Abstract
Background
Delay in the diagnosis of coeliac disease prolongs morbidity and may increase mortality. Little is known about presentations in general practice that may predict a subsequent diagnosis of coeliac disease.
Aim
To examine presentations in general practice during the 5 years prior to diagnosis of coeliac disease.
Design of study
A case-control study with each biopsy-proven coeliac disease case matched by age, sex, and general practice to an average of two controls.
Setting
Thirty-seven general practices in south-east Wales.
Method
Cases were identified via a secondary care clinic and controls recruited from the general practices of cases. General practice clinical records of both cases and controls were analysed to determine frequency of consultations, presenting symptoms, diagnoses, referrals, and investigations during the 5 years prior to diagnosis.
Results
Cases (n = 68) had an increased number of consultations compared with controls (n = 160) during the 5 years prior to diagnosis (mean difference five consultations, P = 0.001). Three clinical features were independently associated with subsequent diagnosis of coeliac disease: depression and/or anxiety (odds ratio [OR] = 2.5, 95% confidence interval [CI] = 1.1 to 5.7, P = 0.031); diarrhoea (OR = 4.5, 95% CI = 2.0 to 10.0, P<0.001); and anaemia (OR = 26.3, 95% CI = 5.7 to 120.6, P<0.001). Both diarrhoea and anaemia remained associated even when data for the year prior to diagnosis was excluded from the analysis.
Conclusion
GPs should consider testing for coeliac disease when patients present often, especially with diarrhoea and/or who are discovered to be anaemic. Further research is required to clarify the role of depression and/or anxiety in the diagnosis of coeliac disease.
Keywords: case-control, coeliac disease, risk factors
INTRODUCTION
Coeliac disease, or gluten-sensitive enteropathy, is a disease of malabsorption characterised by inflammation of the small intestinal mucosa. Symptoms rapidly improve on a strict gluten-free diet.
Early diagnosis is associated with lower standardised mortality ratios.1,2 Treatment improves quality of life and depressive features, and reduces malabsorption, which in turn reduces the risk of complications such as osteoporosis, peripheral neuropathy, micro-and macrocytic anaemia.3–5 Treatment also reduces the risk of malignancy, specifically gastrointestinal carcinoma or lymphoma, which develops in up to 15% of those where the disease is left untreated.6,7 Diagnostic delay is common both in primary and secondary care, and results in protracted morbidity,8 with a median duration of symptoms of 4.9 years before diagnosis.9
Screening studies suggest that many cases remain undetected and the prevalence of people in the UK who are positive on serological screening may be as high as 1 in 100.10,11 While classic symptoms such as diarrhoea are well recognised, the majority of patients present with diverse, non-specific, and vague symptoms that are not often obviously attributable to the gastrointestinal tract or to coeliac disease. Generalised aches and bone pain, reflux oesophagitis, tiredness, migraine, and depressive symptoms are all documented first-presenting symptoms of the condition.12–18 Anaemia may be treated for years with vitamin and iron supplements without identification of the underlying disease.
However, the symptoms linked to coeliac disease are also common in many other conditions and are presented in approximately a third of all consultations in primary care.19 They also represent common reasons for referral to secondary care. Since a high proportion of consultations are potentially influenced by a cluster of problems that could be caused by coeliac disease, understanding the frequency of consultations and the nature of presentations in general practice before a diagnosis of coeliac disease is made may aid earlier diagnosis through identifying hitherto unrecognised pointers to the diagnosis. A case-control study was therefore conducted to examine presentations in general practice during the 5 years prior to diagnosis of coeliac disease.
METHOD
Participants
Eligible cases had biopsy-proven coeliac disease and fulfiled the updated European Society of Paediatric Gastroenterology and Nutrition (ESPGAN) criteria,20 were diagnosed after 1 January 1998, aged over 18 years at time of diagnosis, and registered with a general practice in south-east Wales. The 1 January 1998 was used as a cut-off date for diagnosis to increase the chances of completeness of GP clinical records studied during the 5 years prior to this date. Cases were identified at the coeliac clinic at Llandough Hospital, Cardiff. This is the main secondary care centre for coeliac disease for Cardiff and the Vale of Glamorgan and serves a total adult population of approximately 340 000. Referrals to this clinic arise from both primary and secondary care.
