Abstract
Exposure of mammalian cells to ionizing radiation (IR) induces a complex array of cellular responses including cell cycle arrest and/or apoptosis. IR-induced G1 arrest has been shown to depend on the presence of the tumor suppressor p53, which acts as a transcriptional activator of several genes. p53 also plays a role in the induction of apoptosis in response to DNA damage, and this pathway can be activated by both transcription-dependent and -independent mechanisms. Here we report the identification of a novel transcript whose expression is induced in response to IR in a p53-dependent manner, and that shows homology to the type 2C protein phosphatases. We have named this novel gene, wip1. In vitro, recombinant Wip1 displayed characteristics of a type 2C phosphatase, including Mg2+ dependence and relative insensitivity to okadaic acid. Studies performed in several cell lines revealed that wip1 accumulation following IR correlates with the presence of wild-type p53. The accumulation of wip1 mRNA following IR was rapid and transient, and the protein was localized to the nucleus. Similar to waf1, ectopic expression of wip1 in human cells suppressed colony formation. These results suggest that Wip1 might contribute to growth inhibitory pathways activated in response to DNA damage in a p53-dependent manner.
The tumor suppressor gene p53 is the most frequently inactivated gene in human cancer (1). p53 functions to maintain genomic stability and to prevent tumor formation by activating cell cycle checkpoint pathways and inducing programmed cell death (apoptosis) (2). Mice nullizigous for the p53 gene are more susceptible to tumor development (3), and p53-defective cells show several phenotypes including loss of DNA damage-induced G1 arrest (4), suboptimal induction of apoptosis (5, 6), and centrosome amplification (7). DNA damage induces increased levels of the p53 protein and activates p53’s transactivation function. These events result in the induction of p53-responsive genes including waf1 (8–10), gadd45 (4), cyclin G (11), mdm2 (12), bax (13), and insulin-like growth factor binding protein 3 (14). The transcriptional activation of the cyclin/cdk inhibitor Waf1 has been shown to play a major role in the p53-dependent G1 cell cycle checkpoint (15). However, studies using fibroblasts derived from Waf1-deficient mice have clearly shown that Waf1 is not entirely responsible for the p53-induced G1 arrest (16, 17). The gadd45 gene product has been proposed as a potential DNA repair modulator (18) and mdm2 is suggested to negatively regulate p53 transactivation activity (19), while a clear role for cyclin G has yet to be defined. p53 also induces apoptosis. In some instances, the direct induction of bax by p53 has been shown to regulate apoptosis by its interaction with the survival factor Bcl-2 (20). The recent identification of insulin-like growth factor binding protein 3 as a target of p53 transactivation potentially links the p53-dependent induction of apoptosis with the inhibition of insulin-like growth factor-mediated survival stimuli. These and other still unrevealed p53-responsive genes are potential functional mediators of p53’s actions in cells. To date, waf1 is perhaps the most well-characterized as being a mediator of the G1 cell cycle checkpoint.
In this study we have used differential display methodology to search for genes induced in response to ionizing radiation (IR). We report here the identification of a novel human protein phosphatase, Wip1, which is induced in response to IR, and shows homology to the type 2C protein phosphatases (PP2C). Furthermore, we show that the induction in response to IR correlates with the presence of functional p53 protein. Recombinant wild-type (wt) p53-induced phosphatase (Wip1) shows phosphatase activity in vitro; wip1 mRNA is induced rapidly and transiently in response to IR and the protein localizes to the nucleus. Ectopic expression of Wip1 inhibits colony formation. The results presented here suggest that a novel protein phosphatase, Wip1, might contribute to growth suppression induced in response to DNA damage in a p53-dependent manner.
MATERIALS AND METHODS
Plasmids.
cDNAs encoding p53, waf1, wip1, wip1-del, and wip1-FLAG were cloned into the eukaryotic expression vector pCMVneo-Bam (21) and used for transfection experiments.
Differential Display, Northern Blot Analysis, and Probes.
Total RNA was extracted from cells irradiated with 6.3 Gy or controls for the indicated time using TriZol reagent (GIBCO) as described by the manufacturer. Differential display was performed using the RNA Image kit (GenHunter, Nashville, TN) according to the manufacturer’s instructions. Differentially expressed cDNA fragments were subcloned using the TA system (Invitrogen). Northern blots and 32P-labeled probes were performed as described (21). To detect wip1 mRNA, the cDNA fragment corresponding to nucleotides 2560–2764 was used as 32P-labeled probe. The cDNA library used to clone the full length wip1 cDNA was kindly provided by Toru Miki (National Cancer Institute, Bethesda, MD).
