Abstract
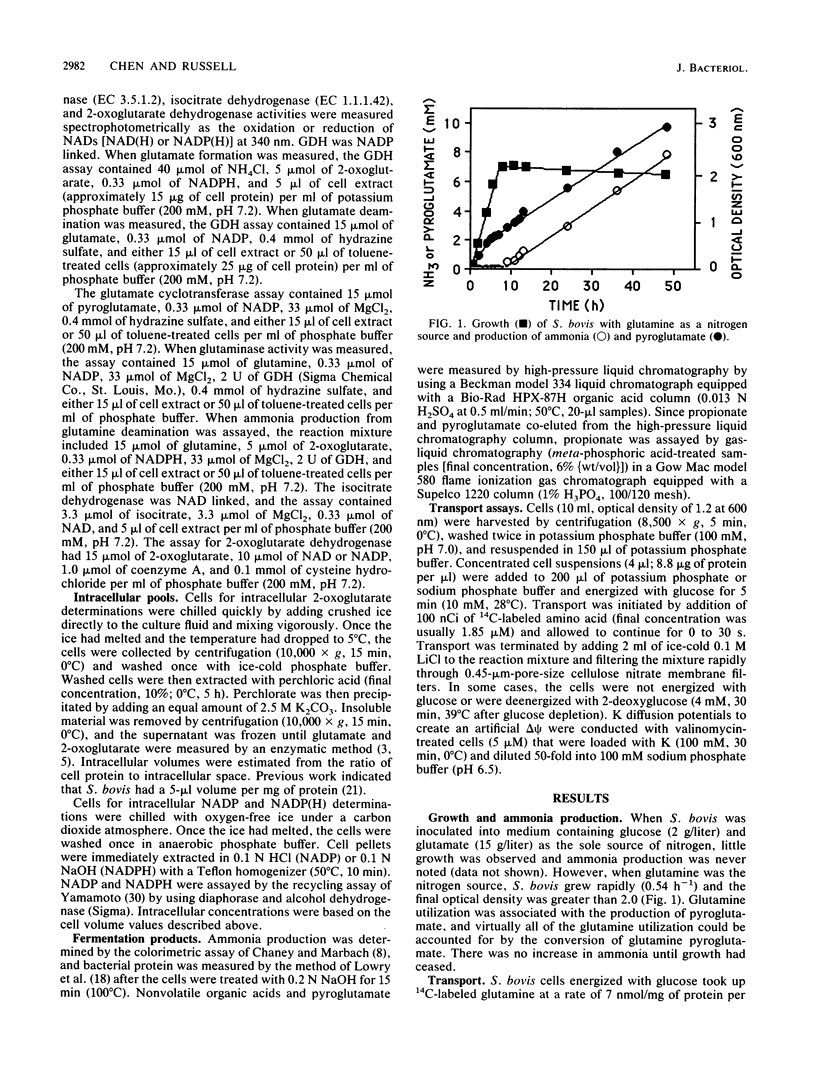
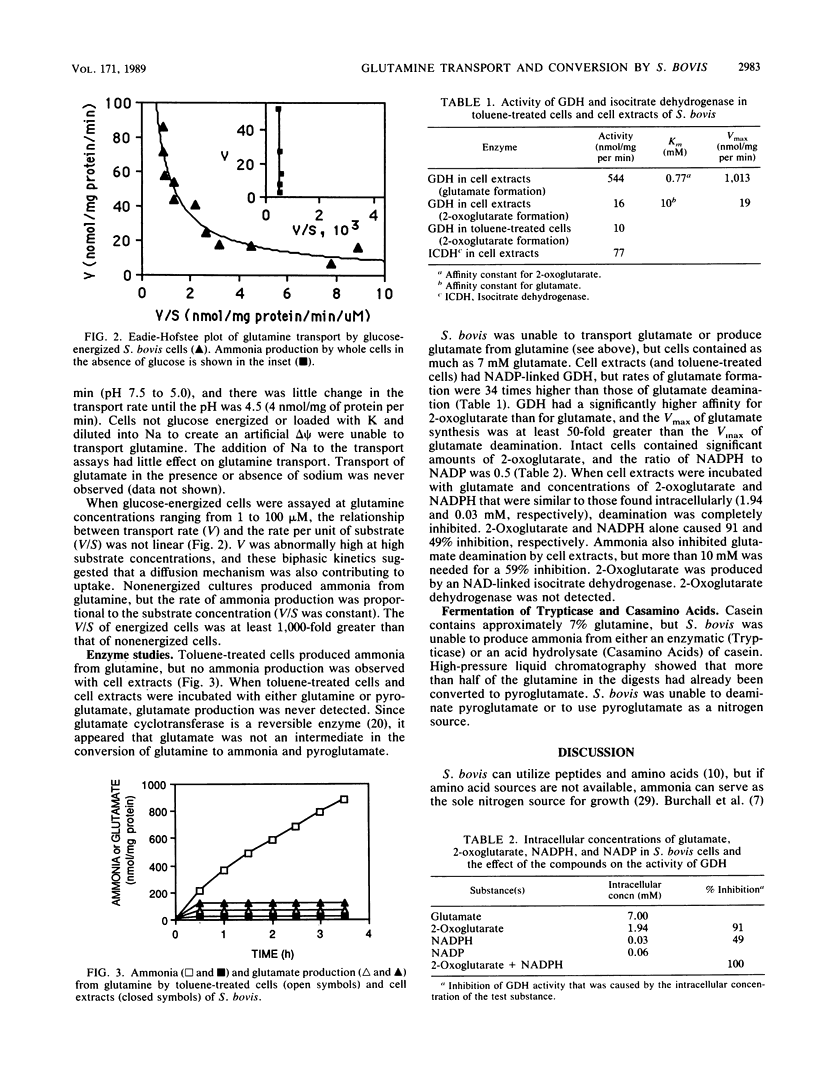
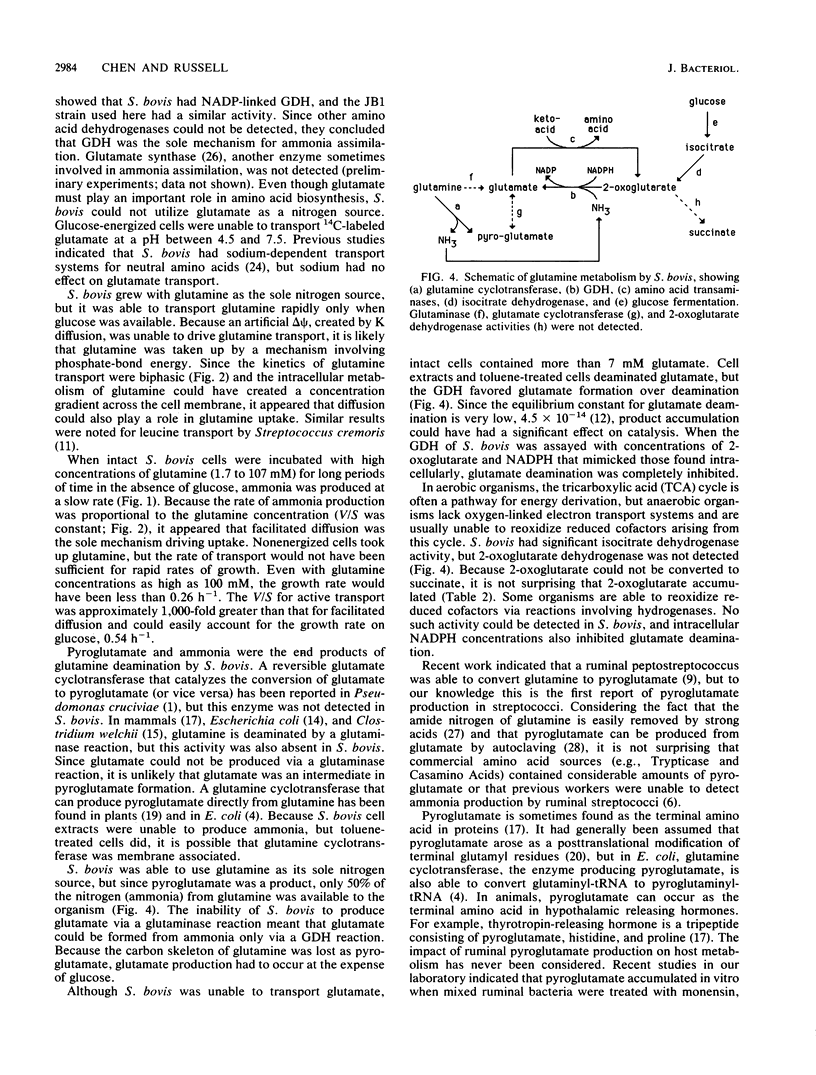
Streptococcus bovis JB1 cells energized with glucose transported glutamine at a rate of 7 nmol/mg of protein per min at a pH of 5.0 to 7.5; sodium had little effect on the transport rate. Because valinomycin-treated cells loaded with K and diluted into Na (pH 6.5) to create an artificial delta psi took up little glutamine, it appeared that transport was driven by phosphate-bond energy rather than proton motive force. The kinetics of glutamine transport by glucose-energized cells were biphasic, and it appeared that facilitated diffusion was also involved, particularly at high glutamine concentrations. Glucose-depleted cultures took up glutamine and produced ammonia, but the rate of transport per unit of glutamine (V/S) by nonenergized cells was at least 1,000-fold less than the V/S by glucose-energized cells. Glutamine was converted to pyroglutamate and ammonia by a pathway that did not involve a glutaminase reaction or glutamate production. No ammonia production from pyroglutamate was detected. S. bovis was unable to take up glutamate, but intracellular glutamate concentrations were as high as 7 mM. Glutamate was produced from ammonia via a glutamate dehydrogenase reaction. Cells contained high concentrations of 2-oxoglutarate and NADPH that inhibited glutamate deamination and favored glutamate formation. Since the carbon skeleton of glutamine was lost as pyroglutamate, glutamate formation occurred at the expense of glucose. Arginine deamination is often used as a taxonomic tool in classifying streptococci, and it had generally been assumed that other amino acids could not be fermented. To our knowledge, this is the first report of glutamine conversion to pyroglutamate and ammonia in streptococci.