA) were performed on at least six data points, producing correlation coefficients ≥ 0.9999 (P < 0.005). Graphs (Figure 1) are shown with data normalized to the minimum values for each parameter to expedite visualization of varying levels of strong-stop cDNA or mRNAs. Statistical significance was evaluated by one-way ANOVA and post-hoc multiple comparison corrections per Bonferroni as appropriate (Sigmastat, Jandel Scientific, Corte Madera, CA). Alpha levels for significance were defined as P < 0.05.
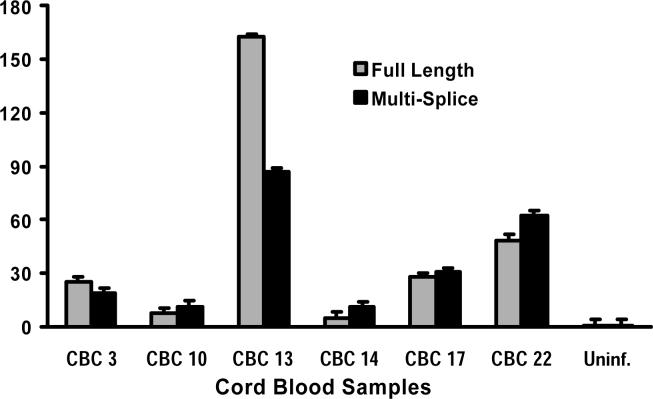
Figure 1. Fetal CBMCs infected by IUT harbor transcriptionally active integrated HIV provirus.
RNA was extracted from fetal CBMCs, positive for integrated provirus and collected from HIV-infected mothers at term. Relative levels of HIV full-length (FL) and multiply spliced (MS) transcripts were determined by real-time PCR. All CBMC samples exhibited detectable levels of both MS and FL HIV mRNA, indicating that infected CBMCs harbored transcriptionally active HIV.
Results
Age, parity and infection characteristics of study volunteers
The maternal age of study volunteers ranged from 22−39 years, parity ranged from 0−4, CD4+ T cell counts ranged from 143 to 588 per mm3 and plasma viral loads from ≤400 to ≥12,800 copies/mL at term. As shown in Table 3, 80% of study volunteers on HAART (16 out of 20 tested) and greater than 50% (3 out of 5 tested) of fetuses with HIV-infected CBMCs either had plasma viral loads less than 400 copies per ml or CD4+ T cell counts greater than 200 per mm3 at term. Plasma viral loads of all infants born to HIV positive mothers during the study period were routinely performed at least twice, once during their first week postpartum (n∼150) and again at 6 weeks. During this 2 year period the rate of MTCT was ≤ 3%.
Table 3.
HIV status of fetal CBMCs vs maternal plasma viral loads & CD4+ T cell counts
| HIV Plasma Viral Load Copies/ml | CD4+ T cell Counts Cells/mm3 | Fetal CBMC Infected | Infection Uninfected | Status Total |
|---|---|---|---|---|
| <400 | >200 | 2 | 5 | 7 |
| >400 | >200 | 1 | 7 | 8 |
| <400 | <200 | 1 | 0 | 1 |
| >400 | <200 | 1 | 3 | 4 |
| NA | 1 | 1 | 2 | |
| TOTAL | 22 |
NA = Not Assayed
Evidence for infection of placental cells of maternal and hematopoietic origin
Detection of HIV DNA merely indicates that HIV has entered the host cell and reverse transcription (RT) has occurred. For productive infection to become established, successful viral integration must follow. Thus, measurements of integrated proviral DNA in placental cells were performed to determine the status of infection in these tissues. Evidence of infection in maternal cells of hematopoietic origin isolated from placental tissues from HIV-infected mothers on HAART (n=22), was detected in all tissue preparations. Real-time PCR measurements of the number of copies of integrated proviral DNA, determined for all placental samples, ranged from 19−467 copies per 106 cell equivalents with a median value of 146 (Table 2).
Table 2.