Cell Lines, Culture Conditions, and Cell Treatment.
Saos-2 and T98G cells were cultured as described by Fiscella et al. (21). Primary fibroblasts were prepared from p53-deficient or normal embryos by standard procedures and were irradiated using 6.3 Gy. Primary keratinocyte cultures were established from newborn p53 (+/+), (+/−), and (−/−) mice and exposed to 50 J/m2 ultraviolet light C (UVC). All other cell lines were cultured as described by Fan et al. (22) and irradiated with 6.3 Gy using a 137Cs source.
Transfections, Colony Inhibition Assay, and Immunofluorescence Experiments.
Exponentially growing cells were transfected by the calcium phosphate coprecipitation method (21). For colony inhibition assays, T98G or Saos-2 cells were transfected either with the pCMVneo-Bam expression vector or with pCMVneo-Bam encoding for p53, waf1, wip1, and a genetically engineered wip1 mutant (wip1-del) in which a stop codon (TAG) was inserted at codon 118, generating a deleted protein lacking amino acids 118–605. Forty-eight hours following transfection, cells were split 1:4 in medium containing G418 (800 μg/ml). Colonies were scored two weeks after beginning selection. For immunofluorescence experiments, cells were fixed in formalin 24 hr after transfection. Following extensive washes with PBS, cells were incubated 1 hr in PBS containing 1% normal goat serum, followed by sequential incubations with the M2 mAb recognizing the FLAG epitope (IBI–Kodak), a goat anti-mouse fluorescein isothiocyanate-conjugated antibody (BioSource International, Camarillo, CA), and 4′, 6′-diamidino-2-phenylindole for DNA staining. Cells were then analyzed by fluorescence microscopy.
Phosphatase Assay.
wip1 cDNA was cloned into the pGEX expression vector (Pharmacia) to express a glutathione S-transferase (GST)–Wip1 fusion protein in Escherichia coli. The expressed protein was purified in a single step on a GST chromatography column (Pharmacia) according to the manufacturer’s protocol and used to perform a phosphatase assay with in vitro [32P]phosphorylated casein as a substrate (23). Briefly, labeled substrate was prepared by incubation of dephosphorylated casein (Sigma) with rabbit muscle cAMP-dependent protein kinase (Sigma) in the presence of [γ-32P]ATP (3,000 Ci/mmol; 1 Ci = 37 GBq). Unincorporated [32P]ATP was removed by gel filtration on a Sephadex G50 Superfine column, and [32P]casein stored at 4°C. Purified GST–Wip1 was diluted in assay buffer (50 mM Tris⋅HCl, pH 7.0/0.1 mM EGTA/0.1% 2-mercaptoethanol/BSA 1 mg/ml). Twenty microliters of sample was preincubated in equal volume of the same buffer containing 60 mM magnesium acetate for 2′ at 30°C. Ten microliters of the substrate was then added, and after 10′ at 30°C the reaction was terminated by the addition of 100 μl of 20% acetic acid. An aliquot of the acid-soluble supernatant was removed and the released 32P was quantitated by liquid scintillation. Assays were performed in the presence or absence of 20 mM magnesium and with or without the inhibitor okadaic acid (GIBCO).
Immunoprecipitation and Western Blot Analysis.
Irradiated (6.3 Gy) or nonirradiated exponentially growing cells were lysed 4 hr after treatment in PBS, 0.5% Nonidet P-40, plus proteinase inhibitors (Complete, Boehringer Mannheim) for 5 min on ice. Nuclei were pelleted and lysed in the same buffer by sonication (Sonifier 450, Branson). Same amount of protein from cytosolic and nuclear fraction were precleared with protein A/G agarose (Oncogene Science), Immunoprecipitation and Western blot analyses were performed as described by Fiscella et al. (21) using either preimmune sera or a polyclonal antisera raised against GST–Wip1, and the enhanced chemiluminescence detecting system (Amersham).
RESULTS
Cloning and Sequence Analysis.
