Abstract
In the U.S., despite available swine influenza virus (SIV) vaccines, multiple influenza subtypes as well as antigenic and genetic variants within subtypes continue to circulate in the swine population. One of the challenges to control and eliminate SIV is that the currently used inactivated influenza virus vaccines do not provide adequate cross-protection against multiple antigenic variants of SIV in the field. We previously generated a recombinant H3N2 swine influenza virus (SIV) based on the influenza A/SW/TX/4199-2/98 virus (TX98) containing an NS1 gene expressing a truncated NS1 protein of 126 amino acids, TX98-NS1Δ126 virus. This recombinant strain was demonstrated to be highly attenuated in swine and showed potential for use as a modified live-virus vaccine (MLV) after intratracheal application in pigs. However, this route of inoculation is not practical for vaccination in the field. In the present study, we first compared intramuscular and intranasal routes of application of the MLV, and found that the intranasal route was superior in priming the local (mucosal) immune response. Pigs were then vaccinated via the intranasal route and challenged with wild type homologous TX98 H3N2 virus, with a genetic and antigenic variant H3N2 SIV (influenza A/SW/CO/23619/99 virus, CO99) and a heterosubtypic H1N1 SIV (influenza A/SW/IA/00239/2004 virus, IA04). The intranasally vaccinated pigs were completely protected against homologous challenge. In addition, MLV vaccination provided nearly complete protection against the antigenic H3N2 variant CO99 virus. When challenged with the H1N1 IA04 virus, MLV vaccinated animals displayed reduced fever and virus titers despite minimal reduction in lung lesions. In vaccinated pigs, there was no serologic cross-reactivity by HI assays with the heterologous or heterosubtypic viruses. However, there appeared to be substantial cross-reactivity in antibodies at the mucosal level with the CO99 virus in MLV vaccinated pigs.
Keywords: Swine influenza virus, modified live-virus vaccine, Het-I, influenza A
1. Introduction
Modified live-virus vaccines (MLV) to protect against influenza virus are now commercially available for human [1] and equine species [2]. The MLV available for these species, based on attenuation of the virus by cold-adaptation, have been demonstrated to be both safe and efficacious [3–6]. However, to date, swine influenza (SIV) vaccines are inactivated and no MLV are commercially available for use in U.S. swine. In addition to the cold-adapted, temperature-sensitive strains used in the available human and equine vaccines, NS-1 deletion mutants have been demonstrated to be highly attenuated in a natural host [7]. In addition, the NS-1 mutant viruses are capable of stimulating a protective immune response as demonstrated against the homologous wild type virus and partial protection against a heterosubtypic virus after intratracheal application [8]. The need for swine vaccines that stimulate broader cross-protection against heterologous SIV is great due to the current milieu of various subtypes and genetic variants within subtypes circulating in the U.S. swine population.
Three major subtypes of swine influenza virus (SIV) currently circulate in US swine populations, H1N1, H1N2, and H3N2, with multiple genetic and antigenic variants within each subtype [9–11]. Until 1998, SIV in North America was relatively stable with only one predominant circulating subtype, known as the classical swine H1N1 (cH1N1) [12]. However in 1998, H3N2 isolates with human, avian, and swine genes were identified in multiple swine populations across the U.S. [13, 14], and reassortants between the classical H1N1 and the newly introduced H3N2 viruses rapidly appeared. The reassortments produced H1 swine viruses (rH1N1) with the hemagglutinin (HA) and neuraminidase (NA) from the cH1N1 swine virus and the internal genes from the H3N2 viruses or the HA from the cH1N1 swine virus and the NA and internal genes from the H3N2 viruses (H1N2) [10, 15–17]. With the acquisition of avian polymerase genes in these viruses, an increase in the rate of genetic change in North American swine influenza isolates appears to have occurred in both H3 and H1 virus subtypes. In addition, human-like H1N1 and H1N2 viruses have been recently isolated from pigs in the U.S. [18] and Canada [19]. In the U.S., swine viruses containing the new human-like HA have combined with the internal genes and/or the N2 NA from the triple reassortant swine viruses. Previous immunity to swine H1 HA is not likely to be protective against the new human-like H1 HA variant.
Antibodies that block binding of the HA protein to host receptors are thought to be responsible for much of the protection conferred by natural or vaccine induced immunity to homologous or antigenically related heterologous viruses, and this is most commonly measured using the hemagglutinin inhibition (HI) assay. However, antibodies raised against non-HI epitopes or other viral proteins [20–23], as well as cell mediated immunity (CMI) (reviewed in[24]), clearly play a role in the heterologous cross-reactive immune responses (Het-I) against influenza virus infection. Exposure to live virus or MLV vaccine is likely to have a more robust response against many viral epitopes through heightened cell-mediated immune activation and through the induction of mucosal immunity. These studies have been predominantly conducted in mice, and reports on the in vivo evaluation of cross-protection between antigenically distinct viruses in a natural host are limited. A clinical study evaluating the efficacy of the live, attenuated cold-adapted influenza vaccine reported significant reductions in clinical disease against a virus antigenically drifted from the vaccine strain [3], suggesting that MLV against influenza virus will have superior cross-reactivity against drifted or heterosubtypic viruses as compared to traditional inactivated vaccines.
In a previous study we showed the ability of an MLV NS-1 deletion mutant (TX98-NS1Δ126) to protect pigs against swine influenza after intratracheal vaccination. To investigate the possible use of the TX98-NS1Δ126 MLV in field situations, we sought to evaluate the efficacy of the MLV given via the intranasal and intramuscular routes against homologous wild type virus. We then evaluated the intranasal administration of the MLV against the homologous wild type virus and a heterologous H3N2 virus, defined by genetic variation in the HA gene and limited cross-reactivity in the HI assay with TX98 antiserum [25]. In addition, the intranasal MLV was evaluated against a heterosubtypic rH1N1. The 6 internal genes of the rH1N1 are more closely related to the triple reassortant H3N2 than those of the cH1N1, although cross-protection induced by the internal gene products of triple reassortant swine H3N2 virus against rH1N1 has not been reported. Here we show the routes and number of applications of the TX98-NS1Δ126 MLV resulting in the establishment of protective immunity against homologous wild type virus. Further, we show that the TX98-NS1Δ126 MLV administered via the intranasal route induces a strong local immune response and protects against homologous and (partially) against heterologous viruses.
2. Materials and Methods
2.1 Viruses and vaccine preparation
The MLV was generated via reverse genetics from A/SW/TX/4199-2/98 H3N2 (TX98) as previously described [7]. The attenuated vaccine virus contains an NS1 gene with a 3′ deletion, producing a protein 126 amino acids in length with a carboxy-terminal truncation (TX98-NS1Δ126). The remaining seven gene segments are wild type from the TX98 virus. The challenge viruses included wild-type TX98 H3N2, the heterologous A/SW/CO/23619/99 H3N2 (CO99), and the heterosubtypic A/SW/IA/00239/2004 rH1N1 (IA04). Vaccine and challenge viruses were grown in embryonated chick eggs. Challenge viruses were passed once through pigs and bronchoalveolar lavage fluid (BALF) containing pig passed viruses were used to inoculate pigs at approximately eight weeks of age. Sham inoculated pigs were given BALF from negative cesarean derived-colostrum deprived pigs at like dilutions as the virus inoculum.
