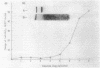
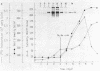
Abstract
Rhodopseudomonas viridis was grown in liquid culture at 30 degrees C anaerobically in light (generation time, 13 h) and under microaerophilic growth conditions in the dark (generation time, 24 h). The bacterium could be cloned at the same temperature anaerobically in light (1 week) and aerobically in the dark (3 to 4 weeks) if oxygen was limited to 0.1%. Oxygen could not be replaced by dimethyl sulfoxide, potassium nitrate, or sodium nitrite as a terminal electron acceptor. No growth was observed anaerobically in darkness or in the light when air was present. A variety of additional carbon sources were used to supplement the standard succinate medium, but enhanced stationary-phase cell density was observed only with glucose. Conditions for induction of the photosynthetic reaction center upon the change from microaerophilic to phototrophic growth conditions were investigated and optimized for a mutant functionally defective in phototrophic growth. R. viridis consumed about 20-fold its cell volume of oxygen per hour during respiration. The MICs of ampicillin, kanamycin, streptomycin, tetracycline, 1-methyl-3-nitro-1-nitrosoguanidine, and terbutryn were determined.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Daldal F. Cytochrome c2-independent respiratory growth of Rhodobacter capsulatus. J Bacteriol. 1988 May;170(5):2388–2391. doi: 10.1128/jb.170.5.2388-2391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Drews G., Giesbrecht P. Rhodopseudomonas viridis, nov. spec., ein neu isoliertes, obligat phototrophes Bakterium. Arch Mikrobiol. 1966 Mar 31;53(3):255–262. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Krieg N. R., Hoffman P. S. Microaerophily and oxygen toxicity. Annu Rev Microbiol. 1986;40:107–130. doi: 10.1146/annurev.mi.40.100186.000543. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marrs B., Gest H. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973 Jun;114(3):1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. doi: 10.1016/0022-2836(82)90216-9. [DOI] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Lottspeich F. The ;heavy' subunit of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the gene, nucleotide and amino acid sequence. EMBO J. 1985 Jul;4(7):1667–1672. doi: 10.1002/j.1460-2075.1985.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Phototrophic growth of halobacteria and its use for isolation of photosynthetically-deficient mutants. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):137–150. doi: 10.1016/s0769-2609(83)80101-x. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Saunders V. A., Jones O. T. Oxidative phosphorylation and effects of aerobic conditions on Rhodopseudomonas viridis. Biochim Biophys Acta. 1973 Jun 28;305(3):581–589. doi: 10.1016/0005-2728(73)90077-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Weyer K. A., Lottspeich F., Gruenberg H., Lang F., Oesterhelt D., Michel H. Amino acid sequence of the cytochrome subunit of the photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1987 Aug;6(8):2197–2202. doi: 10.1002/j.1460-2075.1987.tb02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Growth of Rhodopseudomonas capsulata under anaerobic dark conditions with dimethyl sulfoxide. Arch Biochem Biophys. 1977 Jun;181(2):411–418. doi: 10.1016/0003-9861(77)90246-6. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E., Marrs B. L. Isolation and characterization of enhanced fluorescence mutants of Rhodopseudomonas capsulata. J Bacteriol. 1983 May;154(2):748–755. doi: 10.1128/jb.154.2.748-755.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Cook D. N., Leach F., Armstrong G. A., Alberti M., Hearst J. E. Oxygen-regulated mRNAs for light-harvesting and reaction center complexes and for bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus during the shift from anaerobic to aerobic growth. J Bacteriol. 1986 Dec;168(3):1180–1188. doi: 10.1128/jb.168.3.1180-1188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7613–7617. doi: 10.1073/pnas.83.20.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]