1. Introduction
Although cytomegalovirus (CMV) infections are common, most of the cases in immunocompetent hosts are either asymptomatic or self-limited and require no specific treatment. Infection of the immunocompromised host, however, may result in significant morbidity and mortality even with antiviral therapy (Khare and Sharland, 2001; Pass, 2002). Currently licensed drugs for the treatment of systemic cytomegalovirus infections in the United States include foscarnet, cidofovir and ganciclovir. These compounds are somewhat effective in controlling CMV infections, but emergence of resistance and potentially serious side effects limit their use (Biron, 2006; Boivin et al., 2005; Gilbert and Boivin, 2005). There is also a potential utility for using anti-CMV drugs in patients with congenital CMV infections. Recent data suggests that early treatment of symptomatic congenitally infected infants with intravenous ganciclovir can prevent the progression of hearing loss (Kimberlin et al., 2003). However, safer and more effective orally available compounds are needed to control and improve the outcome of CMV infections.
Animal models of CMV infection utilizing guinea pigs, rats and mice have been very useful in testing new anti-CMV agents (Kern, 2006). However, because of the high species specificity inherent to this virus, testing of new antiviral therapies is restricted to using the specie's own cytomegalovirus. This limitation poses an obstacle for testing of compounds that may show activity only against human cytomegalovirus (HCMV) or with differences in susceptibility between human and animal isolates. One successful approach to overcome this problem has been the use of human tissues such as fetal thymus/liver or retinal tissue surgically implanted in SCID mice and infected with HCMV (Bidanset et al., 2001; DiLoreto et al., 1994; Kern et al., 2001; Mocarski et al., 1993). An alternative approach is to use human tissue culture cells that are placed in a matrix and embedded into mice. For example, hollow fibers containing HCMV infected human cells have been implanted into SCID mice for antiviral testing (Weber et al., 2001) while Chong et al. (Chong et al., 1999) used Gelfoam gelatin sponges as implants. Gelfoam, commonly used for hemostasis in surgical procedures, is a three-dimensional matrix with large interstices capable of supporting the growth of cells (Centra et al., 1992). We therefore explored the utility of using these sponges as carriers of HCMV infected human foreskin fibroblasts and developed a model suitable for evaluation of new anti-HCMV compounds in Gelfoam implanted SCID mice.
2. Material and Methods
2.1 Virus and Viral Cultures
HCMV recombinant virus, HV5.111, a Toledo strain expressing green fluorescent protein (GFP), under the control of the cellular elongation factor 1α (EF1α), was kindly provided by Jeff Vieira (University of Washington, Seattle, WA). The GFP+ HCMV appears to exhibit growth characteristics similar to wild type virus in cell culture (Jarvis et al., 1999, Iwata et al., 1999, personal communication, J. Viera). Since all efficacy comparisons were made with the untreated control implants that were infected with the same GFP+ virus as the drug treated implants, the differences in behavior and/or properties of the recombinant HV5.111 virus compared to wild type are may not be critical for interpretation of antiviral activity in this model. Viral stocks were grown in human foreskin fibroblasts (HFF, American Type Culture Collection, ATCC CRL 1635, Rockville, MD) that were maintained with DMEM (Invitrogen Corporation, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and penicillin/streptomycin 10,000 units/ml (Invitrogen) and stored frozen at −80°C.
For viral yields, the Gelfoam implants were harvested under sterile conditions from the animal and ground with a glass Dounce homogenizer in 2.0 ml of media. Collagenase type IA (Sigma-Aldrich Corporation, St Louis, MO) was then added to the media (1.0 mg/ml) to digest the Gelfoam particles present in the sample. The homogenate was then examined by fluorescent microscopy to determine the number of infected cells or sonicated and serially diluted to inoculate HFF monolayers to determine plaque forming units (pfu). For pfu determination, the inoculum was removed after a 2-hour incubation period at 37°C and the monolayers were overlaid with media consisting of 50% Basal Medium Eagle (Sigma-Aldrich Corporation) and 1.5% methylcellulose. The cultures were incubated for 12 days at 37°C and virus was quantified by counting plaques after staining with crystal violet. The viral CPE was confirmed by immunofluorescence in selected cultures. For statistical analysis, negative cultures were assigned a number corresponding to the limit of detection of the assay (1.3 Log10 pfu/ml).
2.2 Mice
C.B.−17 male SCID mice were obtained at 21-28 days of age from Charles River Laboratories (Wilmington, MA). Animals were housed under AAALAC approved facilities and all procedures were approved by the Institutional Animal Care and Use Committee.
