Abstract
By regulating matrix metalloproteinase (MMP) activity and controlling the breakdown of extracellular matrix components, tissue inhibitors of metalloproteinases (TIMPs) play an important role in the process of tumor invasion and metastasis. The present study was designed to clarify the role of TIMP-2 in nasopharyngeal carcinoma (NPC) patients and to evaluate its importance relative to clinicopathologic parameters. It was carried out in 30 patients with NPC and 20 controls. Tissue biopsies were studied and graded pathologically, and Western blot analysis was performed to assess TIMP-2 protein expression. Clinically, in accordance with TNM classification (T: tumor size, N: lymph node involvement, M: distant metastasis), 8 cases were diagnosed as stage II, 12 as stage III, and 10 cases as stage IV; however, pathologic typing with use of the World Health Organization (WHO) classification revealed the presence of 9 specimens of squamous cell carcinoma (WHO type 1), 6 cases of nonkeratinizing carcinoma (WHO type 2), and 15 cases of undifferentiated carcinoma (WHO type 3). The difference in percentage of TIMP-2 positivity between NPC patients (76.6%) and normal controls (30%) was statistically highly significant (P < .01). In addition, there was a significant positive correlation between TIMP-2 protein positivity and either the clinical staging or the histopathologic typing (P < .01) using Chi-square test (x2), suggesting that TIMP-2 can be used as a marker of the severity of NPC.
Accordingly, we can assume that TIMP-2 may play a role in regional lymph node and/or distant metastasis and in progression of squamous cell carcinoma. Further studies are needed to investigate the role of TIMP-2 as a marker for tumor progression and to evaluate its potential value in the follow-up of patients.
Introduction
Proteolytic degradation of the extracellular matrix (ECM) is a fundamental aspect of cancer development and a key event in the regulation of tumor proliferation and metastasis. Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that are collectively capable of degrading most components of the basement membrane and ECM, facilitating cell migration.[1] Given their ubiquitous presence, MMPs play an important role in tissue breakdown and remodeling during both normal and pathologic conditions.[2] MMPs are secreted as inactive proenzymes and are transformed into active forms after cleavage of a propeptide domain of the molecule.[3] On the basis of their structure, cell localization, and substrate specificity, MMPs are categorized into several groups such as collagenases, gelatinases, stromelysins, and membrane-type MMPs.[4] MMPs are tightly regulated at various levels, including expression level, latent form activation, and the balance between enzyme levels and their inhibitors (TIMPs). TIMPs are the major endogenous regulators of MMPs and consist of 4 homologous members (TIMP 1-4). The balance between MMPs and TIMPs is critical in maintaining the integrity of ECM and its regulatory role in organ development, cell growth and differentiation.[5] Although each TIMP appears capable of inhibiting several MMPs, these proteins exhibit preferential inhibitory capacity (eg, TIMP-1 and -2 selectively inhibit MMP 9 and 2, respectively.[6]
Although early studies have shown TIMPs to have antitumor or antimetastatic effects, more recent reports indicate a dual function, with positive correlation between increased TIMP levels and poor outcome in some human malignancies. The mechanisms supporting the paradoxical positive effect of TIMPs in tumor progression are not completely understood and are the subject of intense investigation. This tumor-promoting activity may be attributable to either excessive proteolytic degradation of ECM, which leads to impairment of tumor cell adhesion and disruption of the cell-matrix interactions required for migration and invasion, or direct influence on cell survival and growth.[7]
The expressions of MMP family members in head and neck squamous cell carcinoma (SCC) tissues are reported widely; however, the correlation of these expressions with clinical features is still controversial.[8–15] Some studies demonstrated that the expressions of MMP family correlated with histologic grade,[9,11,14] tumor invasion,[13] clinical stage,[15] and/or lymph node involvement,[8–13,15] although their results were not uniform. Furthermore, a majority of previous studies analyzed these expressions in patients with a wide variety of tumor (T1–4) and node (N0–3) classifications. There is little known about the predictive value of expression of MMP family members for clinical outcomes and prognosis.
