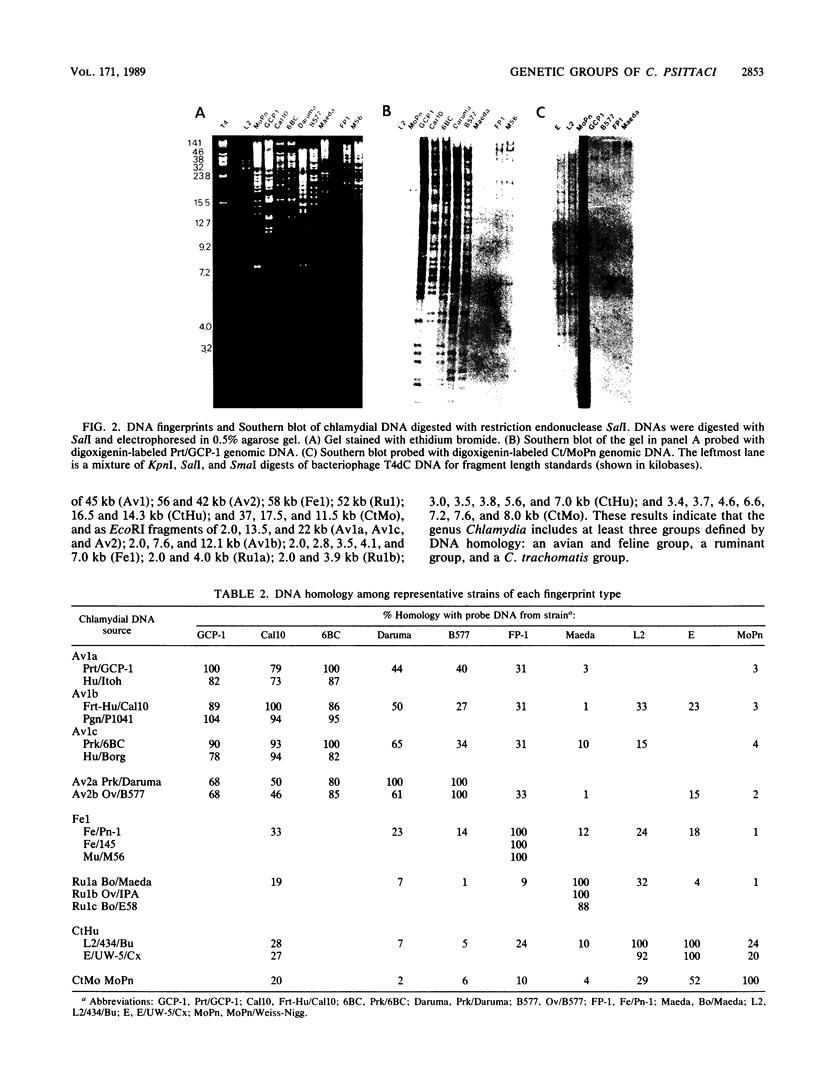
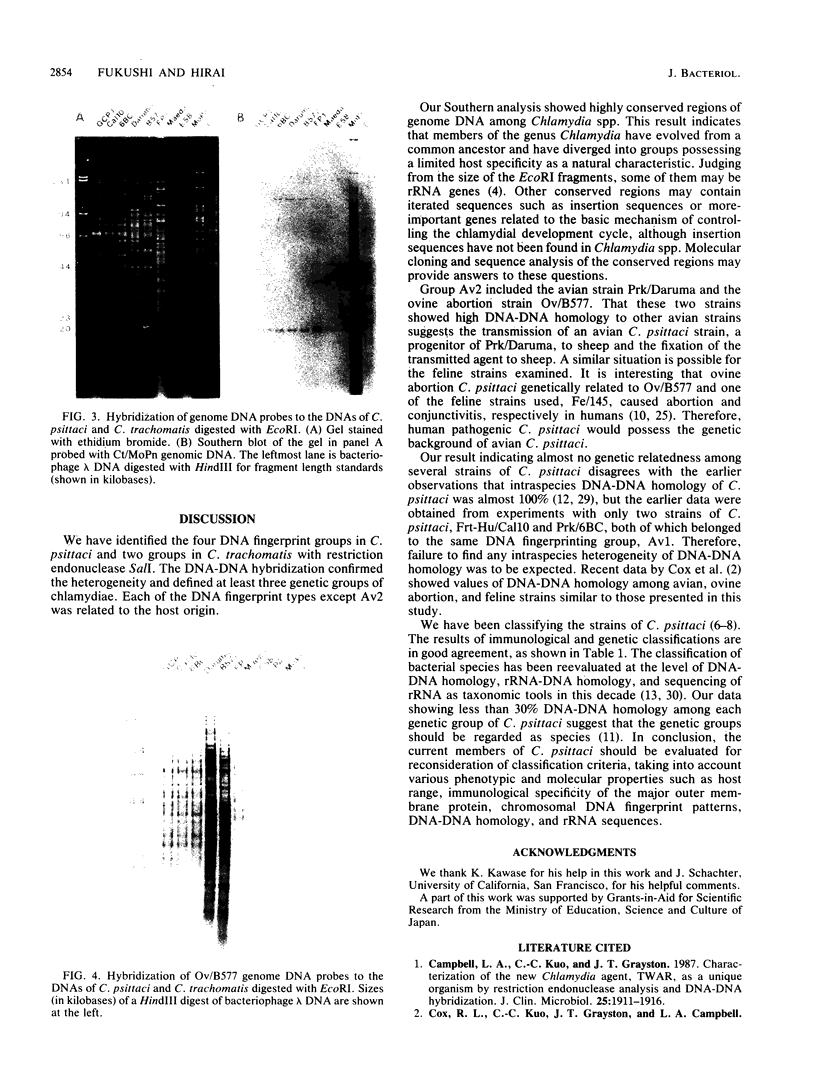
Abstract
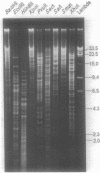
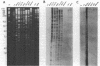
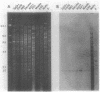
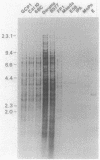
Genetic relationships were reported for Chlamydia psittaci derived from psittacine birds, pigeons, turkeys, humans, cats, muskrats, cattle, and sheep and for C. trachomatis, including representative strains of the three biovars, through physical analysis of genomic DNA including DNA fingerprinting with restriction endonuclease SalI, DNA-DNA hybridization in solution with S1 nuclease, and Southern analysis with genomic DNA probes. A total of 26 strains were divided into four groups of C. psittaci and two groups of C. trachomatis, on the basis of DNA fingerprints. The six groups of Chlamydia spp. were related to host origin: two avian groups (Av1 and Av2), one feline and muskrat group (Fe1), one ruminant group (Ru1), one C. trachomatis biovars trachoma and lymphogranuloma group (CtHu), and one C. trachomatis mouse biovar group (CtMo), although an ovine abortion strain belonged to the avian group Av2. DNA-DNA hybridization assay and Southern analysis with genomic DNA probes indicated three DNA homology groups in the genus Chlamydia: an avian-feline group (groups Av1, Av2, and Fe1), a ruminant group (group Ru1), and a C. trachomatis group (groups CtHu and CtMo). Furthermore, the Southern analysis indicated that the homologous sequences (DNA homology of at least 14%) within the avian-feline group were distributed along the whole genome, whereas the homologous sequences (DNA homology of less than 24%) among the three DNA homology groups were localized in distinct regions of the genome DNA. These results suggest that Chlamydia spp. are derived from a common ancestor and have diverged into various groups showing restricted host ranges as a natural characteristic and that the species C. psittaci should be differentiated into groups related to host origin and DNA homology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Ganem D. Chlamydial rRNA operons: gene organization and identification of putative tandem promoters. J Bacteriol. 