Abstract
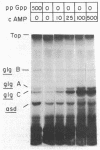
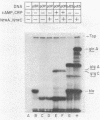
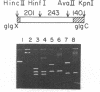
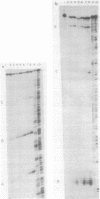
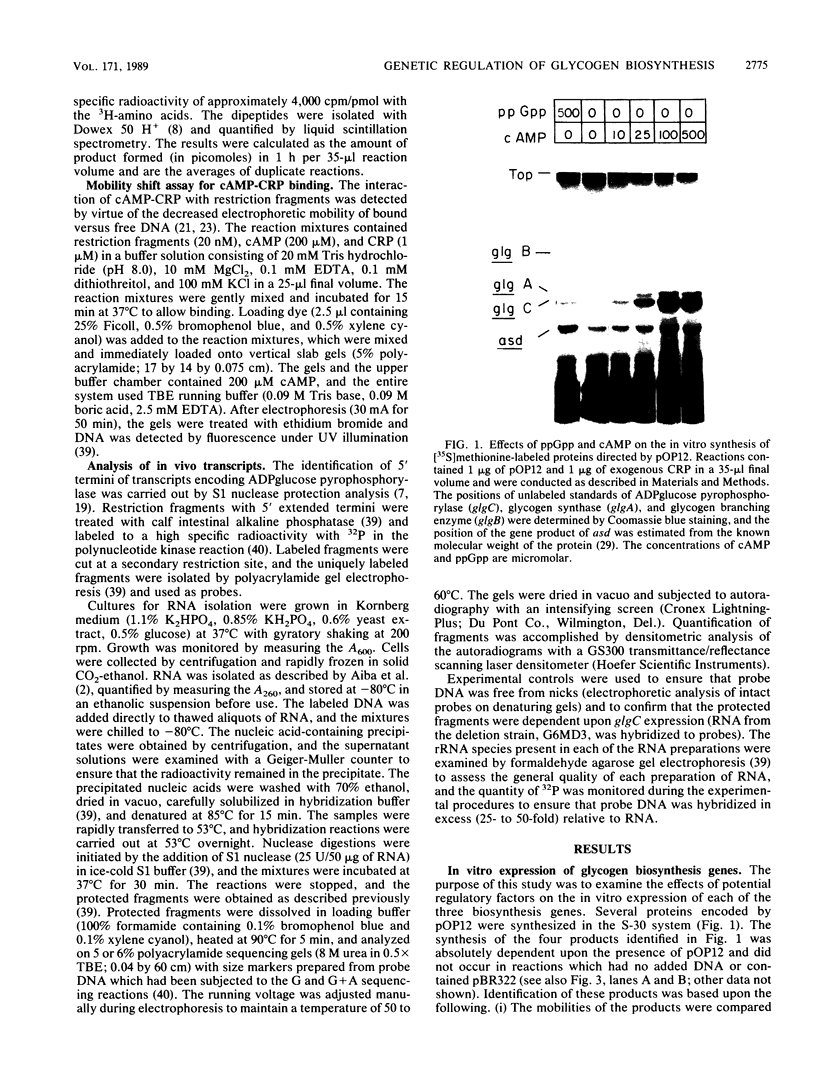
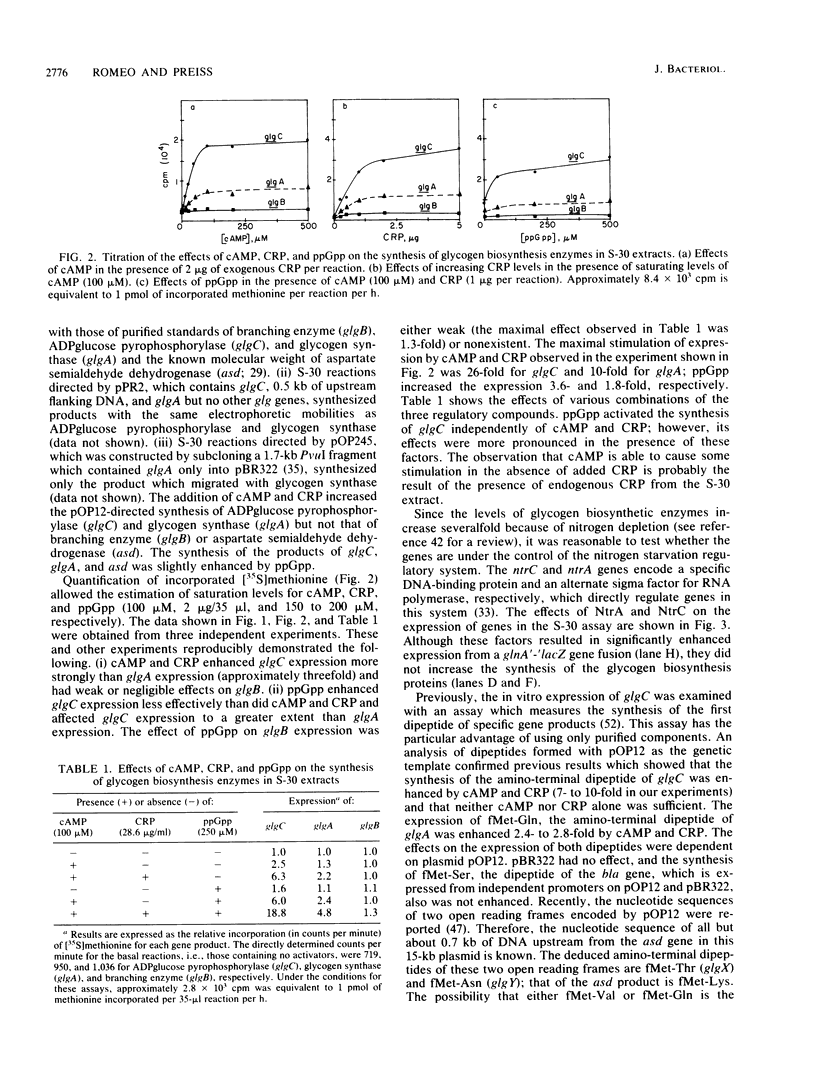
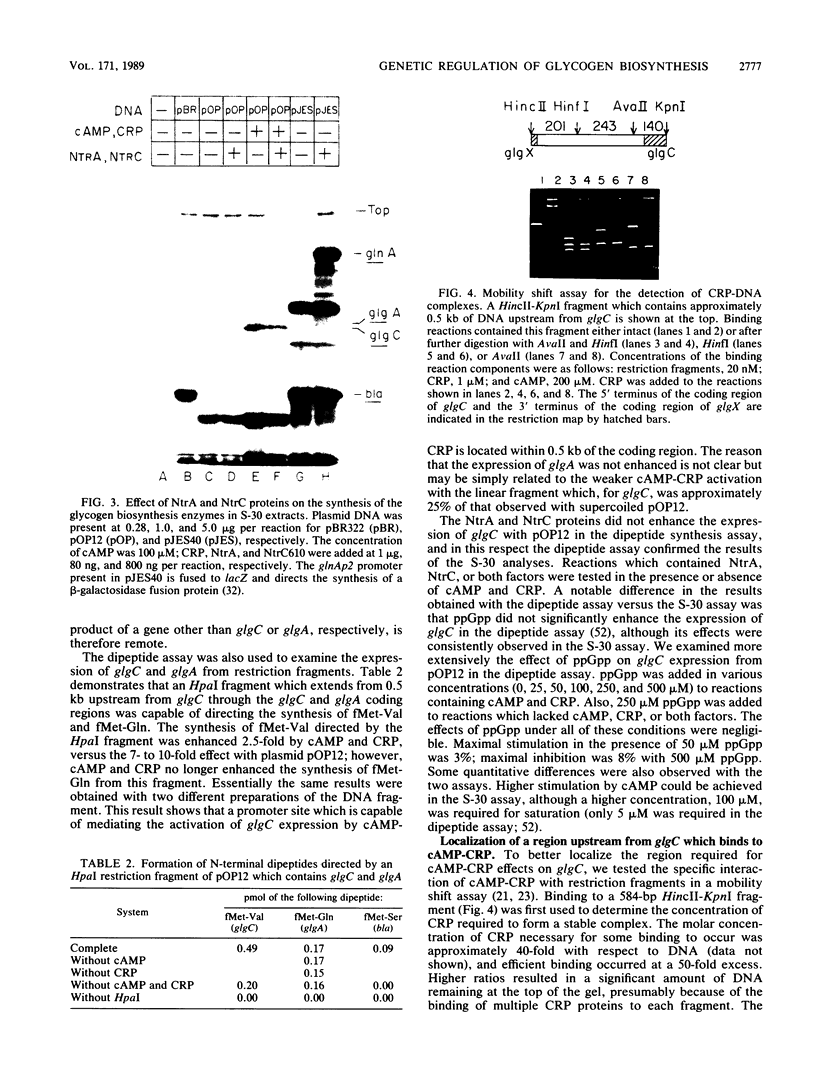
Glycogen accumulation in Escherichia coli is inversely related to the growth rate and occurs most actively when cells enter the stationary phase. The levels of the three biosynthetic enzymes undergo corresponding changes under these conditions, suggesting that genetic control of enzyme biosynthesis may account for at least part of the regulation (J. Preiss, Annu. Rev. Microbiol. 38:419-458, 1984). We have begun to explore the molecular basis of this control by identifying factors which affect the expression of the glycogen genes and by determining the 5'-flanking regions required to mediate the regulatory effects. The in vitro coupled transcription-translation of two of the biosynthetic genes, glgC (ADPglucose pyrophosphorylase) and glgA (glycogen synthase), was enhanced up to 26- and 10-fold, respectively, by cyclic AMP (cAMP) and cAMP receptor protein (CRP). Guanosine 5'-diphosphate 3'-diphosphate stimulated the expression of these genes 3.6- and 1.8-fold, respectively. The expression of glgB (glycogen branching enzyme) was affected weakly or negligibly by the above-mentioned compounds. Assays which measured the in vitro formation of the first dipeptide of glgC showed that a restriction fragment which contained 0.5 kilobases of DNA upstream from the initiation codon supported cAMP-CRP-activated expression. Sequence-specific binding of cAMP-CRP to a 243-base-pair restriction fragment from the region upstream from glgC was observed by virtue of the altered electrophoretic mobility of the bound DNA. S1 nuclease protection analysis identified 5' termini of four in vivo transcripts within 0.5 kilobases of the glgC coding region. The relative concentrations of transcripts were higher in the early stationary phase than in the exponential phase. Two mutants which overproduced the biosynthesis enzymes accumulated elevated levels of specific transcripts. The 5' termini of three of the transcripts were mapped to a high resolution. Their upstream sequences showed weak similarity to the E. coli consensus promoter. These results suggest complex transcriptional regulation of the glycogen biosynthesis genes involving multiple promoter sites and direct control of gene expression by at least two global regulatory systems.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Pastan I. Activation of transcription by guanosine 5'-diphosphate,3'-diphosphate, transfer ribonucleic acid, and novel protein from Escherichia coli. J Biol Chem. 1975 Mar 25;250(6):2189–2195. [PubMed] [Google Scholar]