In this study, we used Western blot analysis to determine whether there is a correlation between TIMP-2 protein positivity and the incidence of nasopharyngeal carcinoma (NPC), clinical staging, and histopathologic typing. A better understanding of the role of TIMP-2 protein may enable us to design new therapeutic approaches for the management of NPC patients.
Subjects and Methods
Our study comprised 30 patients with NPC who attended the Kasr El-Aini ENT Clinic during the period 2004–2006 and 20 controls (during endoscopic sinus operation for sinusitis or allergic nasal polypi and well known to have no malignancy). NPC patients were subjected to full history taking, clinical examination with respect to nasopharyngeal region, computed tomography, magnetic resonance imaging, and nasopharyngoscopy to determine the full extent of the local spread of the tumor. Cases were categorized according to TNM classification.[16]
Sample Collection
Nasopharyngeal biopsies (from the NPC patients and the controls) were divided into 2 parts. The first specimen was studied and graded pathologically as one of the 3 predominant histologic types according to the World Health Organization (WHO) classification,[17] and the second was transferred into a liquid nitrogen container and washed with ice-cold saline. Fat and necrotic tissues were removed and tissues were stored at −80°C until used.
Detection of TIMP-2 Protein Expression
TIMP-2 protein expression was detected using immunoblotting technique (Western blot) according to the method of Sambrook.[18]
Sample preparation
Tissue samples were transferred into a clean Eppendorf tube and solubilized in 500 microliters ice-cold lysing buffer (HEPES 0.1mol/L, glycerol 10%, K2EDTA 1 mol/L, Triton-X-100 10 mol/L, and NaCl 0.5 mol/L). Protease inhibitors (benzamidine 10 mmol/L, beta-mercaptoethanol 10 mmol/L, aprotinin 5 mg/L, and PMSF 0.39 mmol/L) were freshly added to the lysing buffer before use. Solubilized tissues were sonicated on ice using an ultrasonic homogenizer (Ultra-Turax T25, IKA-Lab, Switzerland) for 3 bursts of 1 minute each, then centrifuged for 30 minutes at 18,000 rpm at 4°C. The supernatant was collected, aliquoted, and stored at −80°C until used.
Protein concentration was measured according to the method of Bradford.[19] SDS-polyacrylamide gel electrophoresis of proteins (SDS/PAGE): 1-dimensional slab gel eletrophoresis was done according to the standard protocol of Laemmli[20] with some modifications.
Electrophoretic separation of protein samples was followed by quantitative electrotransfer as described by Sambrook[18] using a mini transcellulose membrane (Hoefer Scientific Instruments, San Francisco, California).
Statistical Methods
Version 10 Statistical Package for Social Science software (SPSS Inc., Chicago, Illinois) was used to analyze the data. Univariate analysis was performed using the Chi-square method to find out the relation between various qualitative data. Comparisons of variables among subgroups were made using the Mann-Whitney test. Pearson's x2 test with continuity correction and Mann-Whitney test were used to assess correlations between TIMP-2 expression and clinicopathologic parameters of nasopharyngeal carcinomas.
Results
The study comprised 20 controls and 30 cases of NPC that were staged clinically according to TNM classification. Eight cases were diagnosed as stage II, 12 cases as stage III, and 10 cases as stage IV; however, histopathologic examination of biopsies taken revealed the presence of 9 specimens classified as WHO type I, 6 specimens classified as WHO type 2, and 15 specimens classified as WHO type 3 (Table 1).
Table 1.
Clinical and Histopathologic Categorization of Nasopharyngeal Carcinoma Cases
| Clinical Staging | Histopathologic Typing | ||
|---|---|---|---|
| Stage II | 8 (26.7%) | WHO type 1 | 9 (30%) |
| Stage III | 12 (40%) | WHO type 2 | 6 (30%) |
| Stage IV | 10 (33.3%) | WHO type 3 | 51 (50%) |
Stage II (T2N0M0)
Stage III (T3N0M0-T1,2 or3,N1M0)
Stage IV (T4N0M0-any T,N2 or N3,.M0-any T, any N,M1)
T1: Tumor confined to 1 site or no tumor visible
T2: Involving 2 sites (both posterosuperior and lateral wall)
T3: Extension into nasal cavity or oropharynx
T4: Tumor invasion of skull or cranial nerve involvement (or both)
N1: Metastasis in a single ipsilateral lymph node < 3 cm
N2: Metastasis in contralateral, bilateral, multiple lymph nodes or > 3 cm but < 6 cm
N2: Metastasis in lymph nodes > 6 cm
M0: No distant metastasis
M1: Distant metastasis.