Real Time PCR Results for Integrated HIV and Y Chromosomal DNA
| Sample Set No. | Integrated Provirus/106 Isolated Placental Cells | Integrated Provirus/106 CBMCs | DYS 14 Ratio | Infant Gender |
|---|---|---|---|---|
| 1 | 142 | N.D. | N.A. | M |
| 2 | 144 | N.D. | N.A. | F |
| 3 | 157 | 8 | N.A. | M |
| 4 | 140 | N.D. | 0.5 | M |
| 5 | 102 | N.D. | N.A. | F |
| 6 | 182 | N.D. | N.A. | F |
| 7 | 323 | N.D. | 0.5 | M |
| 8 | 149 | N.D. | N.A. | F |
| 9 | 158 | N.D. | N.A. | F |
| 10 | 123 | 7 | 0.5 | M |
| 11 | 181 | N.D. | 0.5 | M |
| 12 | 191 | N.D. | N.A. | F |
| 13 | 467 | 35 | 0.5 | M |
| 14 | 58 | 7 | N.A. | F |
| 15 | 168 | N.D. | N.A. | F |
| 16 | 29 | N.D. | N.A. | F |
| 17 | 266 | 12 | 0.5 | M |
| 18 | 19 | N.D. | 0 | F |
| 19 | 19 | N.D. | 0.5 | M |
| 20 | N.D. | N.D. | 0.5 | M |
| 21 | 15 | N.D. | N.A. | F |
| 22 |
285 |
19 |
0.5 |
M |
| Uninfected #1 | 0 | N.A. | N.A. | N.A. |
| Uninfected #2 | 0 | N.A. | N.A. | N.A. |
| Uninfected #3 | 0 | N.A. | N.A. | N.A. |
N.D. = Not Detectable
N.A. = Not Assayed
Evidence for infection of cord blood mononuclear cells of fetal origin
To document evidence of IUT, levels of integrated proviral DNA in fetal CBMCs were determined by PCR. Quantitative measurements of the number of copies of integrated proviral DNA from matched fetal CBMC and maternal Placental tissue samples identified 6 (of 22) infected samples, ranging from 8−35 copies of proviral DNA per 106 cell equivalents, approximately 10% the level of infection measured for infected placental cells (Table 2). No apparent correlation between the IUT and the level of infection in corresponding placental cells was observed.
Concerns of possible contamination of cord blood samples with infected maternal cells were addressed by performing an analysis of Y chromosomal DNA in CBMCs from male fetuses (n=11) by real-time PCR as described in the methods. The ratio of DYS14/β-globin for all male samples tested was 0.5 (p ≤ 0.05), indicating no contamination of maternal cells (maternal microchimerism) within the linear range defined by the PCR conditions (∼1%).
Evidence of transcriptional activity in infected fetal CBMCs
Detection of integrated proviral DNA still only suggests that the virus has the potential for replication but does not characterize whether viral replication is occurring. To precisely characterize the level of infection during IUT, real-time qPCR analysis of RNA extracted from HIV-infected CBMCs was performed to measure relative levels of proviral transcription. Evidence of transcription of both FL (early transcription products) and MS (late transcription products) viral mRNA relative to uninfected controls was measured in all CBMC samples with integrated proviral DNA (Figure 1). These results indicate that transcription of proviral DNA in isolated CBMCs infected by IUT occurs even without prior activation in vitro or in vivo.
Induction of infectious virus by alloactivation of infected fetal CBMCs
During post-transcriptional latency or HAART infected fetal CBMCs harboring transcriptionally active integrated proviral DNA may be unable to produce detectable levels of infectious virus. To address this issue two randomly selected HIV-positive CBMC samples were cocultured for 6 days with γ-irradiated matched maternal peripheral blood mononuclear cell (PBMC) stimulators in vitro to induce viral replication by allogeneic stimulation as has been previously described9. Measurement of significant levels of HIV p24 antigen (≥40 ng/ml) in culture supernatant fluids confirmed that (a) expression of infectious virus could be induced in infected fetal CBMCs harboring transcriptionally active integrated proviral DNA, and (b) infected cells responsive to alloactivation by γ-irradiated maternal stimulator cells are of likely (MHC disparate) fetal origin.