Five potential controls were matched to each case by sex, general practice, and date of birth (within 1-year bands) with the aim of recruiting two controls per case. Exclusion criteria for both cases and controls were unobtainable GP clinical records.
Cases were recruited following a formal diagnosis of coeliac disease and prior to a routine consultation at the hospital coeliac clinic. Once consent was obtained, the patients' GPs were contacted to obtain access to their general practice clinical records, and to enlist the GPs' help in recruiting controls. Potential controls were contacted via a letter from the GP, with an attached information sheet, and a consent form which would allow the research team to extract relevant data from their clinical records. A form indicating non-consent was also included to estimate the proportion that declined to participate. After 2 weeks if less than two control patients per case had consented, the practice was approached once more to identify five additional potential controls.
How this fits in
Earlier diagnosis of coeliac disease reduces morbidity and risk of complications. Diagnosis is often difficult as presenting features may not directly suggest gastrointestinal disease. Presentations in general practice during the 5 years prior to a diagnosis of coeliac disease were examined, and it was found that patients subsequently diagnosed with coeliac disease had an increased number of consultations compared with controls. Diarrhoea and anaemia were significantly predictive of an eventual diagnosis with coeliac disease. Increased awareness of the link between frequent consulting, diarrhoea, anaemia, and coeliac disease may assist GPs in achieving earlier diagnosis of this important and easily treatable condition.
Obtaining and extracting clinical data
Photocopies were made of the general practice clinical records for the consenting case and corresponding controls for the 5 years prior to the date in which coeliac disease was first mentioned in the clinical records of cases. Computer and paper clinical records were copied along with the summary sheet. A research nurse anonymised the clinical records and recorded the number of referrals to secondary care consultants and clinical investigations in primary care for each included year. The clinical records were anonymised using a coding system that was known only to the researcher concerned. Missing clinical records for any period during the 5 years were documented.
Two GPs not involved in obtaining copies of the clinical records and blinded to case-control status extracted and recorded information on to a pro-forma, which listed possible common features of the presentation and investigation of coeliac disease. These features were derived from searching the literature and from expert opinion. The overall number of consultations per annum, as well as the number of times that the specified symptoms and diagnoses presented, were recorded. Comorbidities that could account for a higher than average consultation rate were also noted. Data extraction was protocol driven, with explicit and detailed inclusion and exclusion criteria for the consultations and presenting features.
Data extraction was piloted by the two GPs who each extracted data on the same set of 20 patient clinical records. As a result of this pilot phase, minor changes were made to the data extraction protocol, for example clarification of the inclusion/exclusion criteria and inclusion of extra categories of presenting features.
Statistical analysis
Comparison of consultations
The numbers of consultations were reported for both cases and controls for the 1-year and the whole 5-year period prior to diagnosis. To examine the effect that the 1 year prior to diagnosis may have on the overall number of consultations, it was excluded from the 5-year period. The median and interquartile range were used to describe the skewed distribution of the number of consultations in the cases and controls. Differences between the number of consultations for each case and the average consultations of the case's matched controls were calculated. Since these differences were normally distributed, the mean of the differences were calculated with 95% CIs. A weighted paired t-test that took into account the varying number of controls per case was used to compare the two groups.
Independent predictors of coeliac disease
Conditional logistic regression was used to calculate odds ratios (ORs) and 95% CIs to investigate features that could potentially be more frequent in those subsequently diagnosed with coeliac disease. Two separate models were run; one examining patient's symptoms and diagnoses and another examining GP referrals and investigations. Only variables with expected values of greater than five per cell in either cases or controls, and those significant at the 10% level in univariate analyses were included. Analyses were repeated excluding data from the year prior to diagnosis to identify earlier presenting features of coeliac disease. All analyses were performed using SPSS (version 12) and SAS (version 8.02).