WHO type 1: Squamous cell carcinoma
WHO type 2: Nonkeratinizing carcinoma
WHO type 3: Undifferentiated carcinoma that is lymphoepithelioma and anaplastic carcinoma
Figure 1 shows the percent positivity of TIMP-2 protein in malignant cases. Figure 2 illustrates the comparison between the patients with malignancies and the controls.
Figure 1.
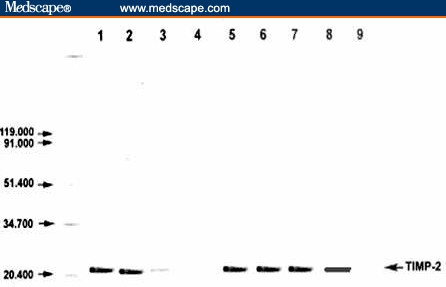
Detection of TIMP-2 protein by Western blot in NPC patients (Lanes 2–8). TIMP-2 protein (21 KDa) was detected at the expected molecular weight (Lanes 2, 3, 5–8). Recombinant TIMP-2 protein positive control (Lane 1) and negative control for the immunodetection system (Lane 9) were included.
Figure 2.
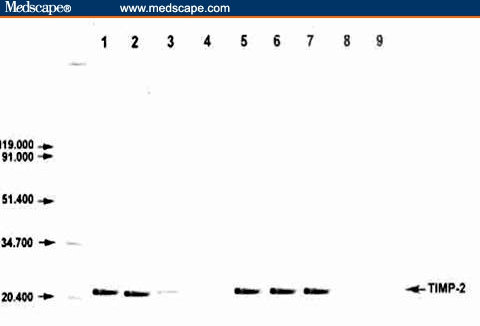
Detection of TIMP-2 protein by Western blot in NPC patients (Lane 2) and controls (Lanes 3–9). TIMP-2 protein (21 KDa) was detected at the expected molecular weight (Lanes 2, 5–7). Recombinant TIMP-2 protein (Lane 1) was included as a positive control.
The difference in percentage of TIMP-2 positivity between NPC patients and controls was statistically highly significant (Table 2), as determined by Chi-square test (x2). There was a significant positive correlation between TIMP-2 protein positivity and either the clinical staging or the histopathologic typing (P < .01) using Chi-square test (x2) (Table 3).
Table 2.
Percent Positivity of TIMP-2 Protein: Comparison Between Nasopharyngeal Carcinoma Patients and Controls
| Positivity of TIMP-2 | Malignant Cases | Controls |
|---|---|---|
| 23/30 | 6/20 | |
| 76.6% | 30% | |
| Pearson's Chi-squared test (x2) | 9.1 | |
| P-value | < .01* | |
Highly significant
Table 3.