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F. Nitrogen metabolism in the sheep; protein digestion in the rumen. Biochem J. 1956 Dec;64(4):705–714. doi: 10.1042/bj0640705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURCHALL J. J., NIEDERMAN R. A., WOLIN M. J. AMINO GROUP FORMATION AND GLUTAMATE SYNTHESIS IN STREPTOCOCCUS BOVIS. J Bacteriol. 1964 Oct;88:1038–1044. doi: 10.1128/jb.88.4.1038-1044.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M. R., Nestor L. The enzymatic conversion of glutaminyl-tRNA to pyrrolidone carboxylate-tRNA. Biochem Biophys Res Commun. 1968 Dec 9;33(5):843–849. doi: 10.1016/0006-291x(68)90238-6. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Chen G. J., Russell J. B. Fermentation of peptides and amino acids by a monensin-sensitive ruminal Peptostreptococcus. Appl Environ Microbiol. 1988 Nov;54(11):2742–2749. doi: 10.1128/aem.54.11.2742-2749.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Strobel H. J., Russell J. B., Sniffen C. J. Effect of hydrophobicity of utilization of peptides by ruminal bacteria in vitro. Appl Environ Microbiol. 1987 Sep;53(9):2021–2025. doi: 10.1128/aem.53.9.2021-2025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Jong S., Konings W. N. Transport of branched-chain amino acids in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1987 Nov;169(11):5193–5200. doi: 10.1128/jb.169.11.5193-5200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- HUGHES