2.2 Experimental design
Two-week-old conventional pigs obtained from a high-health herd free of SIV and porcine reproductive and respiratory syndrome virus (PRRSV) were randomly divided into treatment groups. All pigs were treated with ceftiofur crystalline free acid (Pfizer, New York, NY) to reduce bacterial contaminants prior to the start of the study. Two independent animal studies were conducted.
Comparison of MLV application
Thirty-five pigs divided into seven groups of five pigs each were utilized to evaluate the effects of number of MLV doses and route of administration (Table 1). The groups included: non-vaccinated, sham-challenged controls; non-vaccinated, TX98 challenged controls; 1 dose intramuscular (IM) MLV, TX98 challenged; 2 dose IM-MLV, TX98 challenged; 1 dose intranasal (IN) MLV, TX98 challenged; 2 dose IN-MLV, TX98 challenged; and 2 dose IM wild type TX98, TX98 challenged.
Table 1.
Study design for comparing modified live-virus vaccine route and number of doses for protection against homologous virus.
| Group | Vaccinationπ | Challenge | N |
|---|---|---|---|
| NV/NC | None | Sham | 5 |
| NV/TX98 | None | TX98 H3N2 | 5 |
| 2x IM/MLV/TX98 | 2 dose IM NS1Δ126 | TX98 H3N2 | 5 |
| 1x IM/MLV/TX98 | 1 dose IM NS1Δ126 | TX98 H3N2 | 5 |
| 2x IN/MLV/TX98 | 2 dose IN NS1Δ126 | TX98 H3N2 | 5 |
| 1x IN/MLV/TX98 | 1 dose IN NS1Δ126 | TX98 H3N2 | 5 |
| 2x IM/wtTX98/TX98 | 2 dose IM TX98 | TX98 H3N2 | 5 |
NV = non-vaccinated; NC = Sham-challenged; NS1Δ126 = modified live-virus vaccine with NS1 truncated mutant; IM = intramuscular immunization; IN = intranasal immunization; wt = wild type
The second dose was administered 3 weeks after priming dose
Evaluation of the MLV via the intranasal route
To evaluate intranasal administration of the MLV against homologous wild type, heterologous, and heterosubtypic viruses, 70 pigs were divided into 8 groups (Table 2). The non-vaccinated, sham-challenged and vaccinated, sham-challenged control groups contained 5 pigs per group. All challenged groups contained 10 pigs per group at the start of the experiment. Three pigs died from causes not related to influenza infection prior to the challenge date, leaving 9 pigs in these groups: the non-vaccinated, IA04 challenged, the vaccinated, IA04 challenged, and the vaccinated, CO99 challenged groups. All pigs were vaccinated with 2 IN doses of the MLV.
Table 2.
Study design for evaluating intranasal administration of the modified live-virus vaccine in protecting against homologous and heterologous viruses.
| Group | Vaccination | Challenge | N |
|---|---|---|---|
| NV/NC | None | Sham | 5 |
| MLV/NC | 2 dose IN NS1Δ126 | Sham | 5 |
| NV/TX98 | None | TX98 H3N2 | 10 |
| MLV/TX98 | 2 dose IN NS1Δ126 | TX98 H3N2 | 10 |
| NV/CO99 | None | CO99 H3N2 | 10 |
| MLV/CO99 | 2 dose IN NS1Δ126 | CO99 H3N2 | 9 |
| NV/IA04 | None | IA04 rH1N1 | 9 |
| MLV/IA04 | 2 dose IN NS1Δ126 | IA04 rH1N1 | 9 |
NV = non-vaccinated; NC = Sham-challenged; IN = intranasal immunization; NS1Δ126 = modified live-virus vaccine with NS1 truncated mutant
The second dose was administered 3 weeks after priming dose
Pigs in both studies were vaccinated with 2 mL of TX98-NS1Δ126 or wild type TX98 at a dose of 1 × 106 50% tissue culture infective doses (TCID50) per ml by slowly dripping vaccine in the nose or injecting intramuscularly. The first dose was given at approximately 3 weeks of age and a second dose of vaccine was administered at 6 weeks of age when appropriate. At 8 weeks of age, pigs from each challenge group were challenged with 2 ml of 1 × 105 TCID50/ml of virus appropriate for each group. Challenge viruses were given intratracheally while the pigs were anesthetized with an intramuscular injection of a cocktail of ketamine (8mg/kg), xylazine (4 mg/kg), and Telazol (6 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA). Challenge groups were housed in individual isolation rooms and cared for in compliance with the Institutional Animal Care and Use Committee of the National Animal Disease Center. All animals were humanely euthanized 5 days post infection (dpi) with a lethal dose of pentobarbital (Sleepaway, Fort Dodge Animal Health, Fort Dodge, IA).
2.3 Clinical observation and sampling
To evaluate the efficacy of the MLV given via the intranasal route against homologous and heterologous viruses, pigs were observed daily for clinical signs and rectal temperatures were taken daily from −2 dpi to 5 dpi. Sampling and necropsy were conducted similarly for the initial vaccine application study and the study evaluating the efficacy of intranasal application against homologous and heterologous viruses. Nasal swabs were taken on 0, 3, and 5 dpi, placed in 2 ml minimal essential media (MEM) and frozen at −80°C until study completion. After euthanasia, each lung was lavaged with 50 ml MEM to obtain BALF. Each nasal swab sample was subsequently thawed and vortexed for 15 sec, centrifuged for 10 min at 640 × g and the supernatant passed through 0.45 μm filters to reduce bacterial contaminants. An aliquot of 200 μl of the filtrate was plated onto confluent phosphate buffered saline- (PBS) washed MDCK cells in 24-well plates. After 1 hour incubation at 37°C, 200 μl serum-free MEM supplemented with 1 μg/ml TPCK trypsin and antibiotics was added. All wells were evaluated for cytopathic effect (CPE) between 24 and 48 hours and subsequently frozen. Aliquots of 200 μl from the 24-well frozen-thawed plates were transferred onto confluent MDCK cells in 48-well plates and again evaluated for CPE at between 24 and 48 hours post infection. Ten-fold serial dilutions in serum-free MEM supplemented with TPCK trypsin and antibiotics were made with each BALF sample and virus isolation positive nasal swab filtrate sample. Each dilution was plated in triplicate in 100 μl volumes onto PBS-washed confluent MDCK cells in 96-well plates. Plates were evaluated for CPE between 48–72 hours post infection. At 72 hours, plates were fixed with 4% phosphate-buffered formalin and stained using immunocytochemistry with an anti-influenza A nucleoprotein monoclonal antibody as previously described [26]. A TCID50 was calculated for each sample using the method of Reed and Muench [27].
2.4. Pathologic examination of lungs
At necropsy, lungs were removed and evaluated for the percentage of the lung affected with purple-red consolidation typical of SIV infection. The percentage of the surface affected with pneumonia was visually estimated for each lung lobe, and a total percentage for the entire lung was calculated based on weighted proportions of each lobe to the total lung volume [28]. Tissue samples from the trachea and right cardiac lung lobe and other affected lobes were taken and fixed in 10% buffered formalin for histopathologic examination. Tissues were routinely processed and stained with hematoxylin and eosin. Lung sections were given a score from 0–3 to reflect the severity of bronchial epithelial injury based on previously described methods [25]. The lung sections were scored according to the following criteria: 0.0: No significant lesions; 1.0: a few airways affected with bronchiolar epithelial damage and light peribronchiolar lymphocytic cuffing often accompanied by mild focal interstitial pneumonia; 1.5: more than a few airways affected (up to 25%) often with mild focal interstitial pneumonia; 2.0: 50% airways affected often with interstitial pneumonia; 2.5: approximately 75% airways affected, usually with significant interstitial pneumonia; 3.0: greater than 75% airways affected, usually with interstitial pneumonia. A single pathologist scored all slides and was blinded to the treatment groups.
