Abstract
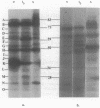
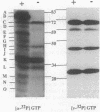
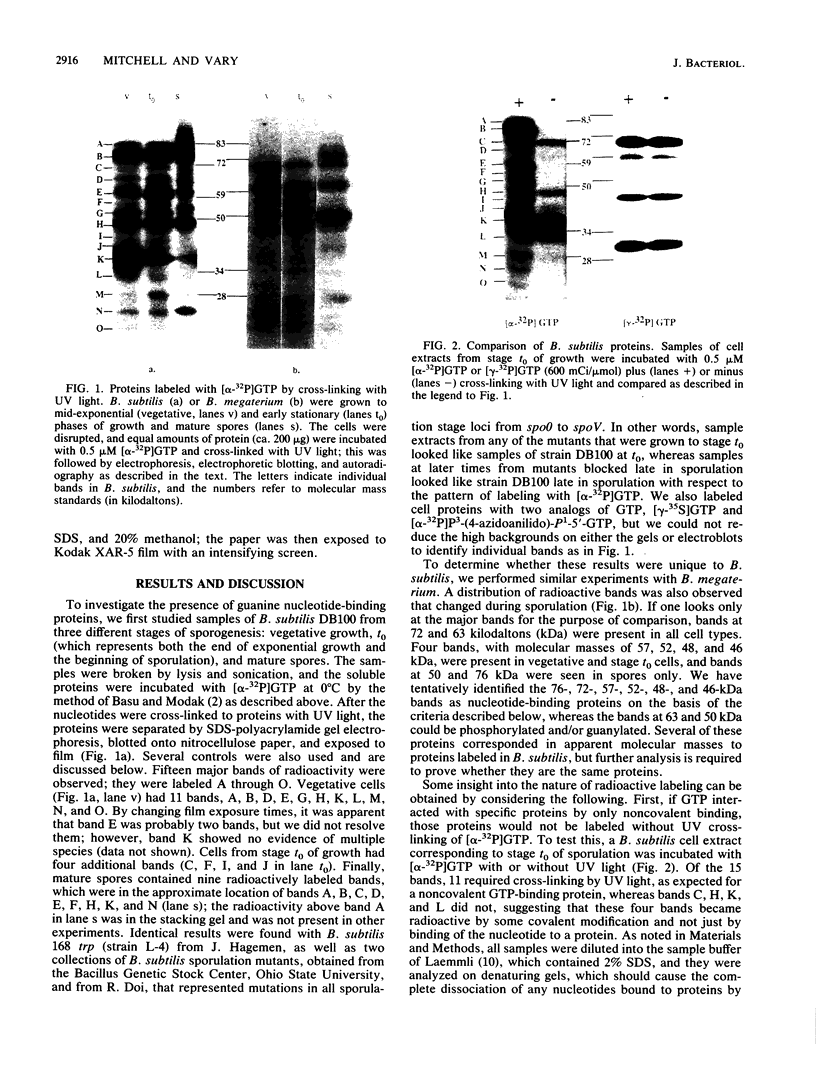
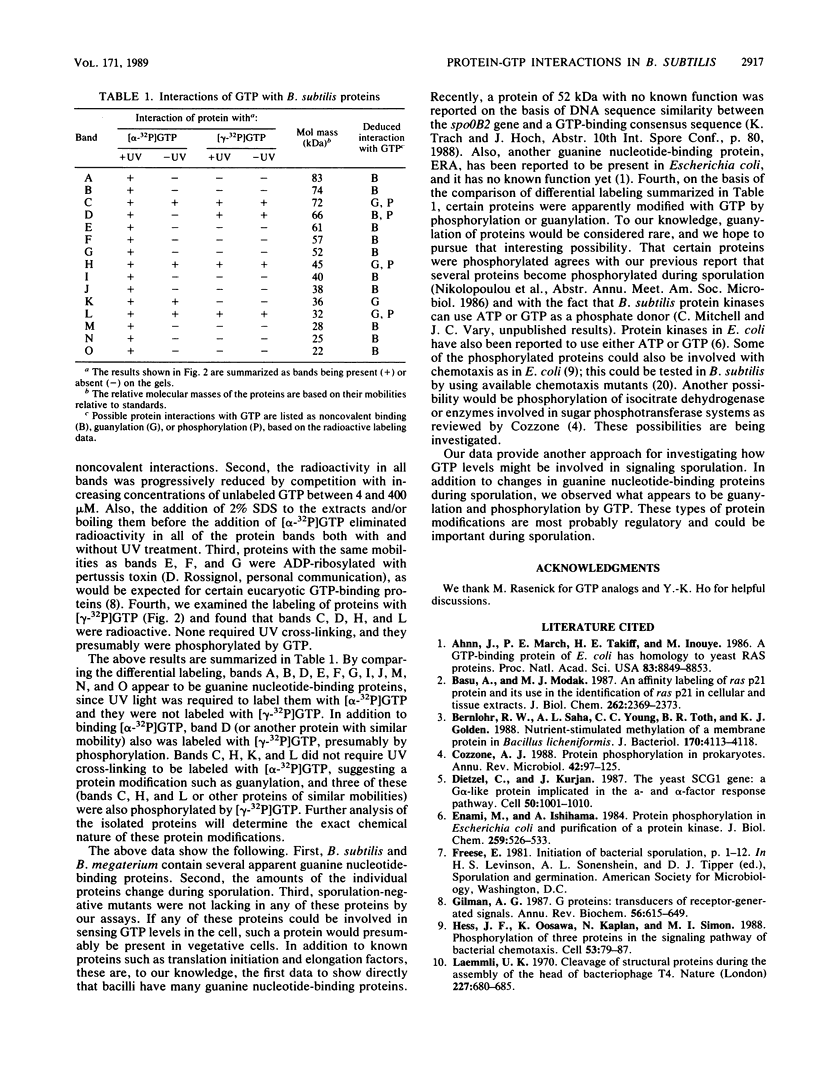
During sporulation of Bacillus subtilis, several proteins were shown to interact with GTP in specific ways. UV light was used to cross-link [alpha-32P]GTP to proteins in cell extracts at different stages of growth. After electrophoresis, 11 bands of radioactivity were found in vegetative cells, 4 more appeared during sporulation, and only 9 remained in mature spores. Based on the labeling pattern with or without UV light to cross-link either [alpha-32P]GTP or [gamma-32P]GTP, 11 bands of radioactivity were apparent guanine nucleotide-binding proteins, and 5 bands appeared to be phosphorylated and/or guanylated. Similar results were found with Bacillus megaterium. Assuming that GTP might be a type of signal for sporulation, it could interact with and regulate proteins by at least three mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnn J., March P. E., Takiff H. E., Inouye M. A GTP-binding protein of Escherichia coli has homology to yeast RAS proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8849–8853. doi: 10.1073/pnas.83.23.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Modak M. J. An affinity labeling of ras p21 protein and its use in the identification of ras p21 in cellular and tissue extracts. J Biol Chem. 1987 Feb 15;262(5):2369–2373. [PubMed] [Google Scholar]
