Abstract
Background
Antimicrobial peptides (AMPs) such as cathelicidins contribute to initial defense of the airway against inhaled pathogens. Recent studies have shown that the hormonally active form of vitamin D3, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) up-regulates AMP gene expression in several established cell lines. Furthermore, serum levels of vitamin D are often deficient in cystic fibrosis (CF) patients.
Methods
We investigated the effect of 1,25(OH)2D3 on AMP mRNA levels in primary cultures of normal human bronchial epithelial (NHBE) cells by real-time PCR, and protein levels by Western blot. Antimicrobial activity of airway surface fluid from these cells was measured by in vitro assay against laboratory strains of bacteria.
Results
Treatment of NHBE cells with 1,25(OH)2D3 (10-8M), resulted in a 10-fold up-regulation of cathelicidin mRNA levels after 12 h, which was augmented 2-fold with co-incubation of 1 mM Calcium. Moreover, 1,25(OH)2D3 induced antimicrobial activity against the airway pathogens Bordetella bronchiseptica and Pseudomonas aeruginosa. 1,25(OH)2D3 induced cathelicidin mRNA expression equally in both normal and CF bronchial epithelial cells.
Conclusions
Elucidation of the effect of 1,25(OH)2D3 on cathelicidin expression in NHBE cells and CF bronchial epithelial cells will aid in the development of novel therapeutic agents for treatment of airway infections in CF.
Keywords: Innate immunity, Vitamin D, Antimicrobial peptide, Airway surface fluid
1. Introduction
The airway epithelium is constantly encountered by pathogens present in ambient air, and a complex defense system containing antimicrobial components has evolved to prevent microbial colonization. These antimicrobial components are present in airway surface fluid (ASF), which include larger proteins such as lysozyme and lactoferrin, as well as the defensins and cathelicidins [1,2]. Human cathelicidin is a cationic antimicrobial peptide (AMP) also known as hCAP18/LL-37. Structurally, all cathelicidins consist of an N-terminal signal sequence, followed by conserved cathelin-like prodomain and variable antimicrobial domain [3,4]. The human precursor peptide is processed by proteolytic cleavage to release the antimicrobial domain, called LL-37 [5]. It exhibits broad spectrum antimicrobial activity against microbes including Gram-positive and Gram-negative bacteria as well as fungi and certain viruses [6]. LL-37 was initially identified in the specific granules of neutrophils, and expression was subsequently identified in monocytes, T cells, and in various epithelia [1,7,8].
While initially isolated as an antimicrobial peptide, LL-37 has been discovered to exhibit numerous other activities, including chemotactic activity for neutrophils, monocytes and some T cells [9], and induction of IL-8 secretion from epithelial cell lines [10]. Furthermore, LL-37 affects dendritic cell (DC) maturation, and can act synergistically with the DC maturation cytokine GM-CSF to activate signal transduction pathways in monocytes [11]. Together, these multiple activities of LL-37 (as reviewed in [12]) suggest that it plays an important, multifunctional role in host defense.
The increasing development of antibiotic resistant bacteria, especially as seen in cystic fibrosis (CF) airway infection [13] has demonstrated the need for more efficient antibiotic therapies. Since bacteria do not generally develop resistance to AMPs such as LL-37, they have been proposed as useful therapeutics [13–16], however technical difficulties in their large-scale production and use as direct antimicrobial agents make them less attractive as therapies [17,18]. Expression of the LL-37 gene can be induced by LPS and interleukin (IL)-1α [19], suggesting that its modulation by exogenous agents could augment the natural innate immune defense against infections.
Recently it was demonstrated that the hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), induced AMPs in number of established cell lines as well as keratinocytes and myeloid cells at concentrations from 10−9 M to 10−7 M [20–23]. However, the effect of 1,25(OH)2D3 on cathelicidin gene expression in primary cultures of airway cells has not been studied. This is important as deficiencies in serum 25-hydroxyvitamin D3 levels have been observed in patients with CF [24], suggesting that exogenous regulation of this AMP gene could lead to antimicrobial therapies.
In this study, we examine the induction of cathelicidin by 1,25(OH)2D3 in primary human bronchial epithelial cells and the resultant increase in antimicrobial activity against airway pathogens of the airway secretions. We also show the increased cathelicidin expression by 1,25(OH)2D3 in CF bronchial epithelial cells, supporting the argument that 1,25(OH)2D3 can augment antibacterial activity in airway epithelia of CF patients, suggesting a novel potential therapy for preventing and treating airway infections.