TIMP-2 Protein Positivity in Relation to Clinical Staging and Histopathologic Typing
| TIMP-2 | Histopathologic Typing | Clinical Staging | |||||
|---|---|---|---|---|---|---|---|
| Stage II | Stage III | Stage IV | WHO 1 | WHO 2 | WHO 3 | ||
| (−) | 4 | 3 | 0 | 4 | 2 | 3 | |
| (+) | 4 (50%) | 9 (75%) | 10 (100%) | 5 (44.4%) | 3 (60%) | 12 (80%) | |
| Pearson's Chi-squared test (x2) | 9.3 | 9.1 | |||||
| P* value | < .01 | < .01 | |||||
P < .01 = highly significant
Discussion
ECM turnover is an event that is tightly regulated. Much of the coordinate physiologic or discoordinate pathologic degradation of the ECM is catalyzed by class of proteases known as metalloproteinases. MMPs are a family of multidomain enzymes that are controlled by a class of specific tissue inhibitors of MMPs (TIMPs). TIMP-2 is an endogenous regulator and a potent inhibitor of MMPs.[21]
Recent in vitro studies[22–24] have clarified the mechanism of cell-mediated MMP-2 activation in tumor tissue, ie, pro-MMP-2 secreted by fibroblasts binds to TIMP-2 in combination with MT1-MMP on the cell surface, and the pro-MMP-2/TIMP-2/MT1-MMP ternary complex is formed. The pro-MMP-2 in the ternary complex is activated by adjacent MT1-MMP that is free from TIMP-2,[22–24] and the activated MMP-2 degrades ECM components. Therefore, on the basis of this theory, the significant association between MMP-2 expression and the expression of either MT1-MMP or TIMP-2 observed here is very reasonable. On the other hand, it is reported that different transcriptional regulation of MMP-2 and MMP-9 genes may be due to the presence of different promoter elements.[25]
In the present study, we attempted to demonstrate a correlation between TIMP-2 protein positivity and the incidence of NPC. We used Western blot analysis to evaluate the expression of TIMP-2 protein in cell lysate from tissue biopsies (from 30 NPC patients and 20 controls). The percent positivity of TIMP-2 protein was statistically highly significant (P < .01) in cancer patients (76.6%) compared with the controls (30%).
This result is consistent with that reported by Katayama and colleagues,[26] who found marked expression of MMP-2 and tissue inhibitor of MMP-2 in early-stage oral SCC. These MMP family members were mainly expressed on the cell surface and in the cytoplasm of tumor cells as well as on some endothelial cells or on the stromal fibroblasts surrounding tumor cells.
In addition, results of TIMP-2 expression in other malignant tumors confirmed the findings of the present study. The expression of TIMP-2 protein was found to be significantly higher in ovarian carcinoma,[27] prostatic adenocarcinoma,[28] thyroid cancer,[29] colorectal cancer,[30] and breast cancer.[31]
In general, TIMP-2 had been believed to suppress tumor invasion and metastasis by inhibiting MMP-2, because TIMP-2 inhibits collagenolysis activity of MMP-2 in vitro.[32] However, according to the model of cell-mediated MMP-2 activation, pro-MMP-2 bound to the TIMP-2/MT1-MMP complex is activated by MT1-MMP that is free from TIMP-2. The activated form of MMP-2 is inactivated by the attachment of TIMP-2.[22–24] Recently, high activation of MMP-2 in tongue SCC samples, detected by gelatin zymographic assay, has been reported to be associated with high expression of TIMP-2 in tumor cells.[15] Therefore, the immunoreactivity of TIMP-2 is likely to be useful for monitoring MMP-2 activation.
Moreover, in an attempt to determine the possible role of TIMP-2 protein expression in regional lymph node and/or distant metastasis and in progression of NPC, we tried to find a correlation between percent TIMP-2 protein positivity and either clinical staging or histopathologic typing. Indeed, we found a positive significant correlation with both clinical staging and histopathologic typing: the percentage of protein positivity increased with their gradual increase (P < .01). Our results are in accordance with those reported in recent immunohistologic studies,[14,33–36] indicating that TIMP-2 plays a positive role in tumor metastasis and that high expression of TIMP-2 correlates with lymphatic invasion and lymph node metastasis. Kallakury and colleagues[37] and Kamayama and colleagues[38] also found a significant correlation between TIMP-2 expression and advanced bladder cancer stage (P < .05).
By contrast, Grignon and colleagues,[39] and Ree and colleagues[40] found that TIMP-2 expression did not correlate with the stage and grade of bladder cancer and breast cancer, respectively, and Oberge and colleagues[41] reported that TIMP-2 protein expression had no significant association with the stage of colorectal cancer and therefore concluded that TIMP-2 protein may be of limited value in colorectal tumor staging.