- Bernlohr R. W., Saha A. L., Young C. C., Toth B. R., Golden K. J. Nutrient-stimulated methylation of a membrane protein in Bacillus licheniformis. J Bacteriol. 1988 Sep;170(9):4113–4118. doi: 10.1128/jb.170.9.4113-4118.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Dietzel C., Kurjan J. The yeast SCG1 gene: a G alpha-like protein implicated in the a- and alpha-factor response pathway. Cell. 1987 Sep 25;50(7):1001–1010. doi: 10.1016/0092-8674(87)90166-8. [DOI] [PubMed] [Google Scholar]
- Enami M., Ishihama A. Protein phosphorylation in Escherichia coli and purification of a protein kinase. J Biol Chem. 1984 Jan 10;259(1):526–533. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez J. M., Dromerick A., Freese E. Response of guanosine 5'-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J Bacteriol. 1981 May;146(2):605–613. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Racine F. M., Vary J. C. Isolation and properties of membranes from Bacillus megaterium spores. J Bacteriol. 1980 Sep;143(3):1208–1214. doi: 10.1128/jb.143.3.1208-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Formation of competent Bacillus subtilis cells. J Bacteriol. 1983 Feb;153(2):813–821. doi: 10.1128/jb.153.2.813-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay L. K., Vary J. C. Biochemical studies on glucose initiated germination in Bacillus megaterium. Biochim Biophys Acta. 1978 Jan 18;538(2):284–292. doi: 10.1016/0304-4165(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]