1987 Dec;169(12):5678–5685. doi: 10.1128/jb.169.12.5678-5685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Immunochemical diversity of the major outer membrane protein of avian and mammalian Chlamydia psittaci. J Clin Microbiol. 1988 Apr;26(4):675–680. doi: 10.1128/jcm.26.4.675-680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi H., Nojiri K., Hirai K. Monoclonal antibody typing of Chlamydia psittaci strains derived from avian and mammalian species. J Clin Microbiol. 1987 Oct;25(10):1978–1981. doi: 10.1128/jcm.25.10.1978-1981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff R. K., Ritter D. B., Watson R. O. Studies on thermal denaturation of DNA from various chlamydiae. J Infect Dis. 1970 Jan;121(1):65–69. doi: 10.1093/infdis/121.1.65. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Anderson I. E., McClenaghan M., Inglis N. F., Williams H., Matheson B. A., West C. P., Rodger M., Brettle P. P. Restriction endonuclease analysis of DNA from two isolates of Chlamydia psittaci obtained from human abortions. Br Med J (Clin Res Ed) 1987 Nov 14;295(6608):1239–1239. doi: 10.1136/bmj.295.6608.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg C. AN UNIDENTIFIED VIRUS WHICH PRODUCES PNEUMONIA AND SYSTEMIC INFECTION IN MICE. Science. 1942 Jan 9;95(2454):49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- OMORI T., ISHII S., MATUMOTO M. Miyagawanellosis of cattle in Japan. Am J Vet Res. 1960 Jul;21:564–573. [PubMed] [Google Scholar]
- Schachter J., Ostler H. B., Meyer K. F. Human infection with the agent of feline pneumonitis. Lancet. 1969 May 31;1(7605):1063–1065. doi: 10.1016/s0140-6736(69)91703-6. [DOI] [PubMed] [Google Scholar]
- Storz J. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J Comp Pathol. 1966 Oct;76(4):351–362. doi: 10.1016/0021-9975(66)90055-7. [DOI] [PubMed] [Google Scholar]
- Timms P., Eaves F. W., Girjes A. A., Lavin M. F. Comparison of Chlamydia psittaci isolates by restriction endonuclease and DNA probe analyses. Infect Immun. 1988 Jan;56(1):287–290. doi: 10.1128/iai.56.1.287-290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]