2.3 Antivirals
Ganciclovir (GCV) (Roche Laboratories, Nutley, NJ) and Cidofovir (CDV) (Gilead Sciences, Foster City, CA) were prepared for animal treatment according to the manufacturer's instructions. GCV was administered twice daily (50 mg/kg/dose) by intraperitoneal injection for a total daily dose of 100 mg/kg. Treatment was begun on day 0 or day 7 after mice were implanted and continued until day 5 or 14 post implantation. CDV was administered once daily (25mg/kg) on days 7-14 post implantation by intraperitoneal injection.
2.4 Infection and implant procedure
Gelfoam (Cardinal Health, Dublin, Ohio) was obtained in strips of 2 cm × 6 cm × 7 mm. The strips were aseptically divided into 5 pieces of ∼4 mm × 6 cm × 7 mm and placed in sterile cryovials. For infection with HCMV, HFFs were first harvested from culture flasks with trypsin, and the cell suspension was centrifuged at 1200 rpm for 10 minutes. The supernatant was discarded and the cell pellet resuspended in fresh media. The cells were counted using a hemocytometer and infected with HCMV with MOIs ranging from 0.01-0.1. The infected cell suspension was placed back in a tissue culture flask containing fresh media and incubated for varying times 37°C. The following day the infected cells were trypsinized, counted and resuspended in media. Approximately 300μl of the cell suspension was dispensed into each cryovial containing a Gelfoam strip and again incubated overnight at 37°C.
For implantation into mice, the Gelfoam strips were loaded into an 11 gauge trocar needle (Popper & Sons, New Hyde Park, NY) using sterile forceps. The mice were anesthetized with sodium pentobarbital and the Gelfoam strips were implanted subcutaneously by entering the area of the upper back and directing the needle towards the lower back down to the dorsal area located just above the hips. One Gelfoam strip was implanted into each mouse in all in vivo experiments.
2.5 Statistics
Means were compared by Student's t-test. All comparisons were two-tailed.
3. Results
3.1 In vitro experiments
To determine the optimal growth conditions of the HCMV recombinant virus infected HFFs on the Gelfoam strips, a series of evaluations were performed in vitro. Factors such as the number of cells used for each strip, the MOI, and the incubation periods were examined. First, the number of cells was determined by seeding the Gelfoam strips with either 1×105 or 5×105 HFFs after infecting them with HCMV at an MOI of 0.01. Higher viral yields were found with the Gelfoam strips seeded with the higher number of infected cells than in those with a lower cell number (data not shown). All further studies were therefore conducted using 5×105 HFFs.
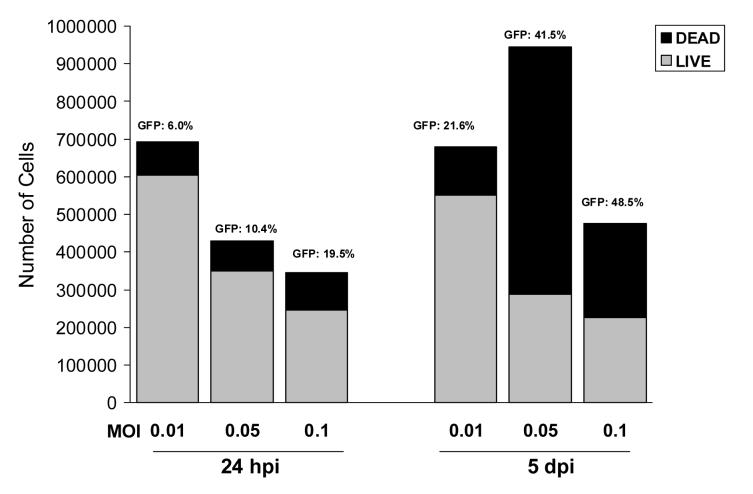
We next examined the effects of varying the MOI. HFF cells were infected at MOIs of 0.01, 0.05, and 0.1. Following an incubation period of 1 or 5 days after seeding, the strips (one per MOI) were digested with media containing collagenase and the total cell counts were obtained. The number of non-viable cells, determined by Trypan Blue exclusion, and the proportion of GFP-expressing cells were then determined. At day 1, a dose response with the highest number of viable cells (87.4%) was seen with the lowest MOI of 0.01 (Figure 1). However, the proportion of GFP+ cells increased with increasing MOIs, ranging from 19.5% with the highest MOI to 6.0% with the lowest MOI. After 5 days of incubation , the proportion and number of GFP+ cells increased with increasing MOIs and also increased compared to day 1. However, the number of viable cells decreased with the higher MOIs, so that only 47.4% were viable after infection at an MOI of 0.1. The Gelfoam strips that were seeded with the cells infected at an MOI of 0.01 exhibited the highest cell viability (80.1%), with an increase in the number of GFP+ cells from 6.0% on day 1 to 21.6%.
Fig 1.
Effect of CMV MOI on virus replication in Gelfoam strips (one per MOI) seeded with 5 ×105 infected fibroblasts. Infected Gelfoam strips examined after a 24-hour period of incubation (left bars), and after 5 days of incubation (right bars); total cell counts, percent of viable cells (gray) and proportion of GFP expressing cells are shown.
