Abstract
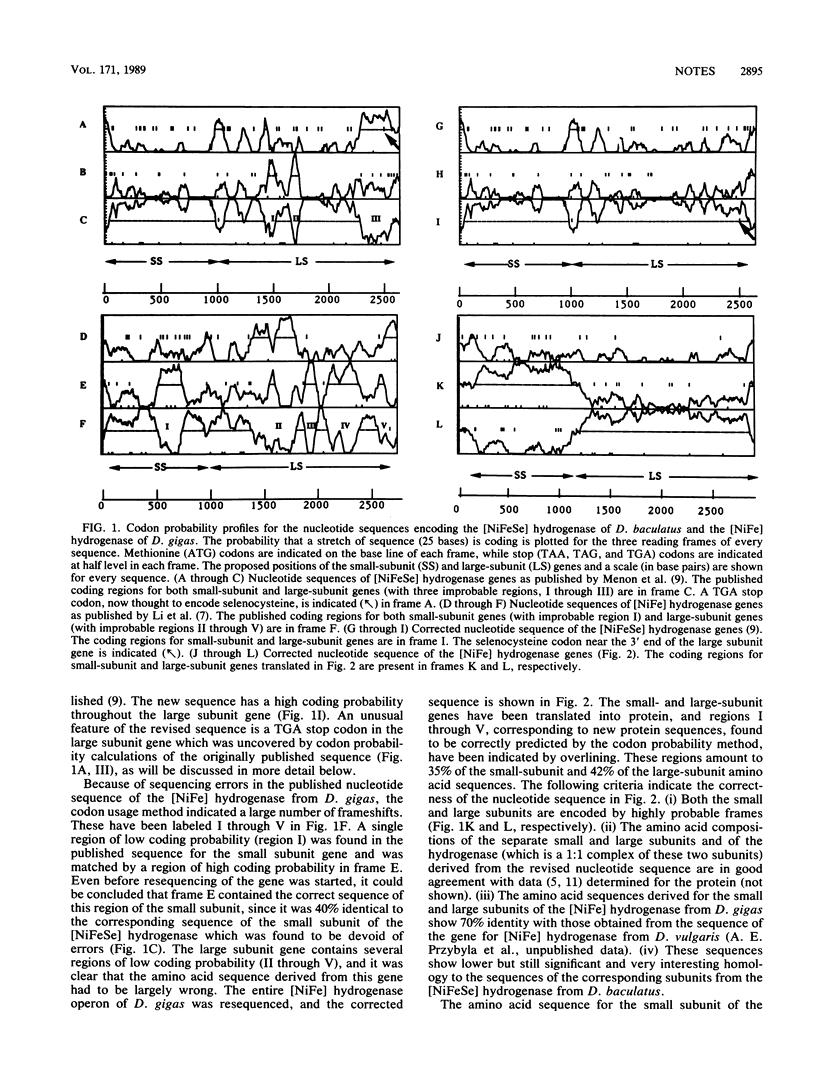
The nucleotide sequences encoding the [NiFe] hydrogenase from Desulfovibrio gigas and the [NiFeSe] hydrogenase from Desulfovibrio baculatus (N.K. Menon, H.D. Peck, Jr., J. LeGall, and A.E. Przybyla, J. Bacteriol. 169:5401-5407, 1987; C. Li, H.D. Peck, Jr., J. LeGall, and A.E. Przybyla, DNA 6:539-551, 1987) were analyzed by the codon usage method of Staden and McLachlan. The reported reading frames were found to contain regions of low codon probability which are matched by more probable sequences in other frames. Renewed nucleotide sequencing showed the probable frames to be correct. The corrected sequences of the two small and large subunits share a significant degree of sequence homology. The small subunit, which contains 10 conserved cysteine residues, is likely to coordinate at least 2 iron-sulfur clusters, while the finding of a selenocysteine codon (TGA) near the 3' end of the [NiFeSe] large-subunit gene matched by a regular cysteine codon (TGC) in the [NiFe] large-subunit gene indicates the presence of some of the ligands to the active-site nickel in the large subunit.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auric P., Gaillard J., Meyer J., Moulis J. M. Analysis of the high-spin states of the 2[4Fe-4Se]+ ferredoxin from Clostridium pasteurianum by Mössbauer spectroscopy. Biochem J. 1987 Mar 1;242(2):525–530. doi: 10.1042/bj2420525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlier Y., Fauque G. D., LeGall J., Choi E. S., Peck H. D., Jr, Lespinat P. A. Inhibition studies of three classes of Desulfovibrio hydrogenase: application to the further characterization of the multiple hydrogenases found in Desulfovibrio vulgaris Hildenborough. Biochem Biophys Res Commun. 1987 Jul 15;146(1):147–153. doi: 10.1016/0006-291x(87)90703-0. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidsness M. K., Scott R. A., Prickril B. C., DerVartanian D. V., Legall J., Moura I., Moura J. J., Peck H. D., Jr Evidence for selenocysteine coordination to the active site nickel in the [NiFeSe]hydrogenases from Desulfovibrio baculatus. Proc Natl Acad Sci U S A. 1989 Jan;86(1):147–151. doi: 10.1073/pnas.86.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchikian E. C., Bruschi M., Le Gall J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1978 May 30;82(2):451–461. doi: 10.1016/0006-291x(78)90896-3. [DOI] [PubMed] [Google Scholar]
- He S. H., Teixeira M., LeGall J., Patil D. S., Moura I., Moura J. J., DerVartanian D. V., Huynh B. H., Peck H. D., Jr EPR studies with 77Se-enriched (NiFeSe) hydrogenase of Desulfovibrio baculatus. Evidence for a selenium ligand to the active site nickel. J Biol Chem. 1989 Feb 15;264(5):2678–2682. [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Li C., Peck H. D., Jr, LeGall J., Przybyla A. E. Cloning, characterization, and sequencing of the genes encoding the large and small subunits of the periplasmic [NiFe]hydrogenase of Desulfovibrio gigas. DNA. 1987 Dec;6(6):539–551. doi: 10.1089/dna.1987.6.539. [DOI] [PubMed] [Google Scholar]
- Lissolo T., Choi E. S., LeGall J., Peck H. D., Jr The presence of multiple intrinsic membrane nickel-containing hydrogenases in Desulfovibrio vulgaris (Hildenborough). Biochem Biophys Res Commun. 1986 Sep 14;139(2):701–708. doi: 10.1016/s0006-291x(86)80047-x. [DOI] [PubMed] [Google Scholar]