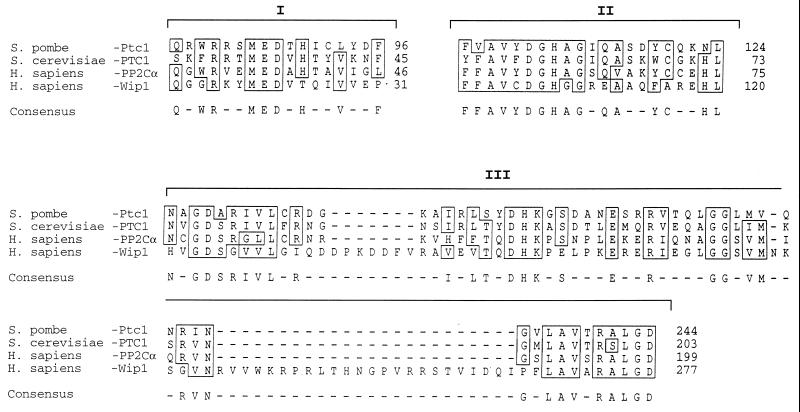
WMN Burkitt lymphoma (BL) cells, carrying a wt-p53 gene, undergo G1 arrest and apoptosis following IR (22). We utilized this cell line and mRNA differential display methodology (24) to search for genes induced in response to IR. Comparative analysis of RNA samples from cells irradiated with 6.3 Gy and nonirradiated controls revealed two cDNA fragments that were enriched after IR. These fragments were used as probes to confirm their inducibility upon γ-irradiation. Because of the critical role of p53 in the response to IR, we also analyzed RNA from the Burkitt lymphoma cell line CA46, carrying a mutant (mut) p53 gene, subjected to the same treatment, to determine whether these cDNAs were induced in a p53-dependent manner. Both probes detected specific mRNAs induced following IR in WMN (wt-p53) but not in CA46 (mut-p53), suggesting a role for wt-p53 in the mechanism(s) responsible for their induction (data not shown). One of the fragments was found to be mdm2, a known gene that is transcriptionally activated by wt-p53 (10, 19). The screening of a cDNA library allowed the cloning of the full-length cDNA for the second and novel fragment. A 605-amino acid ORF was encoded by this novel cDNA. Analysis of the predicted polypeptide sequence revealed three regions with homology to the serine/threonine PP2C phosphatases (Fig. 1). The cDNAs for PP2Cs have been cloned from several species; the sequences of PP2Cs from different organisms show limited similarity except in the three conserved regions shown in Fig. 1, in which the identity varies from 50–77%. These regions are thought to be important in forming the catalytic core and are conserved in all PP2Cs (25).
Figure 1.
Wip1 partial amino acid sequence and protein sequence alignment. wip1 cDNA has an ORF encoding for a protein of 605 amino acids. The three conserved regions among PP2C are outlined (I, II, and III). The figure shows the amino acid sequence alignment within the three identified conserved regions (25) (sequence analysis software from Genetics Computer Group) between human PP2Cα, Wip1, Ptc1 from Schizosaccharomyces pombe, and PTC1 from Saccharomyces cerevisiae. Identical or conserved amino acids are boxed. Numbers on the right identify the amino acid position for each protein. Wip1 carries sequence features typical of this class of phosphatases.
Wip1 Has Properties of a 2C-Like Protein Phosphatase.
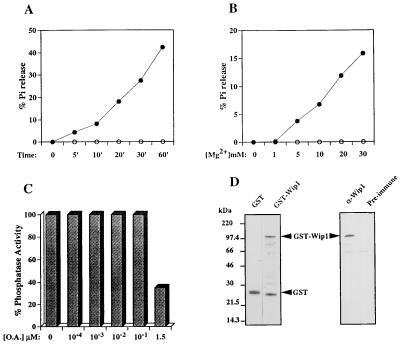
To investigate the possibility that wip1 cDNA encodes a functional protein phosphatase, we generated a GST–Wip1 fusion protein and tested for phosphatase activity in an in vitro assay. GST–Wip1 displayed time-dependent phosphatase activity in vitro (Fig. 2A). The activity of GST–Wip1 was Mg2+-dependent (Fig. 2B), characteristic of type 2C protein phosphatases. In our in vitro assay GST–Wip1 showed insensitivity to okadaic acid (OA) up to 10−1 μM concentrations; contrary to previously reported PP2Cs, Wip1 activity was partially inhibited by μM concentration of OA (Fig. 2C). GST protein alone, similarly expressed and purified, was inactive in our assay, confirming that the detected phosphatase activity was GST–Wip1 specific (data not shown).
Figure 2.