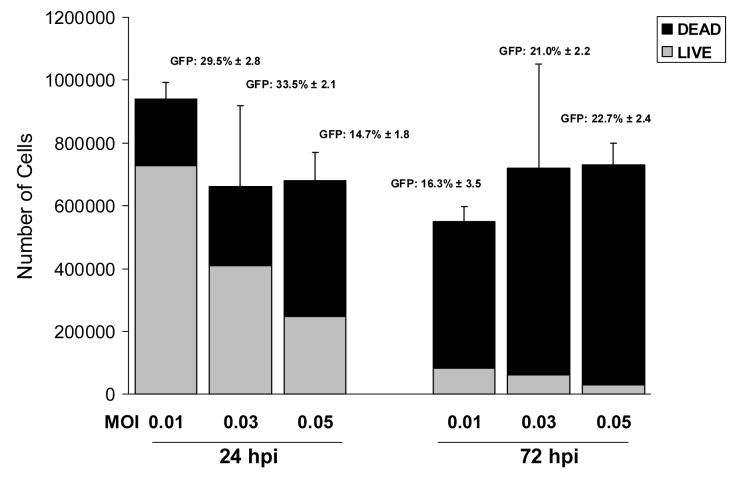
A subsequent experiment was performed to confirm these initial results and to test an intermediate MOI of 0.03. We also examined the effect of increasing the incubation time of the CMV infected HFF cells in vitro from 24 to 72 hours. These parameters were evaluated at 5 days after incubation of the seeded Gelfoam strips (7 to 9 days after infection of the cells). Three strips per MOI were examined. The results (Figure 2) indicated that the Gelfoam strips that were seeded 24 hours after infection exhibited a decline in the number of viable cells with increasing MOIs. Similar to the results obtained in the previous experiment, 77.3% of the cells were viable after infection at an MOI of 0.01 while the percentage of GFP+ cells was 29.5%. Incubation of the Gelfoam strips for 72 hours after CMV inoculation resulted in much higher cell mortality in all 3 viral MOIs examined.
Fig 2.
Effect of time between infection of cells and seeding of the Gelfoam strips with 5 ×105 infected fibroblasts. Mean (± SD) of the total cell counts of 3 Gelfoam strips seeded after an incubation period of 24 hours (left bars) or 72 hours (right bars). The percent of viable cells (gray) and proportion of GFP expressing cells are shown.
3.2 In vivo experiments
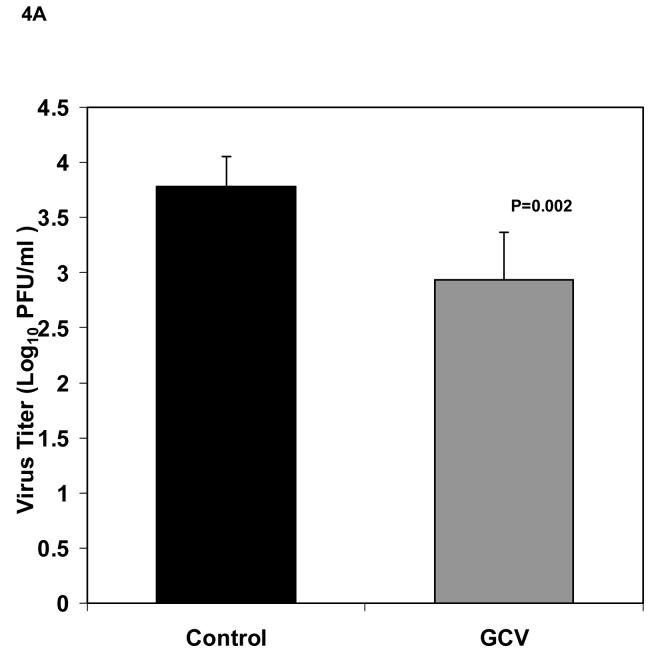
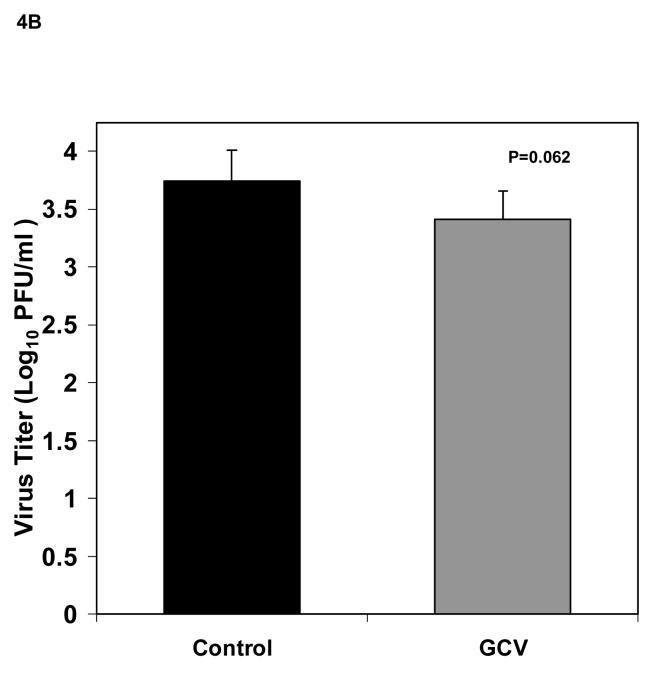
Based on the results of our in vitro experiments, we selected a viral MOI of 0.01 to infect 5×105 fibroblasts that were then seeded to each Gelfoam strip 24 h after infection and implanted into SCID mice the next day. Our initial evaluations in SCID mice indicated that these experimental conditions provided consistent virus titers. Therefore we proceeded to use the model for in vivo antiviral evaluations. The experimental design for the in vivo experiments is illustrated in Figure 3. To determine the effect of antiviral treatment in reducing viral titers in Gelfoam