Comment
The efficacy of accepted measures to limit perinatal transmission of HIV during pregnancy, that include administration of HAART and scheduled Cesarean delivery, has primarily been determined by evidence of a significant reduction in the frequency of MTCT of HIV infection in infants to 1−2%2. Clinical determinations of whether MTCT has occurred by either IUT or intrapartum transmission are routinely made indirectly by measuring how soon detectable levels of plasma borne viral RNA appear postpartum10-12. However, a rigorous examination for direct evidence MTCT and established infection in fetuses delivered to HIV-infected mothers undergoing currently accepted prenatal clinical protocols to limit MTCT has not been conducted. Our findings that 6 of 22 CBMC samples (25%), harboring transcriptionally active and potentially infectious integrated proviral HIV, could be isolated from term fetuses randomly selected from a larger population of 150 delivered by HIV+ women on HAART with a rate of MTCT of 3%, strongly suggest that IUT can indeed occur in some fetuses that may present as HIV-negative postpartum.
Unlike intrapartum transmission or postpartum vertical transmission of HIV, little is known regarding how and when IUT of HIV occurs. 12 13. For this study only CBMCs collected from fetuses delivered during scheduled Cesarean delivery and in the absence of clinical or pathological evidence of abruption were assayed to minimize the confounding effects of maternal microchimerism. To more carefully determine whether infected cells exclusively of fetal origin were examined, PCR analysis of Y-chromosomal DNA in CBMCs from male fetuses was performed (Table 2), thus establishing a detection threshold (≤ 1.3%) for contaminating maternal cells in all fetal cord blood samples. In addition, cellular viral loads of infected placental cells of maternal origin, determined to be ≤0.004% (Table 2), provide strong evidence that CBMCs harboring integrated HIV proviral DNA were of fetal origin and infected transplacentally.
Since the routine administration of HAART, the presence of latently infected cells and cellular reservoirs harboring low levels of persistent viral replication has become an important consideration in the pathogenesis of HIV infection in infected adults. However, the extent to which HAART may influence the establishment of “occult” fetal HIV infection during MTCT has received little attention. For infection to become established, the virus must do more than just enter susceptible cells; it must also successfully integrate and replicate within those cells. Studies employing PCR techniques that measure total HIV DNA in blood samples do not clearly differentiate between integrated and unintegrated proviral DNA in infected cells. Thus, the level of true infection determined by these methods may be potentially overestimated. Likewise, studies that rely solely on PCR determinations of number of copies of HIV RNA in plasma samples may be falsely negative, especially when HIV infection is undetectable due to pre- or post-integration latency. In support of this hypothesis is the finding that PBMCs isolated from 18% of infants born with undetectable plasma viral loads to HIV infected mothers in a recent study harbored unintegrated virus12. There is evidence that unintegrated virus can persist for a limited period in an integration-competent state, and that integration of replication competent provirus will follow only after appropriate immune activation in the infected host cell14. In our study we confirmed infection by measuring levels of both proviral integration and post-integration transcription of HIV in cord blood. Our data thus indicate that IUT may successfully occur before term and in the absence of clinical or pathological evidence of abruptions, maternal microchimerism, intrauterine infection or immune activation, in fetuses that present with HIV-negative serologies at term.
One trivial explanation for this result could be that the infected CBMCs from these fetuses harbor actively transcribing integrated provirus that is replication defective. We therefore confirmed virus infection by measuring HIV p24 levels in two randomly selected co-cultures of matched infected CBMC's and γ-irradiated maternal PBMC stimulator cells as previously described7. Significant levels of HIV p24 (≥40 ng/ml) could be measured in culture supernatant fluids from allo-activated (infected) CBMCs after 5 days (data not shown). Collectively, these data provide strong evidence that productive infection of circulating mononuclear cells can be established by IUT at time points significantly before term in certain fetuses that may appear serologically negative at birth.