2. Material and methods
2.1. Tissue culture
Normal human bronchial epithelial (NHBE) cells (BioWhittaker, Inc., Walkersville, MD, USA), AA (bronchial cells with wt CFTR) and KK (bronchial cells with ΔF508 CFTR) cell lines were cultured in bronchial epithelial growth medium (BEGM; BioWhittaker, Inc.) supplemented with BPE, insulin, HC, retinoic acid, transferrin, triiodothyronine, epinephrine, and hEGF. NHBE (passage-5), AA, or KK cells were seeded onto 6-well tissue culture plate at a density of 0.35×106 cells/well and incubated overnight. Old medium was removed, and fresh medium (2 ml) was added to each well. Cells were incubated in the fresh media for 24 h prior to vehicle (ethanol) or 1,25(OH)2D3 (10-8M) treatment (to deplete growth factors which might interfere in the treatment). Cycloheximide (Sigma, St. Louis, MO, USA) was used at 20 μg/ml and added 30 min prior to the vehicle or 1,25(OH)2D3 treatment. Actinomycin D (Sigma) was added at the same time as vehicle or 1,25(OH)2D3 added [20]. The synergistic role of calcium was determined by pretreating cells with calcium chloride (1.0 mM) and incubated for 24 h before vehicle or 1,25(OH)2D3. All cells were maintained at 37 °C in humidified atmosphere containing 5% CO2.
2.2. Messenger RNA analysis
2.2.1. Semi-quantitative RT-PCR
Total RNA was prepared using QIAshredder and RNeasy Mini Kit, treated with RNase-Free DNase Set (Qiagen, Valencia, CA, USA). Five hundred nanograms of total RNA was reverse transcribed with Superscript III reverse transcriptase by oligo(dT) primers in 20 μl as described by the manufacturer (Invitrogen, Carlsbad, California, USA). First-stranded cDNA was amplified by PCR. PCR primer set for cathelicidin consisted of forward: 5′-GTGACTTCAAGAAGGACGGG-3′; reverse: 5′-GGGTAGGGCACACACTAGGA-3′ and amplified a product of 238 bp. β-Actin was amplified in each reaction using following primer set: forward, 5′-AGTCCTGTGGCATCCACGAAACTA-3′; reverse 5′-CTTCTGCATCCTGTCGGCAATG-3′ and amplified a product of 138 bp. Each PCR reaction contained 3 mM of Mg2+, 0.2 mM of dNTP, 0.4 μl of 5 u/μl Taq polymerase (Invitrogen), and 200 pM of primers. After an initial denaturing step (95 °C for 1 min) 30 cycles of denaturing (94 °C for 1 min), annealing (60 °C for 1 min), and extension (72 °C for 1 min), followed by 7 min at 72 °C for final extension were conducted. PCR products were electrophoresed on 2% agarose gel containing ethidium bromide.
2.2.2. Real-time quantitative PCR (RTQ-PCR)
hBD-1, hBD-2, hBD-3, and cathelicidin mRNA levels were quantified by real-time PCR using MyCycler (Bio-Rad Laboratories, Hercules, CA, USA). A total of 1 μl of cDNA (described above) was analyzed using the final concentration of 100 nM of primers, 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in volume of 20 μl. RTQ-PCR primers used were: cathelicidin forward, 5′-GTCACCAGAGGATTGTGACTTCAA-3′, and reverse, 5′-TTGAGGGTCACTGTCCCCATA-3′; hBD-1 forward, 5′-CGCCATGAGAACTTCCTACCTTCTG-3′, and reverse, 5′-GAATAGAGACATTGCCCTCCACTGC-3′; hBD-2 forward, 5′-GATGCCTCTTCCAGGTGTTTTTGG-3′, and reverse, 5′-TTGTTCCAGGGAGACCACAGGTG-3′; hBD-3 forward, 5′-TATCTTCTGTTTGCTTTGCTCTTC-3′, and reverse, 5′-CCTCTGACTCTGCAATAATATTTCTGTAA-3′; β-actin forward, 5′-AGTCTGTGGCATCCACGAAACTAC-3′, and reverse, 5′-CTTCTGCATCCTGTCGGCAATG-3′; and β2-microglobulin (B2M) forward, 5′-CTCCGTGGCCTTAGCTGTG-3′, and reverse, 5′-TTTGGAGTACGCTGGATAGCCT-3′. The PCR fragments were amplified for 45 cycles (15 s at 95 °C and 1 min at 60 °C). The relative for mRNA expression in each sample was calculated based on its Ct value comparison to Ct of housekeeping gene. The data were presented as 2−ΔΔCt, an arbitrary unit. All amplified products showed single peak in the dissociation curve test. RTQ-PCR was performed in triplicates for each sample. This procedure was conducted in at least three independent experiments.