implants, six mice received ganciclovir at 50 mg/kg/dose twice daily administered by intraperitoneal (IP) beginning immediately after implantation and continued for 5 days while six mice were untreated. No signs of toxicity, such as weight loss or decreased activity, were observed in the ganciclovir treated animals. Mice were then sacrificed on day 5 post implantation, and the Gelfoam strips processed for viral culture. As seen in Figure 4A, viral titers were significantly reduced in treated mice (2.93 ± 0.43 Log10 pfu/ml) compared to the untreated (3.79 ± 0.27 Log10 pfu/ml, P=0.002). To confirm this finding, we repeated this experiment with 12 additional mice that were implanted and treated as described above. Viral titers were again reduced from 3.74 ± 0.27 Log10 pfu/ml in untreated mice to 3.41 ± 0.24 Log10 pfu/ml in treated animals but this time the difference was not significant (P=0.06, Figure 4B). These results suggested that under these conditions, antiviral treatment produced a small reduction in viral titers in this model.
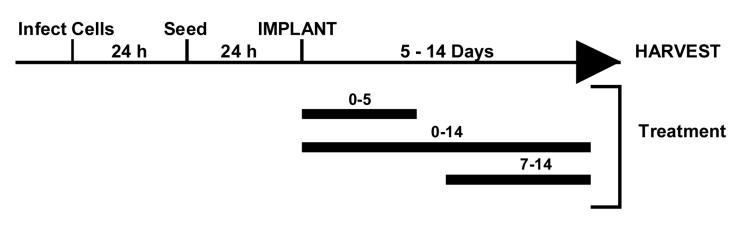
Fig 3.
Time line of a typical experiment using HCMV infected Gelfoam strips implanted in SCID mice. Fibroblasts were infected for 24 h prior to seeding 5×105 infected HFFs to each strip. The cells were then allowed to attach to the strip for 24 h before being implanted into mice. The implanted mice were given antiviral therapy for 0-14 days and the Gelfoam strips harvested at the end of treatment for evaluation of viral titers.
Fig 4.
Viral titers (mean ± standard deviation) of Gelfoam strips harvested from implanted mice (n=6/group) 5 days after implant. (A) Titers from untreated control (black bar) and 100 mg/kg/day ganciclovir treated (gray bar) given IP twice daily, beginning immediately following implantation and continued for 5 days, are shown. (B) Titers from a second experiment using identical conditions. P values are also indicated.