In summary, the results of our study suggest that TIMP-2 may participate in regional lymph node and/or distant metastasis and may be a useful marker of severity of SCC, but further studies are needed to investigate the role of TIMP-2 in tumor progression and to evaluate its potential value in the follow-up of NPC patients.
Footnotes
Readers are encouraged to respond to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
Contributor Information
Amr Ahmed El Badry, Cairo University, Cairo, Egypt.
Amal Abou El-Fadle, Benha University, Benha, Egypt.
Abdel Latif El-Balshy, Benha University, Benha, Egypt.
References
- 1.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 2.Kugler A. Matrix metalloproteinases and their inhibitors. Anticancer Res. 1999;19:1589–1592. [PubMed] [Google Scholar]
- 3.Carmeliet P, Moons L, Lijnen R. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–944. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 4.Curran S, Murray GI. Matrix metalloproteinase in tumour invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 6.Baker AH, Ahonen M, Kahari VM. Potential applications of tissue inhibitor of metalloproteinase (TIMP) overexpression for cancer gene therapy. Adv Exp Med Biol. 2000;465:469–483. doi: 10.1007/0-306-46817-4_41. [DOI] [PubMed] [Google Scholar]
- 7.Kallakury BV, Karikehalli S, Haholu A, Sheehan C, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–3119. [PubMed] [Google Scholar]
- 8.Kurahara S, Shinohara M, Ikebe T, et al. Expression of MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of the oral cavity: correlations with tumor invasion and metastasis. Head Neck. 1999;21:627–638. doi: 10.1002/(sici)1097-0347(199910)21:7<627::aid-hed7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Imanishi Y, Fujii M, Tokumaru Y, et al. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31:895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H, Ueno H, Yamashita K, et al. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human papillary thyroid carcinomas. Cancer Res. 1999;59:467–473. [PubMed] [Google Scholar]
- 11.Riedel F, Gotte K, Schwalb J, Bergler W, Hormann K. Expression of 92-kDa type IV collagenase correlates with angiogenic markers and poor survival in head and neck squamous cell carcinoma. Int J Oncol. 2000;17:1099–1105. doi: 10.3892/ijo.17.6.1099. [DOI] [PubMed] [Google Scholar]
- 12.O-charoenrat P, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001;127:813–820. [PubMed] [Google Scholar]
- 13.Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am J Clin Pathol. 1993;99:18–23. doi: 10.1093/ajcp/99.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizaki T, Maruyama Y, Sato H, Furukawa M. Expression of tissue inhibitor of matrix metalloproteinase-2 correlates with activation of matrix metalloproteinase-2 and predicts poor prognosis in tongue squamous cell carcinoma. Int J Cancer. 2001;95:44–50. doi: 10.1002/1097-0215(20010120)95:1<44::aid-ijc1008>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizaki T, Sato H, Maruyama Y, et al. Increased expression of membrane type 1-matrix metalloproteinase in head and neck carcinoma. Cancer (Phila) 1997;79:139–144. doi: 10.1002/(sici)1097-0142(19970101)79:1<139::aid-cncr20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Classification of malignant tumors. American Joint Committee on Cancer Manual for Staging of Cancer. 3rd edition. Philadelphia: J.B. Lippincott; 1988. pp. 24–62. [Google Scholar]
- 17.Neel HB, Pearson GR, Taylor WF. Application of Epstein Barr virus serology to the diagnosis and staging of North American patients with nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 1983;91:255. doi: 10.1177/019459988309100310. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of micrograms quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;7:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Morgunova E, Tuuttilla A, Bergmann U, Tryggvason K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc Natl Acad Sci U S A. 2002;99:7414–7419. doi: 10.1073/pnas.102185399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai K, Ohuchi E, Aoki T, et al. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinases 2. Cancer Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- 23.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 24.Sato H, Takino T, Kinoshita T, et al. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP) FEBS Lett. 1996;385:238–240. doi: 10.1016/0014-5793(96)00389-4. [DOI] [PubMed] [Google Scholar]