2.5 Serologic and mucosal antibody assays
Serum samples were collected by jugular venipuncture at the following time-points: pre-vaccination, pre-boost, pre-challenge, and at necropsy on 5 dpi. For use in the HI assay, sera were heat inactivated at 56°C and treated to remove non-specific agglutinators with a 20% suspension of Kaolin (Sigma Aldrich, St. Louis, MO) followed by adsorption with 0.5% turkey red blood cells (RBC). The HI assays were done with TX98, CO99, and IA04 viruses as antigens and turkey RBC using standard techniques [29].
An ELISA used to detect SIV-specific antibodies present in the respiratory tract was performed as previously described [30] with slight modifications. The BALF samples from 5 dpi were incubated at 37°C for 1 hr with an equal volume of 10 mMol dithiothreitol (DTT) to disrupt mucus present in the fluids. Independent assays were run using the TX98, CO99, and IA04 as ELISA antigen. Concentrated wild type virus was resuspended in Tris-EDTA basic buffer, pH 7.8, and diluted to an HA concentration of 100 HA units/50 μl. Immulon-2HB 96-well plates (Dynex, Chantilly, VA) were coated with 100 μl of SIV antigen and incubated at room temperature overnight. Plates were blocked for 1 hr with 100 μl of 10% BSA in PBS and washed 3 times with 0.05% Tween 20 in PBS (PBS-T). The assays were performed on each BALF in triplicate. Plates were incubated at room temperature for 1 hr, washed 3 times with PBS-T, then incubated with peroxidase-labeled goat anti-swine IgA (Bethyl, Montgomery, TX) or IgG (Kirkegaard & Perry Laboratories, Gaithersburg, MD) at 37°C for 1 hr. 2, 2′-azino-di(3-ethylbenzthiazoline-6-sulfonate) (ABTS)-peroxide was added as the substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) and optical density (OD) was measured at 405 nm wavelength with an automated ELISA reader. Antibody levels were reported as the mean OD for each triplicate sample, and the mean OD of each treatment group was compared.
2.6 Statistical analysis
Macroscopic pneumonia scores, microscopic pneumonia scores, BALF and nasal swab virus titers, ELISA O.D. readings, and log2 transformations of HI reciprocal titers were analyzed using analysis of variance (ANOVA) with a p-value ≤0.05 considered significant (JMP, SAS institute, Cary, NC). Response variables shown to have a significant effect by treatment group were subjected to comparisons for all pairs using the Tukey-Kramer test. Pair-wise mean comparisons between vaccinated and non-vaccinated groups for virus titers were made using the student’s t-test.
3. Results
3.1. Evaluation of route and number of administrations of the MLV: IM- or IN-MLV application reduced pneumonia from homologous TX98 virus challenge
All pigs were free of influenza virus prior to the start of the experiment by nasal swab sampling. Clinically, the general influenza signs after experimental infections with TX98 virus of 8–10 week old pigs were relatively mild, with almost no detectable coughing or anorexia, even in the non-vaccinated challenged groups. The lungs were removed in toto from each pig and examined for macroscopic evidence of influenza pneumonia. The macroscopic pneumonia was mild, but typical of challenge with TX98 in 8–9 week old pigs. All vaccinated groups had statistically significant reductions in macroscopic pneumonia as compared to the non-vaccinated, challenged controls (Table 3).
Table 3.
Macroscopic lung lesions and virus isolation positive animals in nasal swabs at 3 and 5 dpi and in broncho-alveolar lavage fluid (BALF) at 5 dpi in vaccinated and non-vaccinated pigs.
| Group | % Pneumonia* | Nasal Swab 3 dpi | Nasal Swab 5 dpi | BALF 5dpi |
|---|---|---|---|---|
| NV/NC | 0.1 ± 0.1a | 0/5 | 0/5 | 0/5 |
| NV/TX98 | 2.5 ± 0.6b | 5/5 | 5/5 | 5/5 |
| 2x IM/MLV/TX98 | 0.5 ± 0.1a | 0/5 | 0/5 | 0/5 |
| 1x IM/MLV/TX98 | 1.0 ± 0.4a | 2/5 | 3/5 | 3/5 |
| 2x IN/MLV/TX98 | 0.4 ± 0.2a | 0/5 | 0/5 | 0/5 |
| 1x IN/MLV/TX98 | 0.2 ± 0.1a | 0/5 | 0/5 | 0/5 |
| 2x IM/wtTX98/TX98 | 0.7 ± 0.3a | 0/5 | 0/5 | 1/5 |
Mean ± standard error of the mean. Values within a column with different superscript letters are statistically different at p<0.05.
One dose IN or two doses IM reduced homologous virus levels
All non-vaccinated TX98 challenged pigs shed virus from the nose on both 3 and 5 dpi, and all pigs had virus replicating in the lung on 5 dpi. One or two intranasal doses of the MLV prevented nasal shedding in all pigs challenged with the homologous TX98 at 3 and 5 dpi (Table 3). In addition, infectious virus was not detected in the lungs in any pigs from the 1 or 2 dose IN-MLV vaccinated, TX98-challenged pigs. When given 2 doses, the IM-MLV prevented virus shedding at 3 and 5 dpi and virus replication in the lung at 5 dpi when challenged with TX98. However, 1 dose given IM conferred only partial protection, as 2 of the 5 pigs were positive by nasal swab at 3 dpi, 3 pigs were positive by nasal swab at 5 dpi, and 3 pigs were positive in the lung at 5 dpi. Two doses of the wt-TX98 given IM prevented all of the pigs from shedding virus from the nose and 4 of the 5 pigs from having virus in the lung at 5 dpi.
Application of MLV induced SIV antibody responses in pigs
All pigs were serologically negative prior to the start of the experiment for H1N1 and H3N2 SIV antibodies by HI assay. Only vaccinated pigs seroconverted to the TX98 H3N2 antigen during the course of the study, although to a very low level. At the time of challenge, 3 groups had statistically significant geometric mean reciprocal titers greater than the non-vaccinated, sham-challenged control group: the group that received 1 dose of the MLV intranasally, the group that received 2 doses of the MLV intramuscularly, and the group that received 2 doses of wild type virus intramuscularly, with mean titers of 20, 23, and 46, respectively. Although 3 of the 5 pigs in the group that received 2 doses of the MLV intranasally had begun to sero-convert by the challenge date, the group mean titer was not statistically different than the non-vaccinated control group. At 5 days post challenge, all vaccinated groups had geometric mean titers significantly greater than the non-vaccinated, sham-challenged control group; however none of them were greater or equal to the typical positive cut-off of 40.