Sample size
A sample size calculation was conducted prior to the study and showed that 68 cases and 136 controls were required for 80% power to detect an increase of two consultations per annum at the 5% significance level (from population consultation rate of 4.95 per annum).21 This calculation was based on unmatched cases and controls, thus giving a conservative sample size estimate.
RESULTS
Recruitment
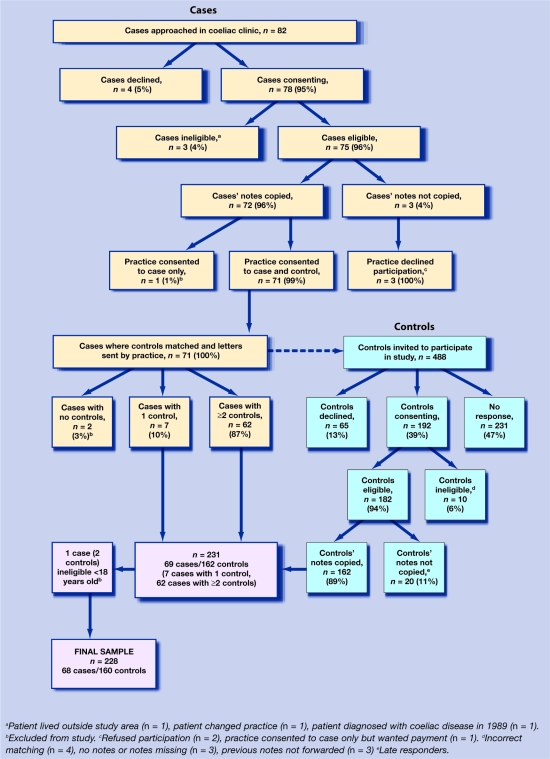
A flow chart of the recruitment process is shown in Figure 1.
Figure 1.
Flow chart of recruitment.
Cases and controls
Two hundred and twenty-eight clinical records were analysed; 68 cases and 160 controls, an average of two controls per case. Cases and controls were well matched (Table 1). There was no notable or significant difference between cases and controls with respect to comorbidity.
Table 1.
Characteristics of cases and controls.
| Characteristics | Cases (n = 68) (%) | Controls (n = 160) (%) |
|---|---|---|
| Age at diagnosis, years | ||
| <44 | 24 (35) | 51 (32) |
| 45–64 | 28 (41) | 66 (41) |
| 65–74 | 12 (18) | 31 (19) |
| ≥75 | 4 (6) | 12 (8) |
| Males | 19 (28) | 44 (28)) |
| Comorbidity present | 21 (31) | 58 (36) |
| Townsend deprivation scoresa(based on 2001 Census)22 | −1.0 (–4.0 to 2.6) | −1.2 (–3.6 to 2.6) |
Median (interquartile range).
The average consent rate among those invited to be a control was 43.4% (standard deviation = 23.1 %), ranging from 0 to 100% for each case. There was a higher consent rate from older controls (P = 0.001) and from practices in less deprived areas (P = 0.016).
Comparison of consultations
There was strong evidence of a higher rate of consultations in cases compared with controls during the 5 years prior to diagnosis (P = 0.001) (Table 2). These differences were also seen for the 1 year prior to diagnosis and excluding the 1 year prior to diagnosis (P<0.001 and P = 0.008 respectively).
Table 2.
Number of general practice consultations in cases and controls during the 5 years prior to diagnosis of coeliac disease.
| Median (IQR) | Meanb (95% CI) | P-value | ||
|---|---|---|---|---|
| Consultations | Cases (n =68) | Controls (n = 160) | Differencea (Case - Control) | |
| 5 years prior to diagnosis | 22 (12–35) | 20 (10–31) | 5.04 (2.09 to 7.99) | 0.001 |
| 1 year prior to diagnosis | 5 (2–9) | 4 (1–7) | 1.59 (0.79 to 2.40) | <0.001 |
| Excluding 1 year prior to diagnosis | 16 (9 to 29) | 15 (8 to 24) | 3.47 (0.92 to 6.01) | 0.008 |
Difference calculated for each case and the average consultations of its matched controls.