2.3. Protein expression analysis
Protein expression was analyzed by Western blot. For LL-37 expression analysis, total cell lysates were prepared and concentration was measured by Bradford assay. Equal amounts of protein from each sample were electrophoresed through SDS-PAGE 8–18% gradient gels. The material in gels was blotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad), and the membrane was blocked with 3% BSA in pH 7.5 TBS buffer for 2 h. After washes (0.02% Tween 20 pH 7.5 TBS), immunolabeling was performed using polyclonal rabbit antibody against hCAP18/LL-37 (1:7500) or β-actin (1:7500) overnight. Alkaline phosphatase-conjugated goat anti rabbit IgG (LL-37, Sigma) or goat anti-mouse (β-actin, Bio-Rad) was applied (1:5000) to the membrane for 2 h after washes. The membrane was washed several times for 3 h, and bound antibodies were visualized by using AP conjugate substrate kit (Bio-Rad). Western blots were repeated three times.
For vitamin D receptor (VDR) expression analysis, a method from Barletta et al. was followed [25]. Briefly, nuclear extracts were prepared following method of Legarda et al. [26]. Protein concentration was measured by Bradford assay. Equal amount of protein (40 μg) was electrophoresed through a 10% SDS-polyacrylamide gel. Proteins were transferred to a PVDF membrane, and the membrane was blocked with PBST (0.5% Tween-20 in PBS) containing 5% nonfat milk at 4 °C for 16 h. After blocking, the blot was incubated with rabbit VDR polyclonal antibody (C-20, Santa Cruz Biotechnology, 1:3000) for 2 h at room temperature, followed by incubation for an additional hour with a goat anti-rat IgG conjugated to horseradish peroxidase (Sigma). After washing with PBST, the ECL Western blotting detection system (NEN) was used to detect the antigen/antibody complex. As a control, VDR Western blots were also analyzed for α-tubulin (Sigma).
2.4. Preparation of airway surface fluid (ASF)
NHBE cells were plated at 0.3×106 cells/well onto Transwell CM inserts (0.45-μm pore size and 24-mm diameter; Costar, Cambridge, MA, USA). Fresh medium was replaced every day with 1 ml on the apical and 2 ml on the basolateral compartment until the cells were grown to confluence. To treat the cells with 1,25(OH)2D3, the apical surface medium was aspirated and washed with fresh medium three times. 1,25(OH)2D3 (10-8M final concentration) was added to the basolateral compartment, and cells were further cultured. The crude ASF was then collected by washing the apical surface of the cells with 140 μl of 10 mM sodium phosphate buffer. The collected solution was centrifuged at 200×g for 10 min, and supernatant was recovered as ASF [27]. ASF was stored at −80 °C.
2.5. Antimicrobial assay
The effect of 1,25(OH)2D3 treatment on the bactericidal activity of ASF of NHBE cells was studied using Bordetella bronchiseptica and Pseudomonas aeruginosa [28–30]. Single colonies of P. aeruginosa were inoculated into LB broth and B. bronchiseptica in Stainer–Scholte medium (SSM) overnight. Aliquots of these cultures were transferred into fresh LB broth and SSM and incubated 3–6 h to obtain mid-log phase of the bacteria growth. 500 CFU of bacteria in 10μl of 10 mM of sodium phosphate buffer was mixed with 90 μl of ASF in a 1.5-ml tube, and the mixture was incubated for 3 h at 37 °C while shaking at 75 rmp. P. aeruginosa was seeded on LB plates overnight, and B. bronchiseptica onto BG Bordet–Gengou (BG) agar plates (Difco Laboratories, Detroit, MI, USA) supplemented with 5% sheep blood for 48 h. CFU were counted to determine activity.
2.6. Statistical analysis
Data are expressed as means±standard error of the mean (SEM). Statistical analysis was performed using two-tailed, unpaired Student's t-test using Microsoft Excel software. Significance was accepted at P<0.05.
3. Results
3.1. Induction of cathelicidin expression by 1,25(OH)2D3 in NHBE cells
To assess the effect of 1,25(OH)2D3 on expression of AMPs, NHBE cells were exposed to 10−8M 1,25(OH)2D3 for 0, 6, 24, and 48 h. Total RNA was collected and used separately for cDNA synthesis. The cDNA was utilized as templates in the RT-PCR and RTQ-PCR reactions for hBD1, 2, 3, and cathelicidin. β-Actin was used as a housekeeping gene control. The PCR reactions were size fractionated on a 2% agarose gel, and the intensity of PCR products of cathelicidin only was increased at 6 and 24 h of 1,25(OH)2D3 exposure (data not shown). To confirm the RT-PCR results, RTQ-PCR was conducted for hBD1, 2, 3, and cathelicidin. Fig. 1A shows that cathelicidin mRNA levels were upregulated whereas mRNA levels of hBD1, 2, and 3 remained at a basal level.
Fig. 1.