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecker P. A., Furlong C. E., Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose synthetase as deduced from the nucleotide sequence of the glg C gene. J Biol Chem. 1983 Apr 25;258(8):5084–5088. [PubMed] [Google Scholar]
- Baecker P. A., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli 1,4-alpha-D-glucan:1,4-alpha-D-glucan 6-alpha-D-(1, 4-alpha-D-glucano)-transferase as deduced from the nucleotide sequence of the glg B gene. J Biol Chem. 1986 Jul 5;261(19):8738–8743. [PubMed] [Google Scholar]
- Bartlett D. H., Frantz B. B., Matsumura P. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5' consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988 Apr;170(4):1575–1581. doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boyer C., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli b alpha-1,4,-glucan: alpha-1,4-glucan 6-glycosyltansferase. Biochemistry. 1977 Aug 9;16(16):3693–3699. doi: 10.1021/bi00635a029. [DOI] [PubMed] [Google Scholar]
- Bridger W. A., Paranchych W. relA Gene control of bacterial glycogen synthesis. Can J Biochem. 1978 Jun;56(6):403–406. doi: 10.1139/o78-063. [DOI] [PubMed] [Google Scholar]
- Cattanéo J., Sigal N., Favard A., Segel I. H. Inhibition par l'actinomycine D et par la p-fluorophénylalanine de l'accumulation de glycogène chez Escherichia coli. Bull Soc Chim Biol (Paris) 1966;48(3):441–445. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chen H. Z., Zubay G. Prokaryotic coupled transcription-translation. Methods Enzymol. 1983;101:674–690. doi: 10.1016/0076-6879(83)01047-2. [DOI] [PubMed] [Google Scholar]
- Das A., Yanofsky C. A ribosome binding site sequence is necessary for efficient expression of the distal gene of a translationally-coupled gene pair. Nucleic Acids Res. 1984 Jun 11;12(11):4757–4768. doi: 10.1093/nar/12.11.