Mean values weighted for differing number of controls per case. IQR = interquartile range.
Identification of predictors of coeliac disease
In total, 26 variables were considered for univariate conditional logistic regression. Eight patient symptoms and diagnoses, referral by GP and five types of investigations were significantly associated at the 10% level, with a subsequent diagnosis of coeliac disease during the 5 years prior to diagnosis (Table 3). An OR of greater than one implies a higher probability in cases and a 95% CI including the value 1 indicates no statistically significant difference between cases and controls. Thus, all associated variables were more common in those with a subsequent diagnosis of coeliac disease. However, the same proportion of patients with coeliac disease were issued with sick certificates as controls (26 versus 24% respectively), and had a similar number of barium radiological investigations (7 versus 8% respectively).
Table 3.
Univariate analyses of features of general practice consultations during the 5 years prior to diagnosis of coeliac disease.
| n (%) with at least one consultation | OR (95% CI) | P-value | ||
|---|---|---|---|---|
| Variable | Cases (n = 68) | Controls (n = 160) | ||
| Patient symptoms and diagnoses | ||||
| Depression/anxiety | 28 (41) | 48 (31) | 1.8 (0.9 to 3.4) | 0.090 |
| Insomnia | 21 (31) | 30 (19) | 2.2 (1.0 to 4.5) | 0.040 |
| Abdominal pain | 29 (43) | 53 (34) | 1.9 (0.9 to 3.8) | 0.080 |
| Diarrhoea | 35 (51) | 28 (18) | 4.6 (2.4 to 8.9) | <0.001 |
| IBS | 11 (16) | 11 (7) | 3.0 (1.1 to 7.9) | 0.030 |
| Gastritis | 21 (31) | 29 (18) | 1.9 (1.0 to 3.8) | 0.060 |
| Headache | 17 (25) | 24 (15) | 2.0 (0.9 to 4.5) | 0.090 |
| Anaemia | 23 (34) | 4 (3) | 25.9 (6.0 to 110.7) | <0.001 |
| GP referral and investigations | ||||
| Referralsa | 61 (90) | 101 (64) | 3.9 (1.7 to 8.7) | <0.001 |
| Endoscopyb | 22 (32) | 16 (10) | 5.3 (2.3 to 12.2) | <0.001 |
| Haematology | 64 (94) | 90 (57) | 13.1 (4.0 to 42.9) | <0.001 |
| Biochemistry | 58 (85) | 100 (63) | 5.1 (2.1 to 12.6) | <0.001 |
| Microbiology | 16 (24) | 15 (9) | 3.6 (1.5 to 8.6) | 0.005 |
| Immunology | 29 (43) | 27 (17) | 4.9 (2.3 to 10.8) | <0.001 |
Includes referrals to any secondary care consultants for any clinical investigations including radiological and laboratory tests.
Includes gastroscopies, sigmoidoscopies and colonoscopies. OR = odds ratio. IBS = irritable bowel syndrome.
These statistically significantly associated factors were subsequently entered in two separate multivariate models. The multivariate conditional logistic regression showed three patient symptoms and diagnoses, referral by GP, and three kinds of investigations independently associated with a subsequent diagnosis of coeliac disease (Table 4). Increased risk of being diagnosed with coeliac disease was associated with patients presenting with depression and/or anxiety, diarrhoea, or being diagnosed with anaemia during the preceding 5 years. Coeliac disease was also associated with being referred at least once during the 5-year period, not necessarily to a gastroenterologist. Haematology or immunology blood tests were also associated with having coeliac disease, as was referral for any kind of endoscopy.
Table 4.