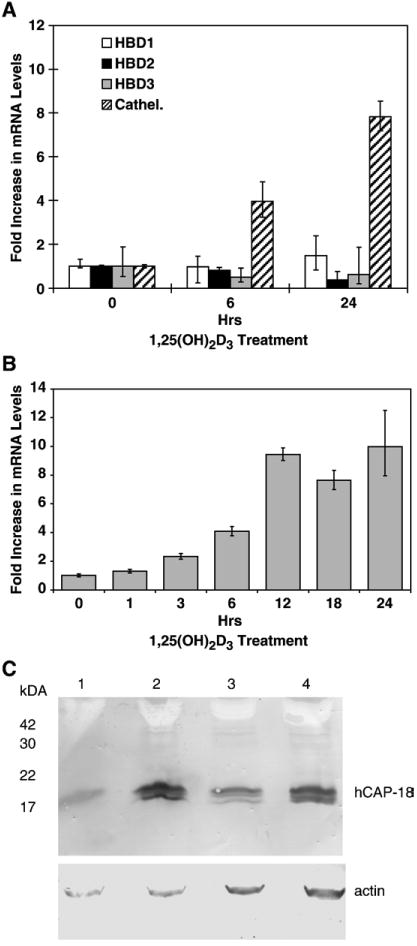
Induction of cathelicidin gene expression in NHBE cells. NHBE cells were treated with 10−8M 1,25(OH)2D3 for times indicated. (A) Total RNA was extracted, and RTQ-PCR was conducted to quantify hBD1, 2, 3, and cathelicidin mRNA levels. (B) Kinetics of cathelicidin mRNA induction was determined as described above. β-Actin was used as a house-keeping gene. Results are represented as the means±SEM (*p<0.05) from at least three independent experiments. (C) NHBE cells were treated with 10-8M 1,25(OH)2D3 or with vehicle for 24 or 48 h. Total cell lysates were analyzed by Western blot to observe hCAP18 expression. The amount of protein from the lysates was determined by Bradford assay, and equal amount of protein was loaded in each well, and a parallel blot was performed with an antibody to β-actin. Lane 1, 24 h, vehicle; lane 2, 24 h, 1,25(OH)2D3; lane 3, 48 h, vehicle; lane 4, 48 h, 1,25(OH)2D3.
To define the kinetics of cathelicidin mRNA induction in response to 1,25(OH)2D3, NHBE cells were treated with 1,25(OH)2D3 in a time course as indicated. RTQ-PCR result showed that the levels of cathelicidin mRNA were significantly (p<0.05) up-regulated within 3 h of exposure, with a maximum induction of 10-fold at 12 to 24 h of incubation (Fig. 1B). Incubation with lower doses (10-9M and below) failed to elicit a significant response (data not shown).
To determine whether the induction of cathelicidin mRNA levels correlated to an increase in protein expression, Western blot analysis was performed. In this experiment, NHBE cells were treated with vehicle or 1,25(OH)2D3, and total cell lysates were collected for the Western blot. After 24 and 48 h of exposure, increased levels of hCAP18 peptide were observed compared with the vehicle-treated sample (Fig. 1C). As with the initial description of this peptide in lung extracts [8], two bands are observed. The larger band may be attributed to the unprocessed precursor.
3.2. Mechanism of cathelicidin mRNA induction by 1,25(OH)2D3 in NHBE cells
To test whether the induction of cathelicidin mRNA by 1,25(OH)2D3 requires new protein synthesis, NHBE cells were pretreated with cycloheximide or vehicle prior to 1,25(OH)2D3 addition to block the new protein expression. The results (Fig. 2) demonstrated that cycloheximide pretreatment inhibited ongoing cathelicidin mRNA induction by 1,25(OH)2D3, indicating that new protein synthesis is necessary for cathelicidin mRNA induction by 1,25(OH)2D3. Similarly, there was no induction observed when 1,25(OH)2D3 was co-incubated with 5μg/ml actinomycin D (data not shown), suggesting that the induction is at the level of transcription.
Fig. 2.
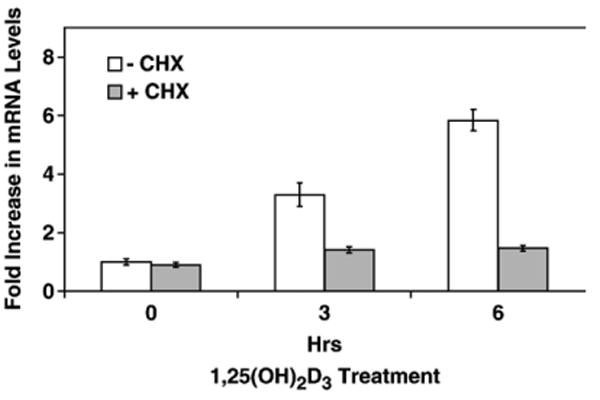
Mechanism of induction of cathelicidin in NHBE cells. NHBE cells were pretreated in the absence (−CHX) or presence (+CHX) of cycloheximide (20 μg/ml) for 30 min followed by 10−8M 1,25(OH)2D3. Total RNA was extracted, and RTQ-PCR was conducted to determine cathelicidin mRNA levels, normalized to β-actin. Graphs show means±SEM (*p<0.05) from at least three experiments.