Nevertheless, how IUT occurs, especially during HAART, remains unknown. Our results demonstrate the presence of cells harboring integrated proviral DNA in all placental tissue samples examined, indicating that prenatal administration of HAART does not completely prevent the presence of infiltrating HIV-infected hematopoietic cells of maternal origin in the placenta. However 80% of study volunteers on HAART either had plasma viral loads less than 400 copies per ml or CD4+ T cell counts greater than 200 per mm3. Furthermore, although levels of anti-retroviral drugs in placental tissues or fetal circulation were not determined, evidence of HIV-infected CBMC samples were detected in 3 separate fetuses delivered by women on HAART with plasma viral loads < 400 per ml and CD4+ T cell counts > 200 per mm3 (Table 3).
These results suggest that even in the presence of adequate prenatal HAART and a MTCT rate of ≤ 3%, HIV is transmitted transplacentally and infects circulating fetal mononuclear cells in greater than 25% of pregnancies. This disparity between the rate of IUT in this report and rates reported by others may be due to at least 3 factors: (a) differences in study sample size, (b) possible contamination with infected maternal lymphocytes, or (c) differences in the methods used for determining HIV infection. Admittedly the sample size (n=22) of this study must be enlarged for accurate estimates of IUT. Nevertheless, because we were able to confirm (by Y chromosomal DNA analysis) absence of maternal microtransfusion (≤1.3%), and due to the fact that γ-irradiated PBMCs of maternal origin could allo-activate and induce the release of infectious virus in infected CBMCs, IUT and antenatal fetal infection was presumed.
In this study our findings of an apparent increased frequency of infected circulating fetal CBMCs in HIV-infected pregnant women on HAART was not confirmed in infants postpartum and therefore raise several important questions: How and when does IUT of HIV occur during pregnancy? Is the infection cleared, if so how? Can an occult infection be established in the fetus that persists post-partum? Are circulating infected fetal cells able to establish extravascular sanctuaries of persistent or latent infection in cryptic fetal tissue compartments? If so, what is the significance of the timing of IUT on the creation of such reservoirs in utero and which tissue compartments are potentially involved? More importantly, how should such infants be followed or managed clinically? Well designed longitudinal studies of HIV negative infants born to HIV-infected mothers are needed to fully appreciate the potential clinical consequences of intrauterine fetal HIV infection on long term immunological and developmental health of these infants.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (AI062383 to JBS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the SMFM 2007 Meeting, San Francisco, CA
No Reprints Available
CONDENSATION
Circulating fetal mononuclear cells harboring actively replicating HIV are present at term in some fetuses from HIV-infected pregnant women receiving HAART and undergoing elective cesarean delivery.