Antibodies present in the BALF were measured using ELISA assays with plates coated with the TX98 wild type H3N2. At 5 days post challenge, 3 of the vaccinated groups had mean O.D. levels of IgG against TX98 that were statistically greater than the non-vaccinated, sham-challenged control group but not different from one another, the groups that received 1 or 2 doses of the MLV intranasally and the group that received 2 doses of the MLV intramuscularly, with O.D. of 1.14, 0.91, and 0.83, respectively. Only 2 of the vaccinated groups had mean O.D. levels of IgA against TX98 that were statistically different from the non-vaccinated, sham-challenged control group, the groups that received 1 or 2 doses of the MLV intranasally, with mean O.D. values of 1.14 and 1.00, respectively. The mean O.D. for the one- and two-dose IN groups were not different from each other.
3.2 Clinical efficacy of IN administration of MLV against homologous, heterologous, and heterosubtypic viruses
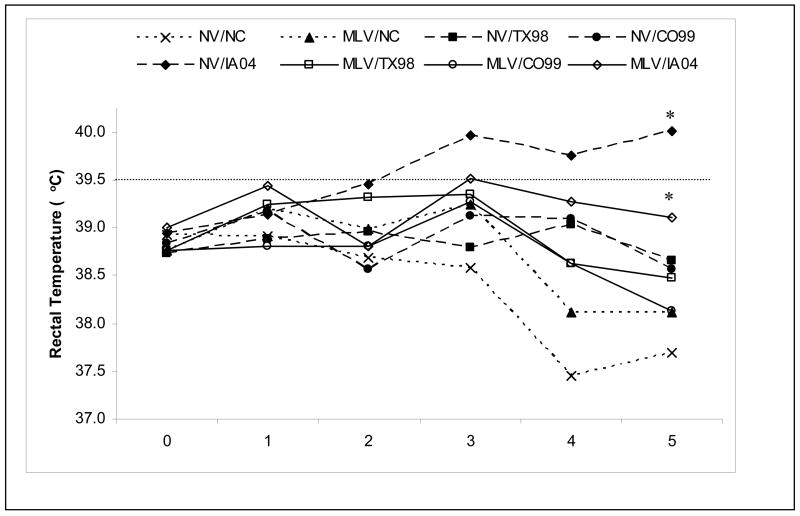
Again, the general clinical impact of the influenza virus infections was relatively mild in 8–10 week old pigs for all the isolates evaluated in this study with almost no detectable coughing or anorexia, even in the non-vaccinated challenged groups. On any day post challenge, the non-vaccinated TX98- and CO99-challenged group means never reached the minimum value for fever (≥39.5°C), calculated as two standard deviations above the mean rectal temperature of all pigs on day zero plus all sham-infected pigs throughout the study. However, many of the non-vaccinated H1N1 IA04-challenged pigs were febrile beginning on 2 dpi and mean rectal temperatures for the group were above the febrile level from 3 dpi until necropsy on 5 dpi (Fig 1). From 2 to 5 dpi, the MLV vaccinated, IA04-challenged pigs had reduced rectal temperatures as compared to the non-vaccinated, IA04-challenged control group, and by 5 dpi the mean rectal temperatures were significantly different (39.1°C versus 40.0°C respectively). There was a trend for all MLV vaccinated and challenged groups to have reduced rectal temperatures compared to their respective non-vaccinated challenge controls by day 4 and 5 post challenge.
Fig. 1.
Mean rectal temperatures from 0 to 5 days post-infection (dpi) in pigs vaccinated 2 times via the intranasal route or non-vaccinated and challenged with homologous and heterologous viruses. Days post-infection are plotted along the X-axis. The cut-off for a febrile response was calculated as two standard deviations above the mean rectal temperature of 0 dpi and sham inoculated pigs from 0 to 5 dpi and is represented by the dashed horizontal line. *Statistical significance at p<0.05 was detected between the non-vaccinated IA04 challenged pigs compared to MLV vaccinated IA04 challenged pigs at 5 dpi.
All isolates induced some level of lung pathology in the non-vaccinated challenged controls, although the severity was different between the IA04 rH1N1 and the H3N2 isolates (Table 4). The MLV significantly reduced the percentage of macroscopic lung pathology in groups challenged with either the homologous TX98 virus as well as the heterologous CO99 H3N2 virus. There was no difference in percentage of pneumonia between the vaccinated and non-vaccinated IA04 groups.
Table 4.
Macroscopic and microscopic pneumonia in non-vaccinated pigs and pigs intranasally vaccinated 2 times with modified live-virus vaccine and challenged with homologous and heterologous SIV.*
| Group | % Pneumonia | Histopathologic Score (0–3) |
|---|---|---|
| NV/NC | 0.1 ± 0.1 | 0.0 ± 0.0 |
| MLV/NC | 0.0 ± 0.0 | 0.0 ± 0.0 |
| NV/TX98 | 6.0 ± 1.8 | 1.2 ± 0.3 |
| MLV/TX98 | 0.1 ± 0.1ψ | 0.0 ± 0.0 ψ |
| NV/CO99 | 6.2 ± 1.2 | 2.1 ± 0.3 |
| MLV/CO99 | 1.1 ± 0.4 ψ | 0.4 ± 0.3 ψ |
| NV/IA04 | 25.9 ± 2.9 | 3.0 ± 0.1 |
| MLV/IA04 | 21.1 ± 2.7 | 2.7 ± 0.2 |
Mean ± standard error of the mean.
Statistically significant reduction as compared to respective non-vaccinated challenged control at p<0.05.
Sections of lung were taken from the right cardiac lobe and/or lobes with macroscopic lesions for microscopic evaluation. Histopathologically, the extent of damage to lung architecture also varied between virus isolates (Table 4). The microscopic lung sections from non-vaccinated TX98-challenged pigs tended to demonstrate only a few airways with epithelial damage in combination with mild focal interstitial pneumonia. The lung sections from non-vaccinated CO99-challenged pigs tended to demonstrate moderate pathology with approximately 50% of the airways affected by bronchiolar epithelial damage and peri-bronchiolar lymphocytic cuffing, often in combination with interstitial pneumonia. In contrast to the H3N2 isolates, the sections of lungs from IA04 rH1N1 non-vaccinated challenged pigs had severe microscopic pathology with greater than 75% of the airways demonstrating epithelial damage in combination with interstitial pneumonia. The IA04 infected lungs were also noted to have a marked infiltration of neutrophils and tracheal epithelial damage. The histopathologic scores for groups vaccinated and challenged with H3N2 viruses were significantly reduced compared to their respective non-vaccinated challenge controls and were no different than the sham-challenged controls. However, the MLV did not reduce microscopic lung pathology in the IA04 challenge group.
IN vaccination with the MLV reduced TX98, CO99, and IA04 virus levels
All pigs were free of influenza virus prior to the start of the experiment by nasal swab sampling. As seen in the first experiment, 2 intranasal doses of the MLV prevented nasal shedding in all pigs challenged with the homologous TX98 at both time points after challenge. Infectious virus was not detected in the lungs in any pigs from the vaccinated, TX98-challenged pigs. Additionally, 8 out of 9 pigs vaccinated and challenged with the antigenic variant CO99 had no detectable virus in the nose on 3 and 5 dpi. Seven of the nine pigs in this group had no detectable virus in the lung at 5 dpi. All pigs challenged with the heterosubtypic IA04 were positive for virus in the nose at 3 and 5 dpi and in the lung at 5 dpi.