To determine whether VDR was expressed in NHBE cells, nuclear extracts were prepared and Western blot analysis was conducted. The results (data not shown) demonstrated that VDR is expressed in NHBE cells.
3.3. Synergistic role of calcium in cathelicidin mRNA induction with 1,25(OH)2D3 in NHBE cells
Calcium interacts with vitamin D, regulating immune processes including TNF-α production [31] and LL-37 expression [23]. To determine the effect of calcium with the addition of 1,25(OH)2D3, we pretreated NHBE cells with CaCl2 (1.0 mM) for 24 h prior to 1,25(OH)2D3 addition. RTQ-PCR data showed that a 100% increase in cathelicidin mRNA levels was observed when cells were pretreated with calcium prior to 1,25(OH)2D3 addition compared with 1,25(OH)2D3 administration alone at 6 h of 1,25(OH)2D3 exposure. A 50% increased induction was observed at 24 h. Pretreatment of calcium alone had no effect in inducing cathelicidin mRNA level (Fig. 3). These results suggested that calcium and 1,25(OH)2D3 have a synergistic role in inducing cathelicidin mRNA. Higher concentrations of calcium (up to 4 mM) with longer pre-incubation time (up to 72 h) was administered to NHBE cells to examine the maximum calcium effect; however, this resulted in a decrease in cell viability, potentially due to calcium toxicity (data not shown).
Fig. 3.
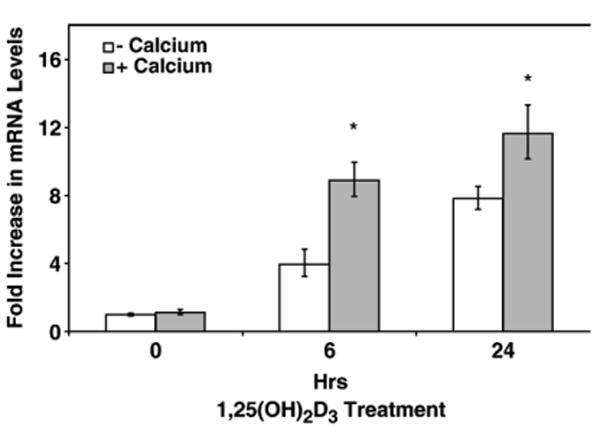
Role of calcium in 1,25(OH)2D3-mediated induction of LL-37. NHBE cells were pretreated with 1.0 mM CaCl2 (+calcium) or vehicle (−calcium) for 24 h followed by 10−8M 1,25(OH)2D3 exposure for increasing times. Total RNA was collected and RTQ-PCR was conducted. Cathelicidin mRNA levels were determined by RTQ-PCR and normalized to β-2-microglobulin. Results show means±SEM (*p<0.05) of three independent experiments.
3.4. Induction of antimicrobial activity by 1,25(OH)2D3 against airway pathogens
To examine whether 1,25(OH)2D3 also induces antimicrobial activity against airway pathogens, NHBE cells were grown in air–liquid interface cultures and treated with 1,25(OH)2D3 or vehicle basolaterally for 24 h. ASF was collected and bacteria were incubated with the ASF followed by seeding on plates to obtain viable colonies. Our results demonstrated that ASF from 1,25(OH)2D3-treated cells exhibited increased antimicrobial activity, indicating cathelicidin up-regulation correlated with antibacterial activity induction. This was observed with both B. bronchiseptica and P. aeruginosa (Fig. 4A). Specifically, we observed up to 47% reduction in viable colonies of B. bronchiseptica and 50% reduction at P. aeruginosa after 24 h of induction.
Fig. 4.