Multivariate analyses of features of general practice consultations prior to diagnosis of coeliac disease.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| For the 5 years prior to diagnosis | |||
| Patient symptoms and diagnoses | |||
| Depression and/or anxiety | 2.5 | 1.1 to 5.7 | 0.031 |
| Diarrhoea | 4.5 | 2.0 to 10.0 | <0.001 |
| Anaemia | 26.3 | 5.7 to 120.6 | <0.001 |
| GP referral and investigations | |||
| Referrals | 2.8 | 1.1 to 7.3 | 0.033 |
| Endoscopya | 2.9 | 1.1 to 7.5 | 0.026 |
| Haematology | 7.6 | 2.2 to 25.8 | 0.001 |
| Immunology | 3.6 | 1.4 to 9.0 | 0.006 |
| Excluding 1 year prior to diagnosis | |||
| Patient symptoms and diagnoses | |||
| Diarrhoea | 3.3 | 1.6 to 6.7 | 0.001 |
| Anaemia | 10.7 | 2.9 to 39.5 | <0.001 |
| GP referral and investigations | |||
| Microbiology | 2.7 | 1.1 to 6.9 | 0.032 |
Includes gastroscopies, sigmoidoscopies and colonoscopies. OR = odds ratio.
Of the 68 cases, 54 (79%), had presented with either diarrhoea, depression and/or anxiety, or had been diagnosed with anaemia, compared with 71 (44%), of the controls. Similarly, 67 (99%) of cases had at least one of the investigations listed in Table 4 or had been referred, compared with 128 (80%) of the controls.
Thirty-five (51%) cases had presented with diarrhoea, 28 (41%) depression and/or anxiety, and 23 (34%) had been diagnosed with anaemia at least once during the 5 years prior to diagnosis. This compares with 31 (19%), 51 (32%) and seven (4%), respectively of the controls. Similarly, 14 (21 %) cases had presented with diarrhoea and depression and/or anxiety compared with 14 (9%) controls, eight (12%) cases presented with depression and/or anxiety and had been diagnosed with anaemia compared with four (3%) controls, 13 (19%) cases had presented with diarrhoea and been diagnosed with anaemia compared with three (2%) controls. Three (4%) cases had all three features compared to three (2%) controls.
Analysis excluding 1 year prior to date of diagnosis
Multivariate analysis using data excluding the 1 year prior to diagnosis was performed to identify early features predictive of a subsequent diagnosis of coeliac disease. Only diarrhoea, anaemia, and microbiology tests were independently associated (Table 4). Supplementary Figure 1 shows the timing of consultations with diarrhoea and anaemia, and the cumulative consultations for cases and controls.
DISCUSSION
Summary of main findings
This study has shown that patients subsequently diagnosed with coeliac disease have an increased number of consultations in general practice during the 5 years prior to diagnosis.
Despite exploring a large number of symptoms and diagnoses previously identified as potential predictors of a diagnosis of coeliac disease, it was found that only diarrhoea, depression and/or anxiety, or a diagnosis of anaemia were significantly independently associated with the subsequent diagnosis of coeliac disease. Diarrhoea and anaemia remained associated even after excluding data for the 1 year prior to diagnosis, indicating that depression and/or anxiety was more prevalent in cases during the year prior to diagnosis.
Patients subsequently diagnosed with coeliac disease were also referred more frequently to secondary care specialists and underwent more investigations in primary care during the 5 years prior to diagnosis compared with controls, but this difference was particularly marked for the 1 year prior to diagnosis. A higher proportion of microbiology investigations were found in cases when data for the year preceding diagnosis was excluded from the analysis, which may be related to the testing of stool specimens.
Strengths and the limitations of the study
Sixty-eight cases were successfully recruited and each case was matched with at least two controls, thus achieving the sample size calculation target. Restricting researcher access to the clinical records of only those patients who have provided written consent may lead to greater bias in contemporary case-control studies compared to studies conducted before this became standard practice. In this study, a high consent rate for sequentially approached cases in the secondary care centre resulted in a low risk of selection bias for case. The sample of cases in this study have comparable demographic characteristics to patients with coeliac disease in other studies.9,22 However, unsurprisingly, a much lower proportion of the potential controls approached posted back signed consent forms, making selection bias among this group more possible.