There were statistically significant reductions in group mean virus titer levels in the MLV groups challenged with TX98, CO99 or IA04. The TX98 virus titers were below detectable limits in all nasal swabs and BALF in the vaccinated group. The CO99 mean titers were reduced for both nasal swab time points and in BALF as compared to the non-vaccinated CO99 challenge control (Table 5; p<0.05). Additionally, the individual virus titers for the two virus-positive animals were reduced at both time points in the nose and in the lung as compared to the group mean for the non-vaccinated CO99 challenge control. At 3 dpi, there was no difference between the MLV vaccinated, IA04 challenged group and the non-vaccinated IA04 challenge control group with regard to nasal shedding. However, by 5 dpi, there were statistically significant reductions in mean virus titers in nasal swabs and BALF between vaccinated and non-vaccinated IA04 challenge groups.
Table 5.
Log10 mean virus titers in nasal swabs at 3 and 5 dpi and in broncho-alveolar lavage fluid (BALF) at 5 dpi in non-vaccinated pigs and pigs intranasally vaccinated 2 times with modified live-virus vaccine challenged with homologous and heterologous SIV.*
| Group | Nasal swab 3 dpi | Nasal swab 5 dpi | BALF 5 dpi |
|---|---|---|---|
| NV/NC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a,b |
| MLV/NC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a,b |
| NV/TX98 | 4.0 ± 0.4b | 5.5 ± 0.1c | 6.0 ± 0.2d |
| MLV/TX98 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| NV/CO99 | 4.5 ± 0.4b | 5.4 ± 0.2c | 6.0 ± 0.1d |
| MLV/CO99 | 0.5 ± 0.5a | 0.5 ± 0.5a | 0.8 ± 0.5b |
| NV/IA04 | 5.0 ± 0.2b | 4.9 ± 0.2c | 6.0 ± 0.1d |
| MLV/IA04 | 4.7 ± 0.3b | 3.7 ± 0.4b | 5.0 ± 0.3c |
Mean ± standard error of the mean. Values within a column with different superscript letters are statistically different at p<0.05.
Intranasal MLV administration induced modest serum HI antibody titers, but robust and cross-reactive local antibody levels
All pigs were negative prior to the start of the experiment for H1N1 and H3N2 SIV antibodies by HI assay. Only pigs vaccinated with the MLV seroconverted by HI assay to the TX98 H3N2 antigen during the course of the study, and there were no statistical differences in geometric mean HI titers between the vaccinated groups at any time point post-vaccination (Table 7). Overall, the HI titers to the vaccine virus were low and 16 of the 33 MLV vaccinated pigs had HI titers below 1:40 at 5 dpi. No pigs seroconverted to the antigenic variant H3N2 CO99 or the heterosubtypic rH1N1 IA04 by HI assay at 5 dpi. By ELISA assay, all MLV vaccinated groups had significant levels of IgG against the parental TX98 H3N2 virus in the serum (Table 7). By contrast, only MLV vaccinated pigs challenged with the heterosubtypic IA04 rH1N1 had significantly increased serum IgG against either CO99 H3N2 or IA04 rH1N1 compared to the negative controls.
Table 7.
Geometric mean hemagglutination inhibition (HI) reciprocal titers and mean O.D. levels for anti-influenza IgG antibody in serum by ELISA at 5 days post-infection against each of the 3 challenge virus antigens in non-vaccinated pigs and pigs intranasally vaccinated with 2 doses of modified live-virus vaccine.*
| TX98 H3N2 | CO99 H3N2 | IA04 rH1N1 | ||||
|---|---|---|---|---|---|---|
| Group | HI | IgG | HI | IgG | HI | IgG |
| NV/NC | <10 | 0.18 ± 0.02a | <10 | 0.10 ± .02a,b | <10 | 0.45 ± 0.05a |
| MLV/NC | 40.0 | 0.85 ± 0.12c | <10 | 0.41 ± 0.07a,b,c | <10 | 0.55 ± 0.05a,b |
| NV/TX98 | <10 | 0.26 ± 0.04a | <10 | 0.13 ± 0.02a | <10 | 0.49 ± 0.06a |
| MLV/TX98 | 24.6 | 0.78 ± 0.13c | <10 | 0.40 ± 0.09b,c | <10 | 0.71 ± 0.09a,b |
| NV/CO99 | <10 | 0.33 ± 0.04a,b | <10 | 0.18 ± 0.02a,b | <10 | 0.55 ± 0.05a,b |
| MLV/CO99 | 23 | 0.66 ± 0.09b,c | <10 | 0.35 ± 0.05a,b | <10 | 0.60 ± 0.05a,b |
| NV/IA04 | <10 | 0.33 ± 0.03a,b | <10 | 0.22 ± 0.02a,b | <10 | 0.46 ± 0.05a |
| MLV/IA04 | 21.4 | 0.95 ± 0.13c | <10 | 0.66 ± 0.11c | <10 | 0.84 ± 0.10b |
Mean ± standard error of the mean. Values within a column with different superscript letters are statistically different at p<0.05.
Antibodies present in the BALF were measured using ELISA assays with plates coated with each of the three viruses (Table 6). The MLV alone induced a strong IgA and IgG antibody response against the parental TX98 H3N2 virus at the respiratory mucosa. Additionally, significant levels of IgG antibodies cross-reacting with the genetic variant CO99 H3N2 were induced by the MLV alone. Trends for elevated IgA levels against CO99 were seen in all vaccinated groups, although the levels were only statistically different at 5 dpi in the vaccinated and CO99- and IA04-challenged groups. Although the MLV alone did not stimulate significant levels of IgG or IgA antibodies that cross-reacted to the IA04 rH1N1, by 5 dpi the pigs vaccinated and challenged with the rH1N1 had begun to produce IgG that reacted to the rH1N1. In addition, vaccination with the MLV and challenge with the rH1N1 appeared to have a booster effect on IgG cross-reacting to CO99 H3N2. This was similar to the trend seen in serum IgG.
Table 6.
Mean O.D. levels for anti-influenza antibody in broncho-alveolar lavage fluid (BALF) at 5 dpi against each of the 3 challenge virus antigens in non-vaccinated pigs and pigs intranasally vaccinated with 2 doses of modified live-virus vaccine.*
| TX98 H3N2 | CO99 H3N2 | IA04 rH1N1 | ||||
|---|---|---|---|---|---|---|
| Group | IgG | IgA | IgG | IgA | IgG | IgA |
| NV/NC | 0.36 ± 0.17a | 0.23 ± 0.08a | 0.30 ± 0.09a | 0.43 ± 0.03a,b | 0.09 ± 0.04a | 0.31 ± 0.05a,b |
| MLV/NC | 1.23 ± 0.13b,c | 1.30 ± 0.13c | 0.84± 0.12b | 0.71 ± 0.06b,c | 0.28 ± 0.09a | 0.30 ± 0.06a,b |
| NV/TX98 | 0.69 ± 0.16a,b | 0.68 ± 0.16a,b | 0.17 ± 0.04a | 0.41 ± 0.02a | 0.10 ± 0.02a | 0.26 ± 0.04a,b |
| MLV/TX98 | 1.16 ±0.07b,c | 0.89 ± 0.06b,c | 0.84 ± 0.15b | 0.63 ± 0.07b,c | 0.26 ± 0.06a | 0.38 ± 0.04b |
| NV/CO99 | 0.31 ±0.15a | 0.34 ± 0.14a | 0.15 ± 0.05a | 0.38 ± 0.02a | 0.09 ± 0.02a | 0.22 ± 0.03a |
| MLV/CO99 | 1.15 ± 0.10b,c | 1.10 ± 0.09b,c | 0.80 ± 0.11b | 0.78 ± 0.07c | 0.25 ± 0.06a | 0.40 ± 0.05b |
| NV/IA04 | 0.46 ± 0.16a | 0.61 ± 0.13a,b | 0.11 ± 0.03a | 0.31 ± 0.01a | 0.08 ± 0.02a | 0.28 ± 0.03a,b |
| MLV/IA04 | 1.45 ±0.04c | 1.25 ± 0.09c | 1.44 ± 0.06c | 0.80 ± 0.06c | 0.68 ± 0.07b | 0.40 ± 0.02b |
Mean ± standard error of the mean. Values within a column with different superscript letters are statistically different at p<0.05.