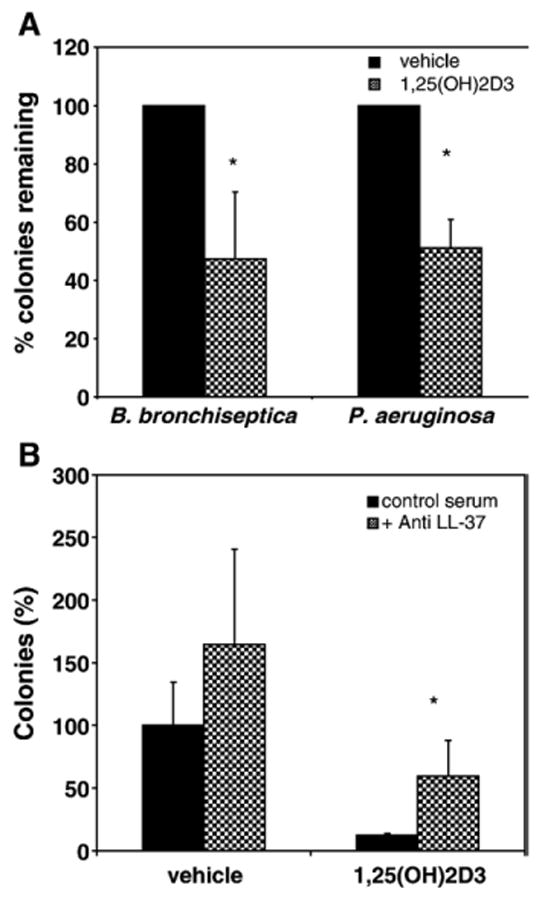
Induction of antibiotic activity of airway surface fluid by 1,25(OH)2D3. NHBE cells were grown on Transwell CM inserts and exposed to 10−8M 1,25(OH)2D3 or vehicle basolaterally for 24 h. Antimicrobial activity of ASL against B. bronchiseptica and P. aeruginosa was determined by counting the number of colonies on 5% BG plates supplemented with 5% sheep blood and LB plates, respectively, after incubation with the ASL collected from the NHBE cells cultured above. (B) Inhibition of antimicrobial activity with LL-37 antibody. ASF was pretreated with 1 µl anti LL-37 antibody (gift of R. Gallo, UCSD) or control serum for 1 h prior to addition of P. aeruginosa in antimicrobial assays as described above. Results are reported as means±SEM (*p<0.05) of at least three independent experiments.
To assess the contribution of LL-37 to this antimicrobial activity, samples of ASF from cultures incubated with 1,25(OH)2D3 or vehicle for 24 h were pre-incubated with either a polyclonal antibody to LL-37 or control serum prior to the assay. The results in Fig. 4B demonstrate a significant decrease in the antimicrobial activity in those samples (both stimulated and unstimulated) pre-incubated with the antibody compared with control serum. There was no significant difference between the stimulated samples pre-incubated with the antibody and the unstimulated samples pretreated with control serum. This indicates that at least a portion of the activity that is induced in the airway surface fluid is due to LL-37.
3.5. Induction of cathelicidin mRNA by 1,25(OH)2D3 in CF and non-CF cell lines
Since bacterial infections in the airway are a major health problem in CF patients, we examined whether 1,25(OH)2D3 could similarly induce cathelicidin expression in epithelial cells exhibiting the CF defect. We stimulated two cell lines, AA (bronchial cells with wt CFTR) and KK (bronchial cells with the homozygous ΔF508 CFTR mutation), with 10-8M 1,25(OH)2D3. Total RNA was collected at increasing times, and cDNA was synthesized for RT-PCR (data not shown) and RTQ-PCR. The results shown in Fig. 5 demonstrate that both normal and CF cell lines exhibited induction of cathelicidin mRNA at the same levels. We observed the up-regulation of cathelicidin mRNA as early as 3 h of exposure, and a maximum induction of 15-fold at 12 h.
Fig. 5.
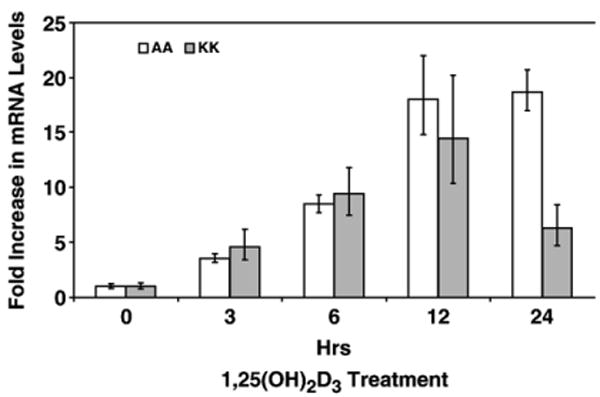
Induction of LL-37 mRNA in normal and CF cell lines. AA and KK cell lines were grown to confluence and exposed to 10−8M 1,25(OH)2D3 for 0, 3, 6, 12, 24, and 48 h. Total RNA was extracted, and RTQ-PCR was performed to determine cathelicidin mRNA levels. β-2-Microglobulin primer sets were used for normalization. Results are represented as the means±SEM (*p<0.05) from at least three independent experiments.