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Magnani J. L., Sughrue M. J., Bergstein P. E., Sternheim W. L. Contribution of cyclic adenosine 3':5'-monophosphate to the regulation of bacterial glycogen synthesis in vivo. Effect of carbon source and cyclic adenosine 3':5'-monophosphate on the quantitative relationship between the rate of glycogen synthesis and the cellular concentrations of glucose 6-phosphate and fructose 1,6-diphosphate in Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8308–8317. [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P. Regulation of ADP-glucose synthetase, the rate-limiting enzyme of bacterial glycogen synthesis, by the pleiotropic nucleotides ppGpp and pppGpp. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1459–1467. doi: 10.1016/s0006-291x(77)80143-5. [DOI] [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Sternheim W. L., Taxman T. L., Ungar J. M., Porter S. E. Evidence for the regulation of bacterial glycogen synthesis by cyclic AMP. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1468–1477. doi: 10.1016/s0006-291x(77)80144-7. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fox J., Kawaguchi K., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli B ADPglucose:1,4-alpha-D-glucan 4-alpha-glucosyltransferase. Biochemistry. 1976 Feb 24;15(4):849–857. doi: 10.1021/bi00649a019. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govons S., Vinopal R., Ingraham J., Preiss J. Isolation of mutants of Escherichia coli B altered in their ability to synthesize glycogen. J Bacteriol. 1969 Feb;97(2):970–972. doi: 10.1128/jb.97.2.970-972.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Ishaque A., Preiss J. Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7.7.27). J Biol Chem. 1976 Dec 25;251(24):7880–7885. [PubMed] [Google Scholar]
- Haugen T., Ishaque A., Chatterjee A. K., Preiss J. Purification of Escherichia coli ADPglucose pyrophosphorylase by affinity chromatography. FEBS Lett. 1974 Jun 1;42(2):205–208. doi: 10.1016/0014-5793(74)80786-6. [DOI] [PubMed] [Google Scholar]
- Haziza C., Stragier P., Patte J. C. Nucleotide sequence of the asd gene of Escherichia coli: absence of a typical attenuation signal. EMBO J. 1982;1(3):379–384. doi: 10.1002/j.1460-2075.1982.tb01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. DNA sequence analysis suggests that expression of flagellar and chemotaxis genes in Escherichia coli and Salmonella typhimurium is controlled by an alternative sigma factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6422–6424. doi: 10.1073/pnas.84.18.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Larsen C. E., Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose:alpha-1,4-glucan, 4-glucosyltransferase as deduced from the nucleotide sequence of the glgA gene. J Biol Chem. 1986 Dec 5;261(34):16256–16259. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leckie M. P., Ng R. H., Porter S. E., Compton D. R., Dietzler D. N. Regulation of bacterial glycogen synthesis. Stimulation of glycogen synthesis by endogenous and exogenous cyclic adenosine 3':5'-monophosphate in Escherichia coli and the requirement for a functional CRP gene. J Biol Chem. 1983 Mar 25;258(6):3813–3824. [PubMed] [Google Scholar]
- Leckie M. P., Tieber V. L., Porter S. E., Roth W. G., Dietzler D. N. Independence of cyclic AMP and relA gene stimulation of glycogen synthesis in intact Escherichia coli cells. J Bacteriol. 1985 Jan;161(1):133–140. doi: 10.1128/jb.161.1.133-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Okita T. W., Rodriguez R. L., Preiss J. Biosynthesis of bacterial glycogen. Cloning of the glycogen biosynthetic enzyme structural genes of Escherichia coli. J Biol Chem. 1981 Jul 10;256(13):6944–6952. [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Preiss J., Yung S. G., Baecker P. A. Regulation of bacterial glycogen synthesis. Mol Cell Biochem. 1983;57(1):61–80. doi: 10.1007/BF00223525. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Romeo T., Kumar A., Preiss J. Analysis of the Escherichia coli glycogen gene cluster suggests that catabolic enzymes are encoded among the biosynthetic genes. Gene. 1988 Oct 30;70(2):363–376. doi: 10.1016/0378-1119(88)90208-9. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Taguchi M., Izui K., Katsuki H. Augmentation of glycogen synthesis under stringent control in Escherichia coli. J Biochem. 1980 Aug;88(2):379–387. doi: 10.1093/oxfordjournals.jbchem.a132983. [DOI] [PubMed] [Google Scholar]
- Urbanowski J., Leung P., Weissbach H., Preiss J. The in vitro expression of the gene for Escherichia coli ADP glucose pyrophosphorylase is stimulated by cyclic AMP and cyclic AMP receptor protein. J Biol Chem. 1983 Mar 10;258(5):2782–2784. [PubMed] [Google Scholar]
- Westman E., Eriksson S., Lås T., Pernemalm P. A., Sköld S. E. Separation of DNA restriction fragments by ion-exchange chromatography on FPLC columns Mono P and Mono Q. Anal Biochem. 1987 Oct;166(1):158–171. doi: 10.1016/0003-2697(87)90558-6. [DOI] [PubMed] [Google Scholar]
- Yu F., Jen Y., Takeuchi E., Inouye M., Nakayama H., Tagaya M., Fukui T. Alpha-glucan phosphorylase from Escherichia coli. Cloning of the gene, and purification and characterization of the protein. J Biol Chem. 1988 Sep 25;263(27):13706–13711. [PubMed] [Google Scholar]