Abstract
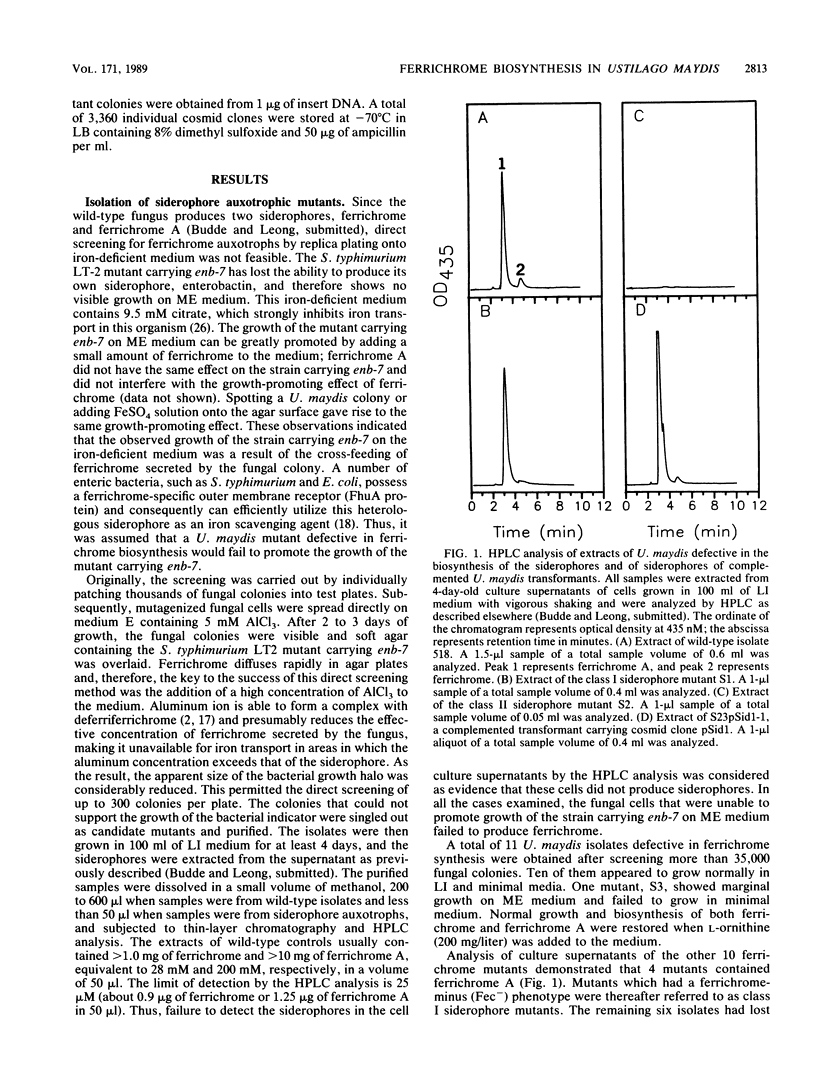
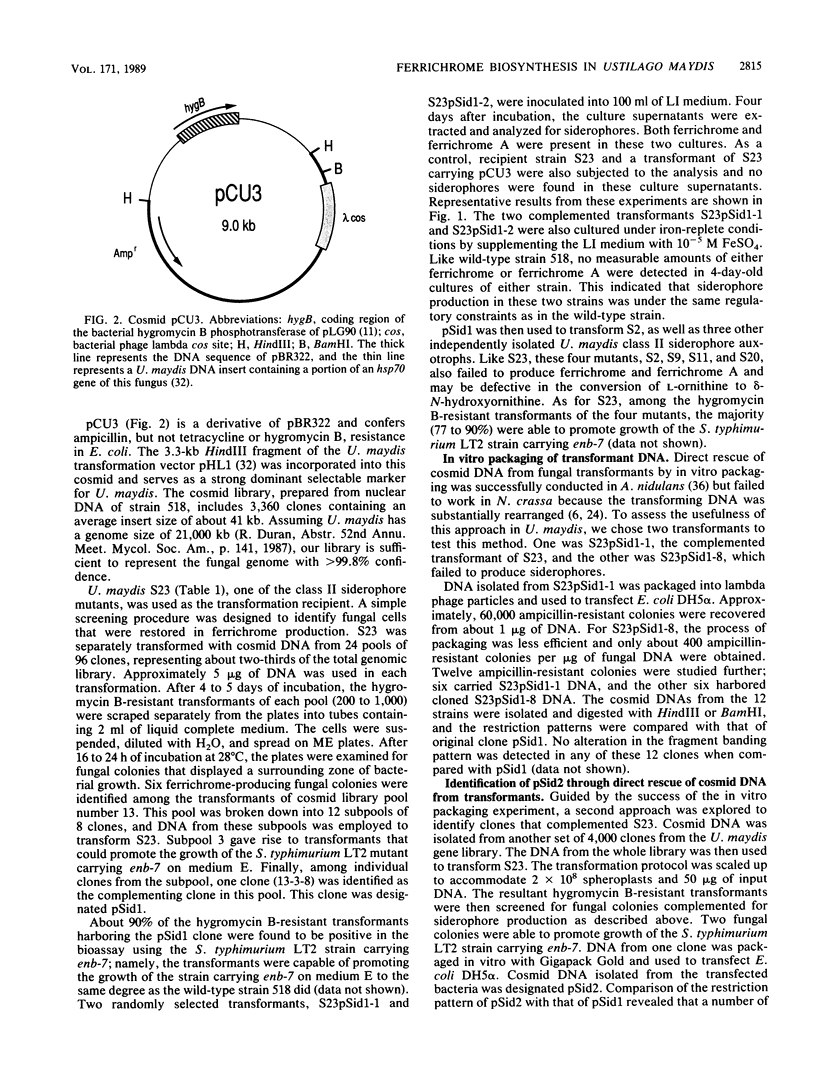
By using a non-enterobactin-producing enb-7 mutant of Salmonella typhimurium LT2 as a biological indicator, a novel screening method was developed for identifying mutants of Ustilago maydis defective in the biosynthesis of the siderophores ferrichrome and ferrichrome A. Two classes of siderophore mutations, both recessive, were isolated after mutagenesis of haploid cells of the corn smut fungus. Class I mutants no longer produced ferrichrome while retaining the ability to produce ferrichrome A; class II mutants were defective in the production of both ferrichrome and ferrichrome A. Genetic and biochemical data suggest that class II mutants are defective in the ability to hydroxylate L-ornithine to delta-N-hydroxyornithine, the first step in the biosynthesis of these siderophores. A genomic library of wild-type U. maydis DNA was constructed in the cosmid transformation vector pCU3, which contains a dominant selectable marker for hygromycin B resistance. Two cosmids, pSid1 and pSid2, were identified in this library by their ability to complement class II siderophore auxotrophs. The production of both siderophores was concomitantly restored in the majority of the resultant transformants. Transforming DNA could be recovered from the fungal, cosmid-containing transformants by in vitro packaging with lambda bacteriophage extracts. Alternatively, the clones could be identified by a sib selection procedure. Cotransformation was found to occur at a high frequency in the fungus and was used to determine that a 2.5-kilobase HindIII-NruI fragment in pSid1 was responsible for complementing the class II siderophore biosynthetic mutation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B. Rhodotorulic acid, a diketopiperazine dihydroxamic acid with growth-factor activity. I. Isolation and characterization. Biochemistry. 1968 Oct;7(10):3734–3739. doi: 10.1021/bi00850a054. [DOI] [PubMed] [Google Scholar]
- Case M. E. Genetical and molecular analyses of qa-2 transformants in Neurospora crassa. Genetics. 1986 Jul;113(3):569–587. doi: 10.1093/genetics/113.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery T. F. Initial steps in the biosynthesis of ferrichrome. Incorporation of delta-N-hydroxyornithine and delta-N-acetyl-delta-N-hydroxyornithine. Biochemistry. 1966 Nov;5(11):3694–3701. doi: 10.1021/bi00875a045. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Herrero M., de Lorenzo V., Neilands J. B. Nucleotide sequence of the iucD gene of the pColV-K30 aerobactin operon and topology of its product studied with phoA and lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):56–64. doi: 10.1128/jb.170.1.56-64.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey J. A., Rambosek J. A. Transformation of Neurospora crassa with the cloned am (glutamate dehydrogenase) gene. Mol Cell Biol. 1984 Jan;4(1):117–122. doi: 10.1128/mcb.4.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S. A., Neilands J. B. Relationship of siderophore-mediated iron assimilation to virulence in crown gall disease. J Bacteriol. 1981 Aug;147(2):482–491. doi: 10.1128/jb.147.2.482-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás M., Klein M. P., Neilands J. B. Solution conformation of ferrichrome, a microbial iron transport cyclohexapeptide, as deduced by high resolution proton magnetic resonance. J Mol Biol. 1970 Sep 28;52(3):399–414. doi: 10.1016/0022-2836(70)90409-2. [DOI] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Paietta J., Marzluf G. A. Plasmid recovery from transformants and the isolation of chromosomal DNA segments improving plasmid replication in Neurospora crassa. Curr Genet. 1985;9(5):383–388. doi: 10.1007/BF00421609. [DOI] [PubMed] [Google Scholar]
- Peet R. C., Lindgren P. B., Willis D. K., Panopoulos N. J. Identification and cloning of genes involved in phaseolotoxin production by Pseudomonas syringae pv. "phaseolicola". J Bacteriol. 1986 Jun;166(3):1096–1105. doi: 10.1128/jb.166.3.1096-1105.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhalla J. E. The formation of diploids of Ustilago maydis on agar medium. Phytopathology. 1969 Nov;59(11):1771–1772. [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Holden D. W., Leong S. A. Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci U S A. 1988 Feb;85(3):865–869. doi: 10.1073/pnas.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Wernars K., Goosen T., Wennekes B. M., Swart K., van den Hondel C. A., van den Broek H. W. Cotransformation of Aspergillus nidulans: a tool for replacing fungal genes. Mol Gen Genet. 1987 Aug;209(1):71–77. doi: 10.1007/BF00329838. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Timberlake W. E., Hondel C. A. A cosmid for selecting genes by complementation in Aspergillus nidulans: Selection of the developmentally regulated yA locus. Proc Natl Acad Sci U S A. 1985 Feb;82(3):834–838. doi: 10.1073/pnas.82.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]