D. E., WILLIAMSON D. H. Some properties of the glutaminase of Clostridium welchii. Biochem J. 1952 Apr;51(1):45–55. doi: 10.1042/bj0510045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E., DOUGHERTY R. W., BRYANT M. P., CELLO R. M. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 1952 Oct;42(4):423–449. [PubMed] [Google Scholar]
- Hartman S. C. Glutaminase of Escherichia coli. I. Purification and general catalytic properties. J Biol Chem. 1968 Mar 10;243(5):853–863. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MESSER M. Enzymatic cyclization of L-glutamine and L-glutaminyl peptides. Nature. 1963 Mar 30;197:1299–1299. doi: 10.1038/1971299a0. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Bottje W. G., Cotta M. A. Degradation of protein by mixed cultures of rumen bacteria: identification of Streptococcus bovis as an actively proteolytic rumen bacterium. J Anim Sci. 1981 Jul;53(1):242–252. doi: 10.2527/jas1981.531242x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Sniffen C. J., Van Soest P. J. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J Dairy Sci. 1983 Apr;66(4):763–775. doi: 10.3168/jds.S0022-0302(83)81856-6. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., MANNING G. B., NELSON W. O. Ammonium salts as a sole source of nitrogen for the growth of Streptococcus bovis. J Bacteriol. 1959 Jul;78(1):147–147. doi: 10.1128/jb.78.1.147-147.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Pyridine Nucleotide Content in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1963 Jan;38(1):45–54. doi: 10.1104/pp.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