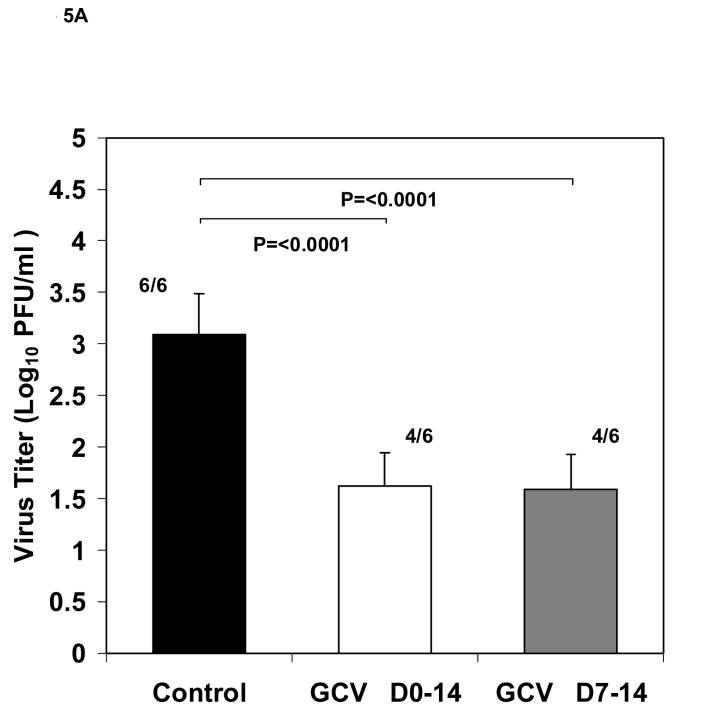
We hypothesized, that during the treatment period of 0-5 days after implantation, the blood supply to the Gelfoam strip in the mice may not be sufficient to allow adequate amounts of the antiviral compound to reach the implant and/or that the duration of treatment may not be adequate. In the next experiment we, therefore, extended treatment to 0-14 days and evaluated a second regimen in which animals began ganciclovir treatment at a later time (day 7) after implantation and continued treatment to day 14. The latter regimen was based on our hypothesis that a highly effective level of ganciclovir might only be available to the implant after one week after transplant due to a higher degree of vascularization. In this experiment, eighteen mice were implanted with Gelfoam strips containing HCMV infected cells and divided into three groups. The results (Figure 5A), showed a significant reduction (P<0.0001) in viral titers between the group treated with ganciclovir from day 0-14 after implantation (1.62 ± 0.32 ) as well as a similar reduction in the group receiving therapy from day 7-14 (1.59 ± 0.32 Log10 pfu/ml vs. the untreated control group 3.09 ± 0.39 Log10 pfu/ml). In addition, 2 of the 6 mice in each treatment group had no detectable virus. These results suggested that vascularization and drug delivery improved overtime, thus improving the effectiveness of therapy and the ability to identify active drugs in this model. The obvious increase in the macroscopic blood supply to the tissues surrounding the implants at 14 days compared to 5 days after implantation is illustrated in Figures 6A and 6B.
Fig 5.
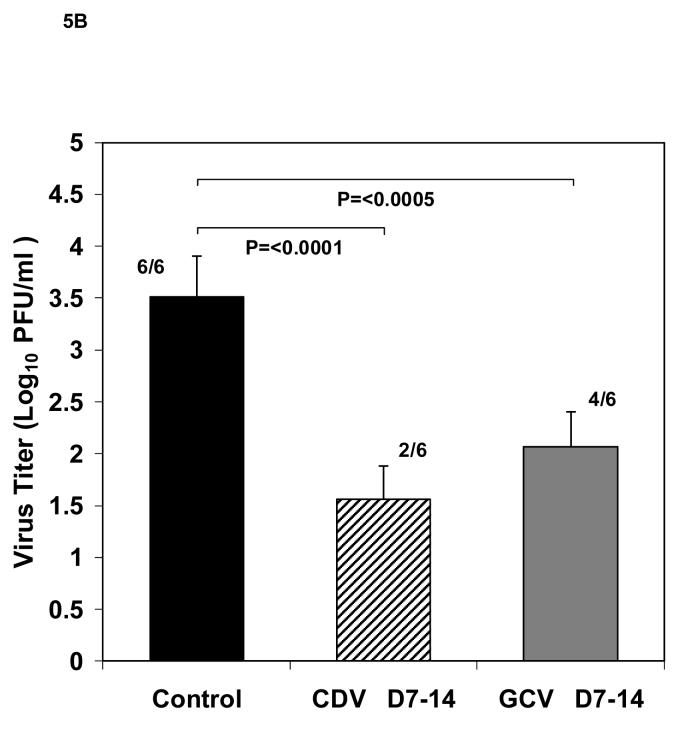
Effect of ganciclovir therapy on viral replication. Viral titers (mean ± standard deviation) of HCMV infected Gelfoam strips from SCID mice (n=6/group) harvested 14 days after implant. (A) Titers from mice receiving antiviral treatment with 100mg/kg/day ganciclovir given IP twice daily, beginning immediately following implantation and continued for 14 days (white bar), or from 7-14 days (gray bar) compared to untreated control (black bar). Numbers shown indicate the number of positive cultures over the total number of cultures. For statistical comparisons, the negative cultures were assigned a number corresponding to the limit of detection of the assay. (B) Titers from mice receiving antiviral treatment with 100 mg/kg/day ganciclovir given twice daily by IP injection from day 7-14 after implant (gray bar) or 25 mg/kg/day cidofovir given daily by IP injection from day 7-14 after implant (striped bar). P values and the number of positive cultures over the total number evaluated are also indicated.
Fig 6.