Biochemical characterization of Wip1 as a PP2C. (A) A typical time course experiment showing time-dependent [32P]casein dephosphorylation in the presence of 20 mM Mg2+ by GST–Wip1 (•) but not by the control GST alone (○). PP2Cs are defined by the requirement for Mg2+ for their activity. B shows that GST–Wip1 phosphatase activity is Mg2+ concentration dependent; no activity was detected in absence of Mg2+, but increased linearly with Mg2+ concentrations ranging from 1–30 mM (incubation time 10 min). Another characteristic of the 2C class of phosphatases is their insensitivity to OA as opposed to PP1 and PP2A. (C) A GST–Wip1 phosphatase assay in presence of 20 mM Mg2+ and increasing concentrations of OA. GST–Wip1 is relatively insensitive to OA concentrations that inhibit PP1 and PP2A (10–100 nM). However, at higher concentrations (1.5 μM) GST–Wip1 activity shows partial inhibition by OA. D Left, shows a Coomassie blue-stained polyacrylamide gel of the purified GST–Wip1 and GST used in the phosphatase assays; on the right, a Western blot of the purified GST–Wip1 protein using a polyclonal antisera raised against a Wip1 carboxy-terminal peptide (GQKKIGNPLLHQRKT) (α-GST–Wip1). Preimmune sera was used as a control. Bands lower than full-length GST–Wip1 detected by Coomassie staining (Fig. 3D Left) are degradation products of the full-length protein and can be detected by Western blot analysis using polyclonal antisera raised against the GST–Wip1 fusion protein (data not shown).
Wip1 Is Induced in Response to IR in a p53-Dependent Manner.
To confirm the wt-p53 dependence for IR induction of wip1, we analyzed several human cell lines whose p53 gene status has been characterized previously. RNA was extracted 4 hr following exposure to 6.3 Gy, and Northern blots from these samples were probed for wip1 expression. We detected a clear correlation between the presence of a wt-p53 and wip1 mRNA induction following IR (Table 1). Indeed, every cell line analyzed that carried a functional p53 showed induction of wip1 mRNA (2–5-fold). In contrast, cell lines carrying mut-p53 failed to show any induction of wip1 mRNA. As a positive control for p53-dependent induction, we quantitated waf1 mRNA; as reported previously, the induction of waf1 transcript occurred in all the cell lines carrying wt-p53. The levels of wip1 mRNA induction were less than those seen for waf1 (Table 1), but similar to those reported for gadd45 (1.8- to 4.6-fold) (29). We also analyzed the expression of wip1 in two cell lines expressing either a human papilloma virus E6 transgene, which abrogates p53 function (27) in a wt-p53 background (HCT116-E6), or a human temperature sensitive p53 transgene (p53-V143A) (28) in a p53-null background (Calu6–143). In contrast to parental HCT116 cells, HCT116-E6 cells expressing human papilloma virus E6 failed to show induction of wip1 mRNA following IR. Calu6–143 cultured at 30°C (permissive temperature), to allow p53-V143A to adopt a wt conformation, exhibited increased levels of wip1 mRNA compared with cells grown at 37°C. In contrast, neither the parental cell line (Calu6) nor a stable transfectant constitutively expressing a mut-p53 (p53-R273H) (data not shown) showed any change in the levels of wip1 mRNA under the same conditions. Furthermore, we examined the expression of wip1 in primary cultures of fibroblasts or keratinocytes derived from normal or p53-deficient mice. When fibroblasts or keratinocytes from p53+/+ mice were treated with IR or UVC, respectively, wip1 mRNA accumulated to levels 2.5–8.4× above control. In contrast, no accumulation was observed in fibroblasts or keratinocytes derived from p53−/− mice (Table 1). These results indicate that the presence of a functional p53 is required for wip1 induction in response to DNA damage.
Table 1.
Relation between induction of wip1 and p53 status
| Cell line | Cell type | p53 status* | G1 arrest | Apoptosis | Fold induction of relative mRNA†
|
|
|---|---|---|---|---|---|---|
| wip1 | waf1 | |||||
| Wild-type p53 status and function | ||||||
| ML-1 | Myeloid leukemia | wt/wt | + | + | 5.0 ± 0.8† | 16.2 |
| WMN | Burkitt lymphoma | wt/wt | + | + | 3.2 ± 0.6 | 4.1 ± 0.5 |
| FWL | Lymphoblastoid | wt/wt | + | + | 2.9 ± 0.4 | 6.0 ± 0.4 |
| Shoemaker | Lymphoblastoid | wt/wt | + | + | 2.6 ± 0.2 | 5.6 |
| NL2 | Lymphoblastoid | wt/wt | + | + | 2.5 ± 0.1 | 6.6 |
| H460 | NSC lung carcinoma | wt/wt | + | 2.2 ± 0.5 | 4.5 | |
| MCF-7 | Breast carcinoma | wt/wt | + | +‡ | 2.0 ± 0.1 | 4.5 |
| Abnormal p53 status and function | ||||||
| CA46 | Burkitt lymphoma | mut/−§ | − | − | 1.0 ± 0.0 | 1.7 ± 1.1 |
| JD38 | Burkitt lymphoma | mut/− | − | − | 1.2 ± 0.1 | 2.1 |
| MC116 | Burkitt lymphoma | mut/− | − | − | 1.2 ± 0.1 | 3.9 |
| Ramos | Burkitt lymphoma | mut/− | − | − | 1.1 ± 0.1 | 1.5 |
| Transfected cell lines | ||||||
| HCT116-CMV¶ | Colon carcinoma | wt/wt | + | 2.0 | 3.5 | |
| HCT116-E6¶ | Colon carcinoma | wt/wt (E6) | − | 1.1 | 1.4 | |
| Calu6 | Lung carcinoma | −/− | 0.7** | 1.0 | ||
| Calu6-143‖ | Lung carcinoma | −/− (tsp53) | 2.5 | 8.0 | ||
| Murine cells | ||||||
| MEF | Fibroblasts | +/+ | 2.5 | |||
| MEF | Fibroblasts | −/− | 1.0 | |||
| PNK | Keratinocytes | +/+ | 8.4‡‡ | |||
| PNK | Keratinocytes | +/− | 4.0 | |||
| PNK | Keratinocytes | −/− | 1.2 | |||
Note: When not determined, the postition was left blank.