- Menon N. K., Peck H. D., Jr, Gall J. L., Przybyla A. E. Cloning and sequencing of the genes encoding the large and small subunits of the periplasmic (NiFeSe) hydrogenase of Desulfovibrio baculatus. J Bacteriol. 1987 Dec;169(12):5401–5407. doi: 10.1128/jb.169.12.5401-5407.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Selenite binding to carbon monoxide oxidase from Pseudomonas carboxydovorans. Selenium binds covalently to the protein and activates specifically the CO----methylene blue reaction. J Biol Chem. 1984 May 10;259(9):5612–5617. [PubMed] [Google Scholar]
- Nivière V., Forget N., Bovier-Lapierre G., Bonicel J., Hatchikian C. Isolation, amino acid analysis and N-terminal sequence determination of the two subunits of the nickel-containing hydrogenase of Desulfovibrio gigas. Biochimie. 1988 Feb;70(2):267–272. doi: 10.1016/0300-9084(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Prickril B. C., Czechowski M. H., Przybyla A. E., Peck H. D., Jr, LeGall J. Putative signal peptide on the small subunit of the periplasmic hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1986 Aug;167(2):722–725. doi: 10.1128/jb.167.2.722-725.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickril B. C., He S. H., Li C., Menon N., Choi E. S., Przybyla A. E., DerVartanian D. V., Peck H. D., Jr, Fauque G., LeGall J. Identification of three classes of hydrogenase in the genus, Desulfovibrio. Biochem Biophys Res Commun. 1987 Dec 16;149(2):369–377. doi: 10.1016/0006-291x(87)90376-7. [DOI] [PubMed] [Google Scholar]
- Shuber A. P., Orr E. C., Recny M. A., Schendel P. F., May H. D., Schauer N. L., Ferry J. G. Cloning, expression, and nucleotide sequence of the formate dehydrogenase genes from Methanobacterium formicicum. J Biol Chem. 1986 Oct 5;261(28):12942–12947. [PubMed] [Google Scholar]
- Sliwkowski M. X., Stadtman T. C. Incorporation and distribution of selenium into thiolase from Clostridium kluyveri. J Biol Chem. 1985 Mar 10;260(5):3140–3144. [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Specific occurrence of selenium in enzymes and amino acid tRNAs. FASEB J. 1987 Nov;1(5):375–379. doi: 10.1096/fasebj.1.5.2445614. [DOI] [PubMed] [Google Scholar]
- Sukenaga Y., Ishida K., Takeda T., Takagi K. cDNA sequence coding for human glutathione peroxidase. Nucleic Acids Res. 1987 Sep 11;15(17):7178–7178. doi: 10.1093/nar/15.17.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M., Fauque G., Moura I., Lespinat P. A., Berlier Y., Prickril B., Peck H. D., Jr, Xavier A. V., Le Gall J., Moura J. J. Nickel-[iron-sulfur]-selenium-containing hydrogenases from Desulfovibrio baculatus (DSM 1743). Redox centers and catalytic properties. Eur J Biochem. 1987 Aug 17;167(1):47–58. doi: 10.1111/j.1432-1033.1987.tb13302.x. [DOI] [PubMed] [Google Scholar]
- Teixeira M., Moura I., Xavier A. V., Huynh B. H., DerVartanian D. V., Peck H. D., Jr, LeGall J., Moura J. J. Electron paramagnetic resonance studies on the mechanism of activation and the catalytic cycle of the nickel-containing hydrogenase from Desulfovibrio gigas. J Biol Chem. 1985 Jul 25;260(15):8942–8950. [PubMed] [Google Scholar]
- Tsibris J. C., Namtvedt M. J., Gunsalus I. C. Selenium as an acid labile sulfur replacement in putidaredoxin. Biochem Biophys Res Commun. 1968 Feb 15;30(3):323–327. doi: 10.1016/0006-291x(68)90454-3. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Brenner S. Nucleotide sequence of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough). Eur J Biochem. 1985 May 2;148(3):515–520. doi: 10.1111/j.1432-1033.1985.tb08869.x. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Walker J. E., Brenner S. Cloning of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough) and determination of the NH2-terminal sequence. Eur J Biochem. 1985 May 2;148(3):509–514. doi: 10.1111/j.1432-1033.1985.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]