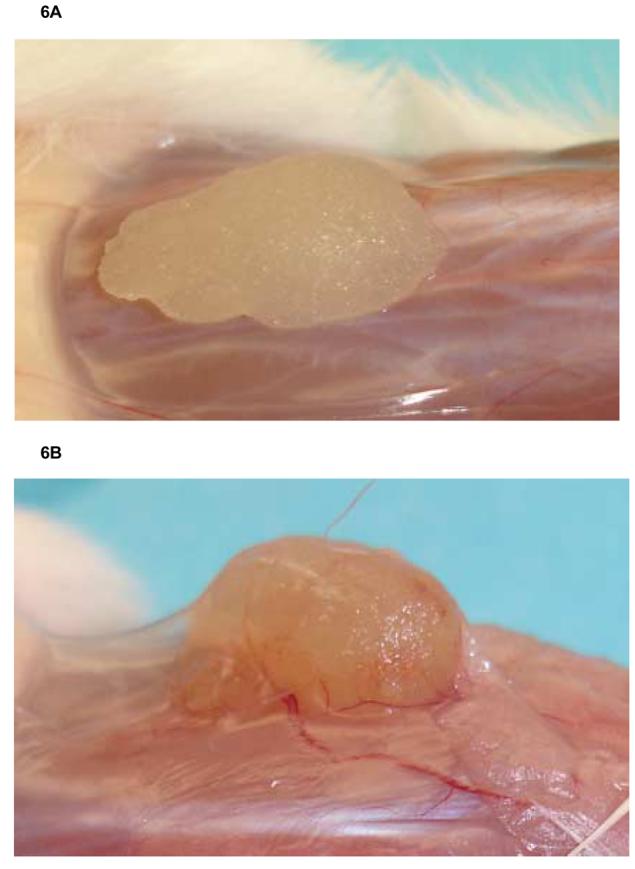
Gelfoam implants from SCID mice shown (A) after 5 days of implantation and (B) after 14 days of implantation. The area surrounding the implant becomes vascularized over time, and a tissue membrane is seen covering the implanted Gelfoam strip. Both untreated and treated groups look similar at all timepoints evaluated.
To further validate the model for antiviral evaluations, we determined the effect of treatment with a second antiviral, cidofovir. In this experiment, 18 mice were implanted with HCMV infected Gelfoam strips as before and divided into three groups. To confirm the results of the previous experiment, 6 mice were treated with ganciclovir on days 7-14 as above while 6 mice received cidofovir at a dose of 25 mg/kg by intraperitoneal injection once a day from day 7-14. Similar to the results obtained in the previous experiment, viral titers (Figure 5B) in the group receiving ganciclovir were significantly reduced to 2.07± 0.62 Log10 pfu/ml (P<0.0005) when compared to the untreated control (3.51± 0.31 Log10 pfu/ml). Cidofovir treatment resulted in an even greater reduction in viral titer (1.56 ± 0.40 Log10 pfu/ml, P<0.0001). In addition, 2 of the 6 animals in the ganciclovir group had no detectable virus while 4 of 6 animals in the cidofovir group had no detectable virus. Taken together, the results presented here indicate that HCMV-infected cells embedded onto Gelfoam strips and implanted in SCID mice provide a useful model for the in vivo evaluation of new antiviral compounds against human cytomegalovirus.
4. Discussion
Cytomegalovirus infections are potentially fatal in immune compromised individuals including those infected congenitally, AIDs patients, and solid organ, bone marrow or hematopoetic stem cell transplants (Gandhi and Khanna, 2004; Hodson et al., 2005; Pass, 2002). Congenital CMV infections are also a leading cause of deafness and mental retardation (Fowler and Boppana, 2006; Pass, 2005). Serious side effects and the poor oral bioavailability of most approved CMV drugs limit their utility (Biron, 2006; Gilbert and Boivin, 2005). Another concern in the management of CMV infections is the emergence of strains with resistance, and especially cross-resistance, to anti-CMV antivirals seen with prolonged antiviral therapy in immunocompromised patients (Erice, 1999; Gilbert and Boivin, 2005; Scott et al., 2007). Clearly, there is a need for new, safer and orally available anti-CMV therapies.
Since HCMV replication only occurs in human cells, pre-clinical evaluation of new antivirals in vivo has been performed in animal models using guinea pig, rat and murine CMV (Kern , 2006). In order to evaluate compounds with activity against HCMV in human cells, models have been developed that utilize human thymus/liver or human retinal tissue implanted under the kidney capsule or the eyes of SCID mice (Bidanset et al., 2001; DiLoreto et al., 1994; Kern et al., 2001; Mocarski et al., 1993). These models are technically challenging and require human fetal tissue. To overcome some of these difficulties, hollow fiber tubes and agarose plugs carrying HCMV infected cells implanted into SCID mice have also been utilized as models for antiviral evaluation (Allen et al., 1992, Weber et al., 2000; Weber et al., 2001). Both models seem less technically demanding than the human transplant models and appear to be useful for antiviral evaluations, but both require surgery whereas the Gelfoam model described here does not. Both of the other models used 5 days of therapy beginning immediately after implantation of the mice whereas the Gelfoam model described here appears to demonstrate better antiviral activity when the drugs is administered from day 7-14 after implant. It is not known whether a similar delayed treatment strategy would be more effective in these models.