Data regarding p53 status, G1 arrest, and apoptosis are taken from ref. 26 and references therein.
Human cell lines with normal or abnormal p53 status were irradiated with 6.3 Gy of IR. Relative values for samples extracted 4 hr after treatment, compared with untreated controls, were determined by densitormetric scanning of Northern blots using PhophoImager model 425 (Molecular Dynamics). wip1 and waf1 values are normalized relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Where shown, data are ±SD from mean value of three experiments.
Apoptosis could be detected but was substantially less than for other cell lines (23).
Only one mutant form of p53 was detected, indicating either that both alleles contained the same mutation or that one allele had been deleted (23).
HCT116 cell line was transfected with either pCMVneo vector or with pCMVneo-E6, encoding for the HPV-E6 protein known to abrogate p53-dependent transactivation (27).
Calu6 cells were transfected with a pCMVneo vector encoding for a human temperature-sensitive p53 (p53-V143A) (28), generating Calu6-143. Calu6-143 expresses p53 either in a mutant or wt conformation when cultured at 37°C or 30°C, respectively.
Induction was determined comparing RNA expression from cells cultured 24 hr at 30°C (permissive temperature) to control cultures at 37°C.
Primary newborn keratinocytes (PNK) were treated with 50 J/m2 UVC. Relative values for samples extracted 24 hr after treatment, compared with untreated controls, were determined by densitometric scanning of Northern blots (W. C. Weinberg and E. Fernandez-Salas, personal communication).
To rule out the possibility that the increased levels of wip1 mRNA were due to the arrest of cell cycle progression induced by p53 as a consequence of DNA damage, we analyzed a colorectal cancer cell line, HT29, lacking a functional p53. HT29 were treated with sodium butyrate, shown to arrest cells in the G1 phase of the cell cycle (W.E.M., unpublished work), and RNA samples were prepared following the treatment. No induction of wip1 mRNA was detected in treated cells, arrested in G1, compared with control cycling cells (data not shown). Thus, wip1 accumulation appears not to be a consequence of a cell cycle arrest, rather a direct consequence of DNA damage.
Time Course Analysis of wip1 Induction Following IR.
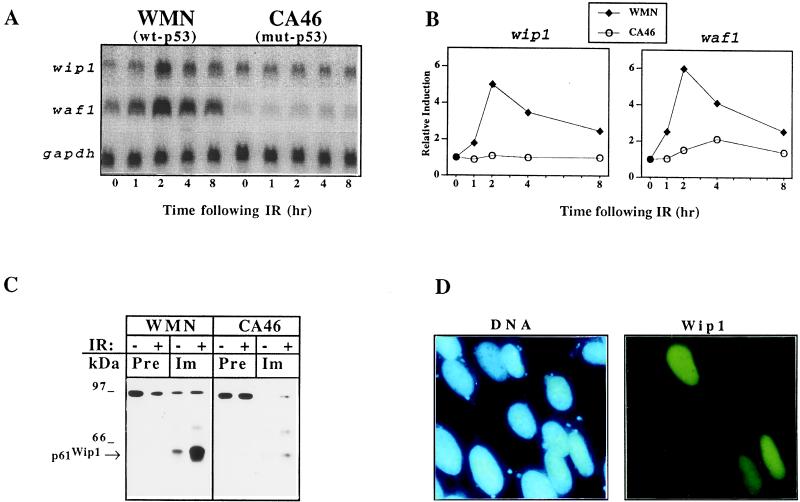
The kinetics of wip1 mRNA induction was assayed in two BL cell lines carrying either wt-p53 (WMN) or mut-p53 (CA46), and a comparison was made with the kinetics of waf1 induction. IR treatment of WMN cells induced a rapid increase in the level of wip1 mRNA with a peak observed at 2 hr, a time when the cell cycle profile of WMN is not dramatically affected by the treatment (data not shown). Thereafter, wip1 mRNA levels declined to near basal levels by 8 hr. In contrast, wip1 mRNA was not induced in CA46 (Fig. 3 A and B). The time course of wip1 mRNA induction was similar to that of waf1 and was akin to the induction pattern observed in another lymphoid cell line we analyzed (FWL), expressing wt-p53 (data not shown).