4. Discussion
Previous studies demonstrated cathelicidin induction by physiological concentrations of 1,25(OH)2D3 in established cell lines from keratinocytes, monocytes, myeloid leukemia and airway submucosal gland, as well as head and neck squamous cell carcinoma [20–23]. These studies were based on the identification of VDRE consensus sequences in the 5′ flanking region of the hBD2 and LL-37 genes. In the current study, we demonstrated that 1,25(OH)2D3 induced cathelicidin expression in primary cultures of bronchial epithelia. In contrast to the previous results by Wang et al. demonstrating induction of cathelicidin and hBD2 [22], we observed that only cathelicidin mRNA and protein expression were up-regulated by 1,25(OH)2D3. This result indicated that AMP induction by 1,25(OH)2D3 is differentially regulated between cell types.
The mechanism that regulates the induction of cathelicidin expression by 1,25(OH)2D3 is still unclear. Recent studies stressed the necessity of the VDRE in the cathelicidin promoter region in the induction of cathelicidin gene expression by 1,25(OH)2D3 in several established cell lines [20,22]. Our results demonstrate the presence of VDR in bronchial epithelial cells, suggesting that a 1,25(OH)2D3 mechanism is present in these cells. However, Gombart et al. demonstrated that ongoing protein synthesis is not necessary for the induction of cathelicidin gene expression by 1,25(OH)2D3 in U937 cells [20]. In contrast, we show that the inhibition of new protein synthesis prior to 1,25(OH)2D3 addition prevented the induction of cathelicidin mRNA (Fig. 2), indicating that cathelicidin mRNA induction by 1,25(OH)2D3 requires new protein synthesis. This difference may reflect the presence of multiple mechanisms for the activation of cathelicidin gene expression in airway cells.
We first demonstrated that induction of antimicrobial peptide gene expression in airway cells with LPS and other inflammatory mediators [32], suggesting that modulation of these genes could be used to increase the host defense capability of the airway. The induction of endogenous cathelicidin expression by cytokines and growth factors has been investigated in several cell lines. LPS and IL-1α treatment of keratinocytes induced cathelicidin expression [19]. In addition, insulin-like growth factor-1 (IGF-1) and butyrate are also known to induce cathelicidin expression in keratinocytes and in colonic epithelial cells, respectively [6,33,34]. However, these agents would not be good candidates as therapeutics because they can be highly toxic or are easily degraded in the physiological environment. 1,25(OH)2D3 used in current research is a relatively safe molecule, and the amount used (10−8M) is also in the range of the physiological environment that would not cause any toxicity, and thus may represent the levels in normal individuals. However, due to fact that vitamin D is absorbed through the plasma membrane, it could be possible to introduce the inducing agent apically, by inhalation of vitamin D. This could allow for the targeted introduction of higher doses without toxicity. Alternatively, one could use analogs of 1,25(OH)2D3 that have been shown in in vivo studies to induce VDR-mediated responses without causing calcemia [35].
Our results demonstrated that ASF collected from 1,25(OH)2D3-treated cells exhibited increased antibacterial activity against airway pathogens (Fig. 4). While much of this antimicrobial activity in the induced cultures could be returned to baseline levels with a specific anti-LL-37 antibody, other genes involved in innate defense might also be induced by 1,25(OH)2D3. The ability to induce the expression of cathelicidin on the mucosal surface of bronchial epithelia could thus provide therapeutic benefits in treating airway infections and disorders.
More than 90% of lung infections in CF are caused by P. aeruginosa [13]. Moreover, there has been an increase in multidrug-resistant CF pathogens including P. aeruginosa [36], and a lack of new antibiotics to address these infections. Thus, developing new therapeutics has become a vital need for CF patients, and antimicrobial peptides and their mimetics are extremely attractive candidates because of their bactericidal activity against multidrug-resistant bacteria [13,37]. Previous studies indicated that ASF from CF airway epithelial cell cultures exhibited reduced antimicrobial activity [30]. However, increased expression of cathelicidin augmented bactericidal activity against P. aeruginosa in a xenograft model of CF bronchial epithelium [38], suggesting that regulation of cathelicidin could be used as part of an antibiotic therapy for CF. Recent clinical studies demonstrated that vitamin D deficiency is common among patients with CF. This deficiency might be caused by poor absorption of vitamin D and the lack of conversion of 25-hydroxyvitamin D3 to 1,25(OH)2D3 [24,39,40]. Therefore, we hypothesized that treatment of CF cultures with exogenous 1,25(OH)2D3 would increase cathelicidin expression, which would consequently increase bactericidal activity. Our results indicated that cathelicidin expression could be induced equally in both normal and CF bronchial epithelial cells (Fig. 5), supporting our hypothesis that regulation of antimicrobial peptide gene expression may be useful to augment current therapies to treat or prevent bacterial infections in CF airways.