Gelfoam is an inexpensive, readily available material with a wide range of applications including surgical hemostasis and drug delivery (Lee and Yalkowsky, 1999; Negvesky et al., 2000; Puterman and Leiberman, 2005). Chong et al. (1999) were first to use Gelfoam sponges in a mouse model of HCMV and studied both SCID and immunocompetent mice. They reported that HCMV replicated to a higher titer and for a longer duration in SCID mice. Their results also found that both ganciclovir and cidofovir were effective in reducing HCMV replication when evaluated in a mouse model using Gelfoam implants, but the timing and duration of treatment are not available. In our studies, we provided further characterization of virus replication and cell viability in the Gelfoam implants, and show evidence that suggests that vascularization of the implant is important for the delivery of the drug to the HCMV infected cells in the Gelfoam implant. Our results indicated that the Gelfoam strips were capable of supporting human cell growth in vitro. HCMV was found to replicate best in Gelfoam strips that contained HFFs infected at the lowest MOI tested (0.01), with increasing MOIs resulting in higher cell death in the period of incubation examined. Lower number of cells or a longer incubation period after infection (and prior to the seeding of the Gelfoam strips) were parameters that also negatively impacted virus replication. Use of the GFP+ Toledo HCMV strain enhanced our ability to quantitate virus and virus infected cells and provided more consistent results than the wild type Toledo strain.
Implantation of the Gelfoam strips into the SCID mouse by a subcutaneous injection was easy and well tolerated. No adverse events such as biting, scratching, secondary infections, or death were observed in the implanted mice. Although a relatively modest titer (∼3.0 Log10 pfu/ml) was obtained from the implanted Gelfoam strips, viral replication was quite consistent, as reflected by the small standard deviations obtained throughout the experiments.
To validate this model for antiviral evaluations, treatment with either ganciclovir or cidofovir was administered to the SCID mice implanted with the Gelfoam strips carrying HCMV infected cells. The onset and duration of therapy were factors that influenced the effectiveness of the antiviral treatment. Only a marginally significant reduction in viral titers was obtained when antiviral therapy was given on days 0-5 after implantation while an extended regimen (from 5 to 14 days), resulted in a significant reduction in viral titers. It appears that the effectiveness of the drugs was influenced by the vascular supply to the implanted Gelfoam strip. Thus treatment from day 7-14 was far more effective than treatment from day 0-5 and this coincides with the increased vascularity of the implant observed over this time. We believe that this increased vascularity would allow a higher drug concentration to be achieved in the transplant which would then lead to increased activity and decreased virus replication. In addition, the antiviral effects demonstrated in this model appeared to be very reproducible. In two experiments ganciclovir reduced viral replication by 1.44 and 1.50 Log10pfu/ml when given from day 7-14. Cidofovir appeared to be more effective than ganciclovir in reducing viral titers in this model, a result that agrees with other studies comparing these antivirals (Neyts and De Clercq, 1994).
Compared to other available models, the model presented here has some benefits such as that it employs commonly available materials, is not labor intensive, and the experiments are relatively quick and inexpensive. One potential disadvantage of the model is that it does not provide information with regards to the antiviral effect in a particular target organ. We believe that this model is promising alternative to the available models for antiviral evaluation of compounds with activity against HCMV
Acknowledgements
Financial support: National Institutes of Health, contract AI 15438 from NIAID
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: None
References
- Allen LB, Li S, Arnett G, Toyer B, Shannon WM, Hollingshead MG. Novel method for evaluating antiviral drugs against human cytomegaovirus in mice. Antimicrob. Agents Chemother. 1992;36:206–208. doi: 10.1128/aac.36.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidanset DJ, Rybak RJ, Hartline CB, Kern ER. Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. J. Infect. Dis. 2001;184:192–195. doi: 10.1086/322015. [DOI] [PubMed] [Google Scholar]
- Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71:154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Boivin G, Goyette N, Gilbert C, Humar A, Covington E. Clinical impact of ganciclovir-resistant cytomegalovirus infections in solid organ transplant patients. Transpl. Infect. Dis. 2005;7:166–170. doi: 10.1111/j.1399-3062.2005.00112.x. [DOI] [PubMed] [Google Scholar]