There is some evidence of a higher rate of consent among older controls. People may also be less likely to consent to researchers accessing their clinical records if they frequently consult, and especially if they have consulted with sensitive problems.23 The average consultation rate per annum for the control group was 4.5, slightly less than the consultation rate for the adult Welsh population of 4.95.21 Given the sensitive nature of mental health conditions, the finding that presentations of depression could be predictive of a subsequent diagnosis of coeliac disease may also be due to selection bias in the control group, as controls with recent presentations with depression may have been less likely to consent.
Comparison with existing literature
The finding that diarrhoea and anaemia are the most important predictors of a subsequent diagnosis of coeliac disease are congruent with studies that examined current symptoms in patients newly diagnosed with coeliac disease through screening. Sanders et al identified 12 new cases among 1200 people they screened for coeliac disease, and found that the diagnosis was more prevalent among those who had iron deficiency anaemia.24 Another screening study found that anaemia was most commonly associated, while gastrointestinal symptoms, especially irritable bowel syndrome (IBS), were not associated with a diagnosis of coeliac disease.8 Neither of these studies found depression commonly present at the time of diagnosis through screening, despite depression being generally more common in coeliac disease.18
Dickey and McMillan found that diarrhoea was the most common symptom present in patients diagnosed in primary care with coeliac disease (34%), followed by iron deficiency without gastrointestinal symptoms (30%). IBS symptoms other than diarrhoea were present in only 6%.25
Implications for future research and clinical practice
Previous studies have suggested that a heightened index of suspicion is important for making the diagnosis, and stress the crucial role of GPs, especially among patients presenting with non-gastrointestinal symptoms.26,27 Indeed, there has been an increase from 28 to 60% in the proportion of patients with coeliac disease where the diagnosis was made in primary care.25
The findings suggest that GPs should consider testing for coeliac disease when patients present often, especially with diarrhoea, and/or who are discovered to be anaemic, as this could lead to an earlier diagnosis of an important and easily treatable condition, thus relieving considerable morbidity. Further research is required to clarify the role of depression in the diagnosis of coeliac disease.
Supplementary Material
Acknowledgments
The study team wish to thank Professor Nigel Stott who chaired the Study Steering Committee. We are also grateful to all the GPs and their practice staff who participated in this study, and to the patients who gave permission for us to use their clinical data for this research.
Supplementary information
Additional information accompanies this paper at http://www.rcgp.org.uk/bjgp-suppinfo
Funding body
Funding was obtained from the RCGP Scientific Foundation Board (reference number SFB/2003/30)
Ethics committee
Ethical approval (reference number 03/5222) was obtained from the South East Wales Local Research Ethics Committees
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Corrao G, Corazza GR, Bagnardi V, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358(9279):356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 2.Seraphin P, Mobarhan S. Mortality in patients with coeliac disease. Nutr Rev. 2002;60(4):116–118. doi: 10.1301/00296640260085859. [DOI] [PubMed] [Google Scholar]
- 3.Kaukinen K, Halme L, Collin P, et al. Coeliac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122(4):881–888. doi: 10.1053/gast.2002.32416. [DOI] [PubMed] [Google Scholar]
- 4.Mustalahti K, Lohiniemi S, Collin P, et al. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002;5(3):105–113. [PubMed] [Google Scholar]
- 5.Walters JR, Banks LM, Butcher GP, Fowler CR. Detection of low bone mineral density by dual energy X-ray absorptiometry in unsuspected suboptimally treated coeliac disease. Gut. 1995;37(2):220–224. doi: 10.1136/gut.37.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson A, Kingstone K. Coeliac disease and malignancies. Acta Paediatr Suppl. 1996;412:78–81. doi: 10.1111/j.1651-2227.1996.tb14259.x. [DOI] [PubMed] [Google Scholar]