4. Discussion
The NS1 protein of influenza virus contributes to virulence by interacting with the host type 1interferon (IFN) antiviral response [31]. The carboxy-terminus is reported to contain the effector domain required for optimally antagonizing the type 1 IFN pathway [32]. The amino terminus is reported to contain the RNA binding domain [33], which may allow the NS1 protein to sequester viral RNA and avoid detection by host cell virus sensors [34]. Swine H3N2 viruses with deletion mutations in the 3′ end of the NS1 gene derived through reverse genetics have been demonstrated to be highly attenuated in vitro and in pigs [7]. The attenuation is due, at least in a major part, to a decreased ability to block antiviral mechanisms, with subsequent host cell upregulation in the expression of type 1 interferons and downstream effectors, such as Mx and protein kinase R [32]. Of the mutants described by Solorzano et al. [7], a virus with an NS1 protein 126 amino acids in length (TX98-NS1Δ126 H3N2) was chosen to be evaluated as an MLV due to its attenuation along with its ability to stimulate immunity in pigs. The TX98-NS1Δ126 H3N2 MLV was previously demonstrated to provide complete protection from homologous challenge as well as partial Het-I against a heterosubtypic cH1N1 when used as an MLV [8]. The earlier study administered the MLV by giving one dose intratracheally and a booster dose intranasally.
In this study, we first evaluated the role of route of administration (IM versus IN) as well as number of doses needed to develop immunity to protect against the homologous wild type virus. The MLV was shown to have complete efficacy against challenge with homologous virus in 1 or 2 doses given intranasally and when 2 doses were given intramuscularly. The intranasal route induced the highest levels of IgA in the BALF in 1 or 2 doses, as well as levels of IgG in BALF equivalent to 2 doses of IM-delivered MLV. Due to the level of protection and stimulation of the mucosal immune response with intranasal administration of the MLV, a second study was conducted to compare the efficacy of 2 doses of the MLV given intranasally to protect against the homologous wild type H3N2, a heterologous H3N2, and a heterosubtypic rH1N1. Since 1 dose versus 2 doses of IN administered MLV was not evaluated against challenge by heterologous or heterosubtypic virus, 2 doses were given in the second study to maximize the efficacy against heterologous viruses. Efficacy was measured by monitoring rectal temperatures, shedding of virus from the nose, replication of virus in the lung, and macroscopic and microscopic lung lesions. In addition, antibody response in the serum and at the respiratory mucosa was measured to evaluate the ability of the MLV to induce cross-reactive antibodies against heterologous and heterosubtypic viruses. The results obtained in the present study demonstrate that the MLV is highly efficient at protecting against a heterologous homotypic H3N2 virus and provides partial protection against a heterosubtypic rH1N1 virus when administered via the intranasal route.
Two doses given intranasally of the TX98-NS1Δ126 H3N2 MLV conferred complete protection from challenge with the wild type TX98 H3N2 virus. Virus was not detected from any pigs in this group at any time point and macroscopic and microscopic lesions were both significantly reduced to minimal or undetectable levels. Live challenge and recovery or natural exposure to influenza virus has been well documented to have improved Het-I over inactivated vaccines [35–38]. To demonstrate the ability of the NS1 deletion mutant in inducing Het-I, vaccinated pigs were challenged with a heterologous H3N2 and a heterosubtypic rH1N1. The TX98-NS1Δ126 H3N2 MLV was effective against the heterologous CO99 H3N2, which is genetically and antigenically distinct from the TX98 H3N2 [25]. Based on virus detection from the nose on 3 and 5 dpi and in the lungs at 5 dpi, 7 of the 9 pigs in the MLV vaccinated, CO99 challenged group demonstrated complete protection. One pig was positive for virus in the nose at 3 and 5 dpi and from the lung at 5 dpi and one pig was positive for virus only from the nose at 3 dpi. Macroscopic and microscopic lesions were significantly reduced as well. There was also a trend for the MLV vaccinated pigs to have reduced rectal temperatures compared to the non-vaccinated, challenged controls toward the end of the evaluation period, although neither the TX98 nor the CO99 challenged control groups were considered febrile based on group mean temperatures. In contrast, the IA04 rH1N1 did induce a febrile response. Remarkably, the MLV protected against pyrexia when challenged with the heterosubtypic rH1N1 IA04.
The presence of cross-reacting antibodies, especially IgA, induced by the MLV at the respiratory mucosa at the time of challenge would suggest their involvement in protection from the heterologous homotypic CO99 H3N2. The MLV induced significantly higher levels of IgA that cross-reacted to CO99 compared to non-vaccinated groups. The 2 pigs from the MLV vaccinated, CO99 challenged group in which virus was detected in the nose or lung had the lowest O.D. values for IgA in the BALF, further suggesting the importance of cross-reactive IgA for Het-I at the respiratory mucosa. In mice, cross-reactive IgA induced by natural infection was shown to be strongly correlated to protection from challenge with a heterologous homotypic virus [35]. Serum HI antibody titer and cross-reactive cytotoxic T-lymphocytes were not correlated with protection from infection, but instead were related to recovery [35]. In addition, IgA was shown to be more cross-reactive than IgG against heterologous virus, and passive transfer of IgA to non-immune mice conferred protection [39], whereas mucosal administration of anti-IgA to immune mice blocked protection from re-infection with the same virus [40]. Pigs immunized with virulent SIV and then challenged with the same virus 42 days later did not have a detectable anamnestic serum antibody response [30]. However, an anamnestic mucosal immune response (rise in IgA and IgG) was detected in the nasal cavity, the site of challenge, indicating that this compartment of the immune system was stimulated [30]. These data support the hypothesis that antibody mediated protection at the mucosal level is important for clearing the respiratory tract of SIV and may not be accurately reflected by systemic antibody levels. This is consistent with our findings reported here.