Figure 3.
Analysis of wip1 expression following IR. (A) wip1 mRNA levels increase after IR in WMN cells and reaches its peak of expression at 2 hr with a time course of induction very similar to waf1, previously shown to be directly induced by wt-p53 (8). No induction was detected in CA46 either for wip1 or for waf1, consistent with the presence of mut-p53 in this cell line. (B) Quantitation of the Northern blot shown in A by using a PhosphorImager model 425 (Molecular Dynamics). (C) Western blot of Wip1 immunoprecipitated from the nuclear fraction of WMN and CA46 cells before and 4 hr after exposure to 6.3 Gy (− or + IR). Detectable amounts of Wip1 were present only in the nuclear fraction of WMN (immunoprecipitation/Western blot of cytosolic fraction not shown), and its levels increased following IR. Immunoprecipitation was performed using either preimmune sera or antisera raised against GST–Wip1. (D) DNA and Wip1 staining of Saos-2 cells transiently transfected with a cDNA encoding for Wip1-FLAG. The fluorescein staining, which is specific for the transfected cells, can be superimposed onto the 4′-6 diamidino-2-phenylindole staining of DNA of the corresponding cells, thus confirming the nuclear localization of Wip1.
Wip1 Localizes to the Nucleus.
The presence of two putative nuclear localization signals suggested that Wip1 might localize to the nucleus. To investigate this possibility, we raised a polyclonal antisera against the GST–Wip1 fusion protein and performed Western blot analysis on immunoprecipitates from nuclear and cytosolic cell fractions. Wip1 was found exclusively in the nuclear fraction of WMN cells (wt-p53), and the levels of nuclear Wip1 increased following IR (Fig. 3C). The apparent molecular mass of Wip1 on SDS/PAGE was ≈61 kDa (p61Wip1, Fig. 3C). Additionally, we generated a recombinant wip1 cDNA encoding for a Wip1 protein tagged with the FLAG epitope at the carboxyl terminus (wip1-FLAG). This construct was transiently transfected into Saos-2 cells, which were then examined by indirect immunofluorescence. Consistent with immunoprecipitation analysis, the Wip1–FLAG protein localized exclusively to the nucleus of transiently transfected cells (Fig. 3D).
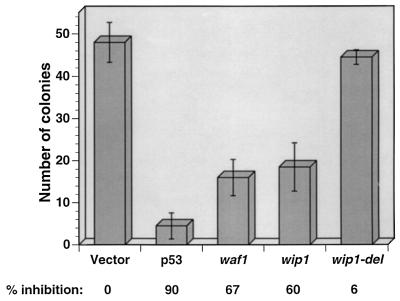
Wip1 Overexpression Leads to Growth Suppression.
The activation of p53 transactivating function following DNA damage leads to growth suppression. To explore the role of wip1 in growth suppression, we transfected a human glioblastoma cell line (T98G), previously shown to arrest in late G1 following p53 overexpression (21), or Saos-2 cells with vectors expressing the neomycin resistance gene and either wt-p53 or wip1 cDNAs. Following transfection, cells were selected in the presence of G418, and resistant colonies were scored two weeks later. The data in Fig. 4 indicate that expression of wip1 results in growth inhibition as indicated by a decreased number of G418-resistant colonies. The activity of Wip1 measured in this assay was similar to the activity observed when waf1 was transfected into T98G cells. As a further control we generated a wip1 mutant (wip1-del) with a stop codon at nucleotide 354, thus deleting amino acids 118–605 including the conserved region III (Fig. 1). When transfected into T98G cells, wip1-del did not suppress growth, indicating that the growth suppression activity of Wip1 may be dependent on its phosphatase activity. Similar results were obtained in Saos-2 cells (data not shown).
Figure 4.