Acknowledgments
The authors would like to thank Dr. Richard Gallo (University of California, San Diego) for the LL-37 antibody and Dr. Scott Randell (University of North Carolina, Chapel Hill) for the AA and KK cell lines. This work was supported by grants from the US Public Health Service, NIH 1R01 HL67871 and 1R01 DE14897 to GD, and 5R01 DK38961 to SC.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol. 2006;306:153–82. doi: 10.1007/3-540-29916-5_6. [DOI] [PubMed] [Google Scholar]
- 2.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L799–805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 3.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 4.Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002;4(3):361–72. doi: 10.1016/s1286-4579(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti M, Gennaro R, Scocchi M, Skerlavaj B. Structure and biology of cathelicidins. Adv Exp Med Biol. 2000;479:203–18. doi: 10.1007/0-306-46831-X_17. [DOI] [PubMed] [Google Scholar]
- 6.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118(4):509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alphadefensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–93. [PubMed] [Google Scholar]
- 8.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95(16):9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171(12):6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 11.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169(7):3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 12.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77(4):451–9. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Parente J, Harris SM, Woods DE, Hancock RE, Falla TJ. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob Agents Chemother. 2005;49(7):2921–7. doi: 10.1128/AAC.49.7.2921-2927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock RE. The therapeutic potential of cationic peptides. Expert Opin Investig Drugs. 1998;7(2):167–74. doi: 10.1517/13543784.7.2.167. [DOI] [PubMed] [Google Scholar]
- 15.Hancock RE, Patrzykat A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord. 2002;2(1):79–83. doi: 10.2174/1568005024605855. [DOI] [PubMed] [Google Scholar]
- 16.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 17.Hancock RE. Host defence (cationic) peptides: what is their future clinical potential? Drugs. 1999;57(4):469–73. doi: 10.2165/00003495-199957040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24(6):536–47. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Erdag G, Morgan JR. Interleukin-1alpha and interleukin-6 enhance the antibacterial properties of cultured composite keratinocyte grafts. Ann Surg. 2002;235(1):113–24. doi: 10.1097/00000658-200201000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 21.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 22.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 23.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080–2. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 24.Hecker TM, Aris RM. Management of osteoporosis in adults with cystic fibrosis. Drugs. 2004;64(2):133–47. doi: 10.2165/00003495-200464020-00002. [DOI] [PubMed] [Google Scholar]
- 25.Barletta F, Dhawan P, Christakos S. Integration of hormone signaling in the regulation of human 25(OH)D3 24-hydroxylase transcription. Am J Physiol Endocrinol Metab. 2004;286(4):E598–608. doi: 10.1152/ajpendo.00214.2003. [DOI] [PubMed] [Google Scholar]
- 26.Legarda D, Klein-Patel ME, Yim S, Yuk MH, Diamond G. Suppression of NF-kappaB-mediated beta-defensin gene expression in the mammalian airway by the Bordetella type III secretion system. Cell Microbiol. 2005;7(4):489–97. doi: 10.1111/j.1462-5822.2004.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizawa K, Suzuki T, Yamaya M, Jia YX, Kobayashi S, Ida S, et al. Erythromycin increases bactericidal activity of surface liquid in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L565–73. doi: 10.1152/ajplung.00316.2004. [DOI] [PubMed] [Google Scholar]
- 28.Bals R, Weiner DJ, Meegalla RL, Accurso F, Wilson JM. Salt-independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am J Respir Cell Mol Biol. 2001;25(1):21–5. doi: 10.1165/ajrcmb.25.1.4436. [DOI] [PubMed] [Google Scholar]
- 29.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88(4):553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 30.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85(2):229–36. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35(1):217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 32.Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci U S A. 1996;93(10):5156–60. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52(5):735–7341. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170(11):5583–9. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 35.Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26(4A):2551–6. [PubMed] [Google Scholar]
- 36.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control. 2003;31 3:S1–S62. [PubMed] [Google Scholar]
- 37.Koczulla AR, Bals R. Antimicrobial peptides: current status and therapeutic potential. Drugs. 2003;63(4):389–406. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- 38.Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest. 1999;103(8):1113–7. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donovan DS, Jr, Papadopoulos A, Staron RB, Addesso V, Schulman L, McGregor C, et al. Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1892–9. doi: 10.1164/ajrccm.157.6.9712089. [DOI] [PubMed] [Google Scholar]
- 40.Lark RK, Lester GE, Ontjes DA, Blackwood AD, Hollis BW, Hensler MM, et al. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am J Clin Nutr. 2001;73(3):602–6. doi: 10.1093/ajcn/73.3.602. [DOI] [PubMed] [Google Scholar]