Intranasal vaccination with the MLV induced a relatively rapid appearance of serum and mucosal IgG antibodies reacting to the heterosubtypic rH1N1 compared to the non-vaccinated, IA04 challenged pigs. Similar results were demonstrated in the previous report by Richt et al. [8], evaluating the TX98-NS1Δ126 H3N2 MLV against a classical swine H1N1 when the vaccine was administered via an intratracheal route followed by an intranasal boost. These results suggest that the immunity induced by the MLV allows for a more rapid development of antibody reacting to heterosubtypic viruses, such as the rH1N1 demonstrated here. The presence of IgG reacting to the IA04 rH1N1 may be responsible for the decrease in shedding and virus replication in the lung, as the amount of virus in nasal swabs and in BALF was reduced by 5 dpi as compared to the non-vaccinated, IA04 challenged controls. This suggests that although the MLV may not prevent heterosubtypic infection, it appears to reduce the duration of shedding and may lead to a more rapid recovery. The ability of a monovalent MLV to reduce fever and virus load due to an infection with a heterosubtypic virus gives this type of vaccine great potential to the swine industry. The Het-I induced by MLV like the one described here may have a significant impact on the epidemiology of novel swine viruses emerging in the population by reducing viral shedding and potentially limiting the spread of such novel viruses.
The ELISAs utilized in this study contain whole virus as antigen, so it is unclear which of the viral proteins are recognized by the antibodies induced by the MLV. A serologic profile of pigs with natural infection induced antibodies against HA, NA, NP, M1, NS1, and NS2, whereas pigs vaccinated with an inactivated vaccine demonstrated IgG primarily against the HA and NP proteins [41]. This, however, may not reflect the antibody profile at the respiratory mucosa, but it does suggest a broader cross-reactive antibody profile with infection of SIV in the natural host. Infection with live virus, MLV or DNA vaccines prime the immune system through intracellular mechanisms [42–45], which may promote a more balanced and effective immune response. It is presumed that the TX98-NS1Δ126 H3N2 MLV evaluated in this study induces a similar balanced immune response, although CMI was not evaluated in this study.
Serum HI antibody titers induced by the intranasal administration of the MLV were low and even below detectability in many pigs. This is in contrast to the high levels of antibody induced at the respiratory mucosa and the IgG in serum detected by ELISA. The HI titers in pigs with intratracheal administration of the MLV were reported to be slightly higher [7, 8] and may reflect a difference in serum HI response between intranasal and intratracheal administration. However, HI titers from pigs vaccinated with either form of respiratory mucosal administration are relatively low compared to HI titers induced by inactivated vaccines administered intramuscularly. In addition, a 2-dose IM administration of the MLV demonstrated the highest serum HI titers of all the MLV vaccinated groups at 5 dpi, but poorly induced the IgA response at the respiratory mucosa. Interestingly, the 2-dose IM administration of wild type TX98 virus induced the highest HI titers and a similar pattern of mucosal antibody production as the 2-dose IM-MLV (data not shown). None of the vaccines utilized in this study were applied with adjuvants. Based on this report and previous experience with adjuvanted inactivated vaccines (Vincent et al., unpublished), the IM administration of the MLV or wild type virus elicits mucosal antibody profiles more similar to IM-administered inactivated vaccines than that elicited by IN or intratracheal administration of the MLV. These results underscore the difference between IM-administered vaccines and IN-administered MLV and their abilities to activate different compartments of the immune system. The IN-MLV induced greater levels of local IgA and was therefore chosen to be the preferred route of administration used in the heterologous and heterosubtypic challenge study. Importantly, serum HI may not be a good indicator of protection or cross-protection in individuals primed by natural exposure or MLV at the respiratory mucosa.
In U.S. rH1N1 and H1N2 swine viruses studied to date, the M, NP, and NS genes are of classical swine H1N1 origin, the PB1 of human origin, and the PA and PB2 genes of avian origin, and these internal genes are similar to the triple reassortant swine H3N2 viruses, including TX98 and CO99 [17, 46]. The M and NP genes have been demonstrated to be conserved between influenza isolates [47, 48], and their proteins are reported to be involved in heterologous immunity (reviewed in [49]). Based on these observations, we hypothesized that the MLV protection against rH1N1 viruses might be improved as compared to the previous results with the cH1N1. The MLV did not appear to have an advantage against the rH1N1 compared to the cH1N1 previously reported [8]. However, the IA04 rH1N1 has been demonstrated to be more virulent than the MN99 cH1N1 [9], indicating that the MLV was tested in an extreme scenario in this study. Notably, it showed a partial protection against this challenge. Although we have demonstrated that the H3N2 MLV has partial protection against heterosubtypic viruses, we would propose that any MLV to reach the market in the swine industry should be targeted against homotypic strains or should be bivalent and include both H3N2 and H1N1 vaccine strains.
Pigs are a natural host for influenza virus and serve as an excellent model for the study of influenza pathogenesis and vaccination strategies. The vaccination and challenge model we have described provides an opportunity to further explore the molecular and cellular control mechanisms for homologous and heterologous immunity induced by MLV vaccines in a natural host. The role of early innate and inflammatory signals as well as the involvement of the humoral and CMI systems can be more carefully examined in future studies utilizing this model. Furthermore, caution is needed when relying on HI cross-reactivity to estimate cross-protection from live or MLV priming, even within the same HA subtypes, as demonstrated by the swine H3N2 viruses used in this model and in recent work with H1 viruses (Vincent, unpublished). With the continual emergence of swine influenza virus genetic and antigenic variants as well as novel subtypes, the swine industry is faced with the challenge of controlling this disease with inactivated vaccines prepared from parent strains with limited representation of the current milieu of viruses circulating among swine. The development of vaccination strategies that induce greater Het-I is necessary to reduce the costly ramifications of this disease. Of equal importance, improved vaccines are needed to reduce the reassortment potential and production of novel viruses due to the large number of farms infected with SIV, the large number of antigenic variants circulating within each of the subtypes of SIV in the U.S., and the constant pressure from human and wildlife reservoirs.