Wip1 inhibits cell growth. T98G cells were transfected with either pCMV-neo, or pCMV-neo containing p53, waf1, wip1, and wip1-del cDNAs. Following transfection cells were selected in medium containing G418 (800 μg/ml), and two weeks later colonies were scored. Shown are the mean values ± SD of three different experiments.
DISCUSSION
DNA damaging agents such as ionizing radiation induce increased levels of the tumor suppressor protein p53, which in turn activates the expression of several genes containing p53-responsive elements. The induction of p53 is responsible for the activation of G1 arrest and p53 possibly contributes to G2 arrest (4, 30, 31). p53 can also induce apoptosis (5, 6). Studies on waf1-deficient murine cells in contrast to human cancer cells have revealed that the G1 checkpoint activated by p53 in response to IR is not totally lost in these cells (15–17). Therefore, other p53-dependent mechanism(s) probably contribute to G1 arrest in response to IR. p53-dependent induction of apoptosis has been shown to be activated by both transactivation-competent and transactivation-defective p53 (32, 33), however, the mechanism underlying this function as well as the mechanism of the p53-induced G2/M checkpoint has not been elucidated.
In this study we report the cloning of a novel human protein phosphatase, Wip1. The Wip1 sequence shows significant region-specific homology to PP2C phosphatases. In vitro, recombinant Wip1 displays characteristics of the PP2Cs, namely Mg2+ dependence and relative insensitivity to OA. The expression of wip1 increases rapidly and transiently following IR, with a pattern very similar to waf1. Because of the role of p53 in radiation response pathways, we investigated the relationship between wt-p53 and wip1. We found that the induction of wip1 expression correlates with the presence of wt-p53. This induction was observed in both human and mouse cell lines, indicating that wip1 regulation by p53 appears to be conserved. Disruption of p53 function by coexpression of the human papilloma virus E6 protein completely abrogates the IR-dependent induction of wip1. In addition, a p53-null cell line transfected with a human temperature sensitive p53 transgene exhibits increased levels of wip1 mRNA only at the permissive temperature where p53 adopts a wt conformation. Most importantly, accumulation of wip1 mRNA in response to IR or UVC treatment occurs only in primary cultures from normal mice but not from mice with disrupted p53 gene. These observations strongly suggest that a functional p53 is essential for the induction of wip1. To analyze whether the induction of Wip1 is directly or indirectly dependent on the expression of wt-p53, we have cloned and sequenced 20 kb of the Wip1 gene. Using sequence analysis, in vitro binding and reporter assays of the promoter region and the first intron we did not find a p53 responsive element. It is known for other p53 responsive genes such as GADD45 and MDM2 that p53 responsive elements are located in introns rather than in 5′ flanking promoter sequences; therefore analysis of the full sequence of the Wip1 gene is required to identify p53 responsive elements.
At this time we cannot exclude the possibility that other pathways could activate wip1 as has been shown for waf1 (34), and it is not clear whether the induction of wip1 mRNA is transcriptionally controlled. Although the experiments on colony inhibition indicate that Wip1 may play a role in the control of cellular growth, at this time we do not know whether Wip1-mediated growth inhibition results from its activity on a specific checkpoint of the cell cycle. New data obtained using stable cell lines expressing an inducible wip1 transgene show that Wip1 expression might lead to a slower entry into S phase, with a concomitant increase in underphosphorylated pRb (S.S., W.E.M., and E.A., unpublished data). However, the mechanisms responsible for Wip1-mediated inhibition of cell growth remain unknown. One possibility is through Wip1-dependent dephosphorylation and inactivation of transcription factors or critical active kinase complexes that promote cell cycle progression. Several transcription factors have been shown to be regulated by phosphorylation in both positive and negative fashion (35) either directly or indirectly. For example, the retinoblastoma gene product pRb, a key component of the G1 cell cycle checkpoint, is thought to switch from an active to an inactive form by changes in its phosphorylation status. pRb has been shown to be underphosphorylated following p53 activation and this correlates with the inhibition of cyclin/cdk complexes activity by Waf1. In addition, protein phosphatases have been implicated in the regulation of pRb phosphorylation status (36–38), thus suggesting a role for active pRb dephosphorylation in the promotion of the G1 checkpoint. Furthermore, it has been shown that protein phosphatases may play a role in growth inhibitory pathways. The findings we report here further emphasize that protein dephosphorylation may play a critical role in p53’s cell activities. Characterization of the relevant in vivo substrates of Wip1 and downstream cellular responses affected by Wip1 activation could lead to a fuller understanding of the role of Wip1 in the p53 pathway response to DNA damage.