References
- 1.Thorne C, Newell ML. Prevention of mother-to-child transmission of HIV infection. Curr Opin Infect Dis. 2004;17:247–52. doi: 10.1097/00001432-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 2.study EC. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 3.Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. Jama. 2002;288:189–98. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 4.Ellis J, Williams H, Graves W, Lindsay MK. Human immunodeficiency virus infection is a risk factor for adverse perinatal outcome. Am J Obstet Gynecol. 2002;186:903–6. doi: 10.1067/mob.2002.123407. [DOI] [PubMed] [Google Scholar]
- 5.CDC U.S. Public Helath Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1--infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR. :51. [PubMed] [Google Scholar]
- 6.Ellis JE, Ansari AA, Fett JD, et al. Inhibition of progenitor dendritic cell maturation by plasma from patients with peripartum cardiomyopathy: role in pregnancy-associated heart disease. Clin Dev Immunol. 2005;12:265–73. doi: 10.1080/17402520500304352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundstrom JB, Ellis JE, Hair GA, et al. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007 doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams KM, Lambert NC, Heimfeld S, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102:3845–7. doi: 10.1182/blood-2003-05-1570. [DOI] [PubMed] [Google Scholar]
- 9.Moriuchi H, Moriuchi M, Fauci AS. Induction of HIV-1 replication by allogeneic stimulation. J Immunol. 1999;162:7543–8. [PubMed] [Google Scholar]
- 10.Kalish LA, Pitt J, Lew J, et al. Defining the time of fetal or perinatal acquisition of human immunodeficiency virus type 1 infection on the basis of age at first positive culture. Women and Infants Transmission Study (WITS). J Infect Dis. 1997;175:712–5. doi: 10.1093/infdis/175.3.712. [DOI] [PubMed] [Google Scholar]
- 11.Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–7. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 12.Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. Jama. 2001;285:709–12. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- 13.Newell ML. Current issues in the prevention of mother-to-child transmission of HIV-1 infection. Trans R Soc Trop Med Hyg. 2006;100:1–5. doi: 10.1016/j.trstmh.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Oswald-Richter K, Grill SM, Leelawong M, Unutmaz D. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur J Immunol. 2004;34:1705–14. doi: 10.1002/eji.200424892. [DOI] [PubMed] [Google Scholar]
- 15.Miller RK, Polliotti BM, Laughlin T, et al. Role of the placenta in fetal HIV infection. Teratology. 2000;61:391–4. doi: 10.1002/(SICI)1096-9926(200005)61:5<391::AID-TERA14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–74. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Vidricaire G, Tardif MR, Tremblay MJ. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J Biol Chem. 2003;278:15832–41. doi: 10.1074/jbc.M210470200. [DOI] [PubMed] [Google Scholar]
- 18.Bacsi A, Ebbesen P, Szabo J, et al. Pseudotypes of vesicular stomatitis virus-bearing envelope antigens of certain HIV-1 strains permissively infect human syncytiotrophoblasts cultured in vitro: implications for in vivo infection of syncytiotrophoblasts by cell-free HIV-1. J Med Virol. 2001;64:387–97. doi: 10.1002/jmv.1063. [DOI] [PubMed] [Google Scholar]
- 19.Mognetti B, Moussa M, Croitoru J, et al. HIV-1 co-receptor expression on trophoblastic cells from early placentas and permissivity to infection by several HIV-1 primary isolates. Clin Exp Immunol. 2000;119:486–92. doi: 10.1046/j.1365-2249.2000.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David FJ, Tran HC, Serpente N, et al. HIV infection of choriocarcinoma cell lines derived from human placenta: the role of membrane CD4 and Fc-Rs into HIV entry. Virology. 1995;208:784–8. doi: 10.1006/viro.1995.1212. [DOI] [PubMed] [Google Scholar]
- 21.Parry S, Zhang J, Koi H, Arechavaleta-Velasco F, Elovitz MA. Transcytosis of Human immunodeficiency virus 1 across the placenta is enhanced by treatment with tumour necrosis factor alpha. J Gen Virol. 2006;87:2269–78. doi: 10.1099/vir.0.81071-0. [DOI] [PubMed] [Google Scholar]
- 22.Thorne C, Newell ML. Mother-to-child transmission of HIV infection and its prevention. Curr HIV Res. 2003;1:447–62. doi: 10.2174/1570162033485140. [DOI] [PubMed] [Google Scholar]
- 23.Cibulka NJ. Mother-to-child transmission of HIV in the United States. Many HIV-infected women are now planning to have children. What are the risks to mother and infant? Am J Nurs. 2006;106:56–63. doi: 10.1097/00000446-200607000-00029. quiz 64. [DOI] [PubMed] [Google Scholar]
- 24.Nesheim SR, Henderson S, Lindsay M, Zuberi J, Grimes V, et al. Anonymous, ed. Morbidity and Mortality Weekly Report. Centers for Disease Control,; Atlanta: 2004. Prenatal HIV testing and antiretroviral prophylaxis at an urban hospital-Atlanta, Georgia, 1997−25. [PubMed] [Google Scholar]