- 7.Trier JS. Coeliac sprue. N Engl J Med. 1991;325(24):1709–1719. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- 8.Hin H, Bird G, Fisher P, et al. Coeliac disease in primary care: case finding study. BMJ. 1999;318(7177):164–167. doi: 10.1136/bmj.318.7177.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders DS, Hurlstone DP, Stokes RO, et al. Changing face of adult coeliac disease: experience of a single university hospital in South Yorkshire. Postgrad Med J. 2002;78(915):31–33. doi: 10.1136/pmj.78.915.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A, Catassi C. Current approaches to diagnosis and treatment in celiac disease: an evolving spectrum. Gastroenterology. 2001;120(3):636–651. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 11.West J, Logan RF, Hill PG. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003;52(7):960–965. doi: 10.1136/gut.52.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourne JT, Kumar P, Huskisson EC. Arthritis and coeliac disease. Ann Rheum Dis. 1985;44(9):592–598. doi: 10.1136/ard.44.9.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slot O, Locht H. Arthritis as presenting symptom in silent adult coeliac disease. Two cases and review of the literature. Scand J Rheumatol. 2000;29(4):260–263. doi: 10.1080/030097400750041424. [DOI] [PubMed] [Google Scholar]
- 14.Cuomo A, Romano M, Rocco A, et al. Reflux oesophagitis in adult coeliac disease: beneficial effect of a gluten free diet. Gut. 2003;52(4):514–517. doi: 10.1136/gut.52.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickey W. Diagnosis of coeliac disease at open-access endoscopy. Scand J Gastroenterol. 1998;33(6):612–615. doi: 10.1080/00365529850171882. [DOI] [PubMed] [Google Scholar]
- 16.Carnevale V, Filabozzi P, Cela P, Scillitani A. Tiredness: a feature of coeliac disease. Age Ageing. 2000;29(5):462–463. doi: 10.1093/ageing/29.5.462. [DOI] [PubMed] [Google Scholar]
- 17.Serratrice J, Disdier P, de Roux C, et al. Migraine and coeliac disease. Headache. 1998;38(8):627–628. doi: 10.1046/j.1526-4610.1998.3808627.x. [DOI] [PubMed] [Google Scholar]
- 18.Ciacci C, Iavarone A, Mazzacca G, De Rosa A. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol. 1998;33(3):247–50. doi: 10.1080/00365529850170801. [DOI] [PubMed] [Google Scholar]
- 19.Royal College of General Practitioners, Office of population censuses and surveys, Department of health. Morbidity statistics from general practice fourth national study, 1991–1992. London: HMSO; 1995. [Google Scholar]
- 20.Walker-Smith JA, Guandalini S, Schmitz J, et al. Revised criteria for diagnosis of coeliac disease. Report of the working group of the european society of paediatric gastroenterology and nutrition. Arch Dis Child. 1990;65(9):917–918. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John G. General practice morbidity database. Cardiff: Health Solutions Wales; 1992. [Google Scholar]
- 22.Hawkes ND, Swift GL, Smith PM, Jenkins HR. Incidence and presentation of coeliac disease in South Glamorgan. Eur J Gastroenterol Hepatol. 2000;12(3):345–349. doi: 10.1097/00042737-200012030-00013. [DOI] [PubMed] [Google Scholar]
- 23.Robling MR, Hood K, Houston H, et al. Public attitudes towards the use of primary care patient record data in medical research without consent: a qualitative study. J Med Ethics. 2004;30(1):104–109. doi: 10.1136/jme.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders DS, Patel D, Stephenson TJ, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003;15(4):407–413. doi: 10.1097/00042737-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Dickey W, McMillan SA. Increasing numbers at a specialist coeliac clinic: contribution of serological testing in primary care. Dig Liver Dis. 2005;37(12):928–933. doi: 10.1016/j.dld.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Sanders DS. Coeliac disease: is case finding the correct ethical and logistical approach? Gut. 2003;52(7):1070–1071. doi: 10.1136/gut.52.7.1070-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell RJ. What's the case for case-finding in primary care for coeliac disease? Dig Liv Dis. 2006;38(7):468–470. doi: 10.1016/j.dld.2006.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.