- 25.Huhtala P, Tuuttila A, Chow LT, Lohi J, Keski-Oja J, Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991;266:16485–16490. [PubMed] [Google Scholar]
- 26.Katayama A, Bandoh N, Kishibe K, Takahara M, Ogino T, Nonaka S, Harabuchi Y. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res. 2005;11:3257–3264. doi: 10.1158/1078-0432.ccr-0864-02. [DOI] [PubMed] [Google Scholar]
- 27.Cai W, Song JD. [Expression of matrix metalloproinase-2 with tissue inhibitor of metalloproteinase-2 in ovarian tumors.] Ai Zheng. 2002;1:91–94. [PubMed] [Google Scholar]
- 28.Ross RK, Paganini-Hill A, Hender BE. Epidemiology of bladder cancer. In: Skinner DC, Leiskovsky G, editors. Diagnosis and Management of Genitourinary Cancer. London and Philadelphia: W.B. Saunders Company; 1988. pp. 25–40. [Google Scholar]
- 29.Korem S, Kraiem Z, Shiloni E, Yehezkel O, Sadek O, Resnick MB. Increased expression of matrix metalloproteinase-2: a diagnostic marker but not prognostic marker of papillary thyroid cancer. Isr Med Assoc J. 2002;4:247–251. [PubMed] [Google Scholar]
- 30.Chan CC, , Menages M, Orzechowski HD. Increased matrix metalloproteinase-2 concentration and transcript expression in advanced colorectal carcinoma. Int. J. Colorectal Dis. 16:133–140. doi: 10.1007/s003840100287. [DOI] [PubMed] [Google Scholar]
- 31.Nakopoulou L, Katsaroi S, Giannopoulou I. Correlation of tissue matrix metalloproteinase-2 inhibitor with proliferative activity and patient's survival in breast cancer. Mod Pathol. 2002;15:26–34. doi: 10.1038/modpathol.3880486. [DOI] [PubMed] [Google Scholar]
- 32.Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2) J Biol Chem. 1989;264:17374–17378. [PubMed] [Google Scholar]
- 33.Gohji K, Fujimoto N, Ohkawa J, Fujii A, Nakajima M. Imbalance between serum matrix metalloproteinase-2 and its inhibitor as a predictor of recurrence of urothelial cancer. Br J Cancer. 1998;77:650–655. doi: 10.1038/bjc.1998.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanayama H, Yokota K, Kurokawa Y, Murakami Y, Nishitani M, Kagawa S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer (Phila) 1998;82:1359–1366. [PubMed] [Google Scholar]
- 35.Visscher DW, Hoyhtya M, Ottosen SK, et al. Enhanced expression of tissue inhibitor of metalloproteinase-2 (TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. Int J Cancer. 1994;59:339–344. doi: 10.1002/ijc.2910590308. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi R, Noguchi T, Takeno S, Kubo N, Uchida Y. Immunohistochemical detection of membrane-type-1-matrix metalloproteinase in colorectal carcinoma. Br J Cancer. 2000;83:215–218. doi: 10.1054/bjoc.2000.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinase-2 and tissue inhibitors of matrix metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;10:3113–3119. [PubMed] [Google Scholar]
- 38.Kanayama H. Matrix metalloproteinases and bladder cancer. J Med Invest. 2001;48:31–43. [PubMed] [Google Scholar]
- 39.Grignon DJ, Sakrw Toth M, Ravery V, et al. High levels of tissue inhibitor of metalloproteinase-2 expression are associated with poor outcome in invasive bladder cancer. Cancer Res. 1996;56:1654–1659. [PubMed] [Google Scholar]
- 40.Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland IM, Foddstad O. High levels of mRNA for TIMP-1 and TIMP-2 in primary breast cancer are associated with development of distant metastasis. Clin Cancer Res. 1997;3:1623–1628. [PubMed] [Google Scholar]
- 41.Oberge A, Hoykhtya M, Tavelin B, Stenling R, Lindmar KG. Limited value of preoperative serum analysis of matrix metalloproteinases (MMP-2, MMP-9) and tissue inhibitors of matrix metalloproteinases (TIMP-1, TIMP-2) in colorectal cancer. Anticancer Res. 2000;20:1085–1091. [PubMed] [Google Scholar]