Acknowledgments
The authors thank Michelle Harland, Deb Adolphson, and Deb Clouser for technical assistance as well as Brian Pottebaum and Jason Huegel for assistance with animal care. We thank Susan Brockmeier for critical review of the manuscript. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. This work is partially supported by NIH grant U01 AI070469-01 and by an NIH funded Center to Investigate Virus Immunity and Antagonism (CIVIA, U19 AI62623) to AG-S. The authors wish to disclose that Mount Sinai School of Medicine owns patent positions for reverse genetics of influenza virus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004 Jul;103(1–2):177–85. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Townsend HG, Penner SJ, Watts TC, Cook A, Bogdan J, Haines DM, et al. Efficacy of a cold-adapted, intranasal, equine influenza vaccine: challenge trials. Equine veterinary journal. 2001 Nov;33(7):637–43. doi: 10.2746/042516401776249354. [DOI] [PubMed] [Google Scholar]
- 3.Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, et al. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004 Oct 1;39(7):920–7. doi: 10.1086/423001. [DOI] [PubMed] [Google Scholar]
- 4.Buonagurio DA, O’Neill RE, Shutyak L, D’Arco GA, Bechert TM, Kazachkov Y, et al. Genetic and phenotypic stability of cold-adapted influenza viruses in a trivalent vaccine administered to children in a day care setting. Virology. 2006 Apr 10;347(2):296–306. doi: 10.1016/j.virol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Chambers TM, Holland RE, Tudor LR, Townsend HG, Cook A, Bogdan J, et al. A new modified live equine influenza virus vaccine: phenotypic stability, restricted spread and efficacy against heterologous virus challenge. Equine veterinary journal. 2001 Nov;33(7):630–6. doi: 10.2746/042516401776249291. [DOI] [PubMed] [Google Scholar]
- 6.Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpaa R, Salmi A, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. The Pediatric infectious disease journal. 2006 Jul;25(7):590–5. doi: 10.1097/01.inf.0000220229.51531.47. [DOI] [PubMed] [Google Scholar]
- 7.Solorzano A, Webby RJ, Lager KM, Janke BH, Garcia-Sastre A, Richt JA. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J Virol. 2005 Jun;79(12):7535–43. doi: 10.1128/JVI.79.12.7535-7543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richt JA, Lekcharoensuk P, Lager KM, Vincent AL, Loiacono CM, Janke BH, et al. Vaccination of Pigs Against Swine Influenza Viruses Using a NS1-Truncated Modified Live Virus Vaccine. J Virol. 2006 Nov;80(22):11009–18. doi: 10.1128/JVI.00787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent AL, Lager KM, Ma W, Lekcharoensuk P, Gramer MR, Loiacono C, et al. Evaluation of hemagglutinin subtype 1 swine influenza viruses from the United States. Vet Microbiol. 2006 December;118:212–22. doi: 10.1016/j.vetmic.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Webby RJ, Rossow K, Erickson G, Sims Y, Webster R. Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res. 2004 Jul;103(1–2):67–73. doi: 10.1016/j.virusres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Choi YK, Goyal SM, Joo HS. Prevalence of swine influenza virus subtypes on swine farms in the United States. Arch Virol. 2002 Jun;147(6):1209–20. doi: 10.1007/s00705-002-0788-4. [DOI] [PubMed] [Google Scholar]
- 12.Easterday BC, Reeth KV. Swine Influenza. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. 8. Ames: Iowa State University Press; 1999. pp. 277–90. [Google Scholar]
- 13.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, et al. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol. 1999 Oct;73(10):8851–6. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, et al. Emergence of H3N2 reassortant influenza A viruses in North American pigs. Vet Microbiol. 2000 May 22;74(1–2):47–58. doi: 10.1016/s0378-1135(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 15.Gramer MR. Reassortant human/swine H1N1 and H1N2 influenza virus infections in US swine. 37th Annual Meeting of the American Association of Swine Veterinarians; 2006 March 4–7 2006; Kansas City, Missouri. 2006. pp. 463–4. [Google Scholar]
- 16.Janke BH, Harmon KM, Yoon KJ, Erickson GA, Webby RJ. Relative prevalence of reassortant H1N1 swine influenza viruses with avian polymerase genes and classic H1N1 viruses with swine polymerase genes. 47th Annual Conference of the American Association of Veterinary Laboratory Diagnosticians; 2004 October 21–15, 2004; Greensboro, NC. 2004. p. 146. [Google Scholar]
- 17.Karasin AI, Landgraf J, Swenson S, Erickson G, Goyal S, Woodruff M, et al. Genetic characterization of H1N2 influenza A viruses isolated from pigs throughout the United States. J Clin Microbiol. 2002 Mar;40(3):1073–9. doi: 10.1128/JCM.40.3.1073-1079.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gramer MR. SIV: An update on circulating strains, advances in diagnostic tests and interpretation of test results. 38th Annual Meeting of the American Association of Swine Veterinarians; 2007 March 3–6, 2007; Orlando, FL. 2007. [Google Scholar]
- 19.Karasin AI, Carman S, Olsen CW. Identification of human H1N2 and human-swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005) J Clin Microbiol. 2006 Mar;44(3):1123–6. doi: 10.1128/JCM.44.3.1123-1126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein SL, Lo CY, Misplon JA, Lawson CM, Hendrickson BA, Max EE, et al. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997 Feb 1;158(3):1222–30. [PubMed] [Google Scholar]
- 21.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997 Jun;71(6):4347–55. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozdzanowska K, Maiese K, Furchner M, Gerhard W. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology. 1999 Feb 1;254(1):138–46. doi: 10.1006/viro.1998.9534. [DOI] [PubMed] [Google Scholar]
- 23.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001 Jun;75(11):5141–50. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006 Jan;12(1):48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richt JA, Lager KM, Janke BH, Woods RD, Webster RG, Webby RJ. Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J Clin Microbiol. 2003 Jul;41(7):3198–205. doi: 10.1128/JCM.41.7.3198-3205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitikoon P, Nilubol D, Erickson BJ, Janke BH, Hoover T, Sornsen S, et al. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet Immunol Immunopathol. 2006 doi: 10.1016/j.vetimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 28.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32(6):648–60. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 29.Palmer DF, Coleman MT, Dowdle WR, Schild GC. Advanced laboratory techniques for influenza diagnosis. Washington, D.C.: U. S. Department of Health, Education, and Welfare; 1975. [Google Scholar]
- 30.Larsen DL, Karasin A, Zuckermann F, Olsen CW. Systemic and mucosal immune responses to H1N1 influenza virus infection in pigs. Vet Microbiol. 2000 May 22;74(1–2):117–31. doi: 10.1016/s0378-1135(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998 Dec 20;252(2):324–30. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Basler CF, Williams BR, Silverman RH, Palese P, Garcia-Sastre A. Functional replacement of the carboxy-terminal two-thirds of the influenza A virus NS1 protein with short heterologous dimerization domains. J Virol. 2002 Dec;76(24):12951–62. doi: 10.1128/JVI.76.24.12951-12962.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, et al. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J Virol. 2000 Dec;74(24):11566–73. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006 Nov 10;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 35.Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984 Apr;14(4):350–6. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 36.Schulman JL, Kilbourne ED. Induction of Partial Specific Heterotypic Immunity in Mice by a Single Infection with Influenza a Virus. Journal of bacteriology. 1965 Jan;89:170–4. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Reeth KV, Brown I, Essen S, Pensaert M. Genetic relationships, serological cross-reaction and cross-protection between H1N2 and other influenza A virus subtypes endemic in European pigs. Virus Res. 2004 Jul;103(1–2):115–24. doi: 10.1016/j.virusres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Webster RG, Askonas BA. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980 May;10(5):396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 39.Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, et al. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991 Jun;21(6):1337–44. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 40.Renegar KB, Small PA., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991 Apr;65(4):2146–8. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim WI, Wu WH, Janke B, Yoon KJ. Characterization of the humoral immune response of experimentally infected and vaccinated pigs to swine influenza viral proteins. Arch Virol. 2006 Jan;151(1):23–36. doi: 10.1007/s00705-005-0615-9. [DOI] [PubMed] [Google Scholar]
- 42.Flynn KJ, Riberdy JM, Christensen JP, Altman JD, Doherty PC. In vivo proliferation of naive and memory influenza-specific CD8(+) T cells. Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8597–602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherle PA, Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–28. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor PM, Askonas BA. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986 Jul;58(3):417–20. [PMC free article] [PubMed] [Google Scholar]
- 45.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 46.Choi YK, Goyal SM, Farnham MW, Joo HS. Phylogenetic analysis of H1N2 isolates of influenza A virus from pigs in the United States. Virus Res. 2002 Aug;87(2):173–9. doi: 10.1016/s0168-1702(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 47.Gorman OT, Bean WJ, Kawaoka Y, Donatelli I, Guo YJ, Webster RG. Evolution of influenza A virus nucleoprotein genes: implications for the origins of H1N1 human and classical swine viruses. J Virol. 1991 Jul;65(7):3704–14. doi: 10.1128/jvi.65.7.3704-3714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991 Oct;65(10):5491–8. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis. 2005 Aug;58(4):195–207. [PubMed] [Google Scholar]