Acknowledgments
We thank Dr. W. C. Weinberg and Dr. E. Fernandez-Salas for generously providing us with unpublished data from Northern blots of keratinocytes mRNA. This research was sponsored in part by the National Cancer Institute, Department of Health and Human Services, under contract with Advanced BioScience Laboratories.
ABBREVIATIONS
- wt
wild type
- mut
mutant
- Wip1
wt-p53-induced phosphatase
- IR
ionizing radiation
- PP2C
type 2C class of protein phosphatases
- GST
glutathione S-transferase
- OA
okadaic acid
- UVC
ultraviolet light C
Footnotes
References
- 1.Hollestein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 2.Bates S, Vousden K H. Curr Opin Genet Dev. 1996;6:12–19. doi: 10.1016/s0959-437x(96)90004-0. [DOI] [PubMed] [Google Scholar]
- 3.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 4.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 5.Lowe S W, Schmitt S W, Smith B A, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 6.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wylie A H. Nature (London) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 7.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude G F. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 8.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 9.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto K, Beach D. EMBO J. 1994;13:4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barak Y, Yuven T, Haffner R, Oren M. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyashita T, Reed J C. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 14.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger B R, Kley N. Nature (London) 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 15.Waldman T, Kinzler K W, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 16.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 17.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 18.Smith M L, Chen I T, Zhan Q, Bae I, Chen C Y, Gilmer T M, Kastan M B, O’Connor P M, Fornace A J. Science. 1994;266:1376–1379. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Bayle J H, Olson D, Levine A J. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 20.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 21.Fiscella M, Ullrich S J, Zambrano N, Shields M T, Lin D, Lees-Miller S P, Anderson C W, Mercer W E, Appella E. Oncogene. 1993;8:1519–1528. [PubMed] [Google Scholar]
- 22.Fan S, El-Deiry W S, Bae I, Freeman J, Jondle D, Bhatia K, Fornace A J, Jr, Magrath I, Kohn K W, O’Connor P M. Cancer Res. 1994;54:5824–5830. [PubMed] [Google Scholar]
- 23.McGowan C H, Cohen P. Methods Enzymol. 1988;159:416–418. doi: 10.1016/0076-6879(88)59041-9. [DOI] [PubMed] [Google Scholar]
- 24.Liang P, Pardee A B. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 25.Klumpp S, Hanke C, Donella-Deana A, Beyer A, Kellner R, Pinna L A, Schultz J E. J Biol Chem. 1994;269:32774–32780. [PubMed] [Google Scholar]
- 26.Zhan Q, Fan S, Bae I, Guillof C, Liebermann D A, O’Connor P M, Fornace A J., Jr Oncogene. 1994;9:3743–3751. [PubMed] [Google Scholar]
- 27.Kessis T D, Slebos R J, Nelson W G, Kastan M B, Plunkett B S, Han S M, Lorincz A T, Hedrick L, Cho K R. Proc Natl Acad Sci USA. 1993;90:3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Guo X Y, Hu G-Y, Liu W-B, Shay J W, Deisseroth A, B. EMBO J. 1994;13:2535–2544. doi: 10.1002/j.1460-2075.1994.tb06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae I, Smith M L, Saeed Sheikh M, Zhan Q, Scudiero D A, Friend S H, O’Connor P M, Fornace A J., Jr Cancer Res. 1996;56:840–847. [PubMed] [Google Scholar]
- 30.Stewart N, Hicks G G, Paraskevas F, Mowat M. Oncogene. 1995;10:109–115. [PubMed] [Google Scholar]
- 31.Agarwal M L, Agarwal A, Taylor W R, Stark G R. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbatini P, Lin J, Levine A J, White E. Genes Dev. 1995;9:2184–2192. doi: 10.1101/gad.9.17.2184. [DOI] [PubMed] [Google Scholar]
- 33.Haupt Y, Rowan S, Shaulian E, Vousden K H, Oren M. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- 34.Michieli P, Chedid M, Lin D, Pierce J H, Mercer E W, Givol D. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 35.Boulikas T. Crit Rev Eukaryotic Gene Expression. 1995;5:1–77. [PubMed] [Google Scholar]
- 36.Chao R, Wasiuddin K, Yusuf A H. J Biol Chem. 1992;267:23459–23462. [PubMed] [Google Scholar]
- 37.Buquet-Fagot C, Lallemand F, Charollais R-H, Mester J. J Cell Physiol. 1996;166:631–636. doi: 10.1002/(SICI)1097-4652(199603)166:3<631::AID-JCP18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Dou Q P, An B, Will P L. Proc Natl Acad Sci USA. 1995;92:9019–9023. doi: 10.1073/pnas.92.20.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]