- Centra M, Ratych RE, Cao GL, Li J, Williams E, Taylor RM, Rosen GM. Culture of bovine pulmonary artery endothelial cells on Gelfoam blocks. Faseb. J. 1992;6:3117–3121. doi: 10.1096/fasebj.6.12.1521742. [DOI] [PubMed] [Google Scholar]
- Chong KT, Modiratt S, Pagano P, Hinshaw R. A novel gelfoam mouse model for human cytomegalovirus (HCMV) replication; Abstracts 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. 1999. p. 441. abstract number 1956. [Google Scholar]
- DiLoreto D, Jr., Epstein LG, Lazar ES, Britt WJ, del Cerro M. Cytomegalovirus infection of human retinal tissue: an in vivo model. Lab. Invest. 1994;71:141–148. [PubMed] [Google Scholar]
- Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 1999;12:286–297. doi: 10.1128/cmr.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J. Clin. Virol. 2006;35:226–231. doi: 10.1016/j.jcv.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect. Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Boivin G. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 2005;49:873–883. doi: 10.1128/AAC.49.3.873-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, Vimalachandra D, Craig JC. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005;365:2105–2115. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- Iwata M, Vieira J, Byrne M, Horton H, Torok-Storb B. Interleukin-1 (IL-1) Inhibits growth of cytomegalovirus in human marrow stromal cells: Inhibition is reversed upon removal of IL-1. Blood. 1999;94:572–578. [PubMed] [Google Scholar]
- Jarvis MA, Wang CE, Meyers HL, Smith PP, Corless CL, Henderson GJ, Vieira J, Britt WJ, Nelson JA. Human cytomegalovirus infection of caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J. Virol. 1999;73:4552–4560. doi: 10.1128/jvi.73.6.4552-4560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER. Pivotal role of animal models in the development of new therapies for cytomegalovirus infections. Antiviral Res. 2006;71:164–171. doi: 10.1016/j.antiviral.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Kern ER, Rybak RJ, Hartline CB, Bidanset DJ. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir. Chem. Chemother. 2001;12(Suppl 1):149–156. [PubMed] [Google Scholar]
- Khare MD, Sharland M. Cytomegalovirus treatment options in immunocompromised patients. Expert. Opin. Pharmacother. 2001;2:1247–1257. doi: 10.1517/14656566.2.8.1247. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, Jacobs RF, Vaudry W, Pass RF, Kiell JM, Soong SJ, Whitley RJ. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- Lee Y, Yalkowsky SH. Systemic absorption of insulin from a Gelfoam ocular device. Int. J. Pharm. 1999;190:35–40. doi: 10.1016/s0378-5173(99)00237-9. [DOI] [PubMed] [Google Scholar]
- Mocarski ES, Bonyhadi M, Salimi S, McCune JM, Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc. Natl. Acad. Sci. U S A. 1993;90:104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negvesky GJ, Butrus SI, Abifarah HA, Lee YC, Yalkowsky SH. Ocular gelfoam disc-applicator for pupillary dilation in humans. J. Ocul. Pharmacol. Ther. 2000;16:311–315. doi: 10.1089/jop.2000.16.311. [DOI] [PubMed] [Google Scholar]
- Neyts J, De Clercq E. New inhibitors of cytomegalovirus replication: in vitro evaluation, mechanism of action, and in vivo activity. Verh. K Acad. Geneeskd Belg. 1994;56:561–592. [PubMed] [Google Scholar]
- Pass RF. Cytomegalovirus infection. Pediatr. Rev. 2002;23:163–170. doi: 10.1542/pir.23-5-163. [DOI] [PubMed] [Google Scholar]
- Pass RF. Congenital cytomegalovirus infection and hearing loss. Herpes. 2005;12:50–55. [PubMed] [Google Scholar]
- Puterman M, Leiberman A. Gelfoam plug tympanoplasty concomitant with removal of retained ventilation tubes. Int. J. Pediatr. Otorhinolaryngol. 2005;69:57–60. doi: 10.1016/j.ijporl.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Scott GM, Weinberg A, Rawlinson WD, Chou S. Multidrug resistance conferred by novel DNA polymerase mutations in human cytomegalovirus isolates. Antimicrob. Agents Chemother. 2007;51:89–94. doi: 10.1128/AAC.00633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber O, Bender W, Eckenberg P, Goldmann S, Haerter M, Hallenberger S, Henninger K, Reefschlager J, Trappe J, Witt-Laido A, RuebsamenWaigmann H. Inhibition of murine cytomegalovirus and human cytomegalovirus by a novel non-nucleosidic compound in vivo. Antiviral Res. 2001;49:179–189. doi: 10.1016/s0166-3542(01)00127-9. [DOI] [PubMed] [Google Scholar]
- Weber O, Reefschlager J, Rubsamen-Waigmann H, Raddatz S, Hesseling M, Habich D. A novel peptide aldehyde with activity against human cytomegalovirus in two different in vivo models. Antivir. Chem. Chemother. 2000;11:51–59. doi: 10.1177/095632020001100105. [DOI] [PubMed] [Google Scholar]